1 Laboratory of molecular biology, Research Centre for Medical Genetics, 115478 Moscow, Russia
2 Department of Physiology, I.M. Sechenov First Moscow State Medical University (Sechenov University), 119048 Moscow, Russia
3 Zhengzhou Research Institute, Harbin Institute of Technology, 450007 Zhengzhou, Henan, China
4 Federal Research Center for Problems of Chemical Physics and Medicinal Chemistry of RAS, 142432 Chernogolovka, Russia
Abstract
The new synthesized water-soluble derivatives of C60 fullerenes are of a great interest to researchers since they can potentially be promising materials for drug delivery, bioimaging, biosonding, and tissue engineering. Surface functionalization of fullerene derivatives changes their chemical and physical characteristics, increasing their solubility and suitability for different biological systems applications, however, any changes in functionalized fullerenes can modulate their cytotoxicity and antioxidant properties. The toxic or protective effect of fullerene derivatives on cells is realized through the activation or inhibition of genes and proteins of key signaling pathways in cells responsible for regulation of cellular reactive oxygen species (ROS) level, proliferation, and apoptosis.
The 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay was used to assess cells viability. Flow cytometry analyses was applied to measure proteins levels in human embryonic lung fibroblasts (HELF) cells. HELF is a standard, stable and well described human cell line that can be passaged many times. Quantitation of ROS was assessed using H2DCFH-DA. Fluorescence images were obtained using microscopy. Expression of BCL2, CCND1, CDKN2A, BRCA1, BAX, NFKB1, NOX4, NRF2, TBP (reference gene) was analyzed using real-time Polymerase chain reaction (PCR).
We found that high and low concentrations of fullerene C60 derivatives with the five residues of potassium salt of 6-(3-phenylpropanamido)hexanoic (F1) or 6-(2-(thiophen-2-yl)acetamido)hexanoic (F2) acid and a chlorine atom attached directly to the cage cause diametrically opposite activation of genes and proteins of key signaling pathways regulating the level of oxidative stress and apoptosis in HELF. High concentrations of F1 and F2 have a genotoxic effect, causing NADPH oxidase 4 (NOX4) expression activation in 24–72 hours (2–4 fold increase), ROS synthesis induction (increase by 30–40%), DNA damage and breaks (2–2.5 fold 8-oxodG level increases), and activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) (by 40–80%) against the background of reduced NF-E2-related factor 2 (NRF2) expression (by 20–45%). Low concentrations of F1 and F2 produced a cytoprotective effect: in 24–72 hours they reduce the oxidative DNA damage (by 20–40%), decrease the number of double-strand DNA breaks (by 20–30%), increase the level of anti-apoptotic proteins and enhance the antioxidant response activating the NRF2 expression (NRF2 gene expression increases 1.5–2.3 fold, phosphorylated form of the NRF2 protein increases 2–3 fold).
Obtained results show that in low doses studied fullrens may serve as perspective DNA protectors against the damaging genotoxic factors.
Keywords
- water-soluble fullerene derivatives
- antioxidant activity
- gene expression
- concentration-dependent biological response of HELF
Carbon nanomaterials are of great interest in the field of nanotechnology due to their unique properties, they have unique electrical, thermal, and optical characteristics that make them promising materials for drug delivery, bio-imaging, biosonding, and tissue engineering [1, 2]. Surface functionalization of fullerene derivatives changes their chemical and physical properties, which increases their solubility, biosafety, and suitability for application in biological systems [3, 4, 5]. Fullerene derivatives covalently attached to drugs via a linker were proved to be more effective than the naive drug [6]. Water-soluble fullerene derivatives with attached residues of amino acid amides represent one of the most promising classes of water-soluble fullerenes.
Fullerene derivatives are considered as human immunodeficiency virus inhibitors [7], contrast agents for magnetic resonance imaging [8], antioxidants [9], antibacterial agents [10], vectors for targeted therapy [11], and sensitizers for photodynamic therapy [12]. The variety of carbon atoms used for fullerene derivatives synthesis, various surface modifications of fullerenes, and numerous synthesis methods provide a wide range of fullerene derivatives [4]. Among all fullerenes types, C60 fullerene derivatives are most widely used [13].
Various factors may be responsible for the carbon nanomaterials toxicity. The cytotoxicity of fullerene derivatives can be affected by their size, shape, aspect ratio, and residual contamination of the final product with their synthesis byproducts, determining the nanocompounds effects on cellular processes both in the cytoplasm and in the nucleus [5, 6, 7, 14]. After surface functionalization fullerenes can demonstrate direct biological effects, e.g., antioxidant activity [5]. In biomedicine, it has been found that fullerene C60 derivatives can be used as powerful free radical scavengers with antioxidant and antibacterial effects [15]. Both pure and modified fullerenes, due to their small size (less than 1 nm), can penetrate into the intracellular space or accumulate on the cell membrane, threatening cellular functions and integrity [16]. In addition, fullerene derivatives can damage lipids and DNA and cause cell death, since they are able not only to scavenge but also to stimulate reactive oxygen species (ROS) synthesis [17]. It has been shown that carbon nanomaterials increase ROS level, which affects the metabolic activity of macrophages, damages the mitochondrial membrane, and leads to the apoptosis [18].
Although a wide range of water-soluble fullerene derivatives have been synthesized before, most of them are represented by complex inseparable mixtures of compounds, such as, for example, fullerenols [19]. Individual water-soluble compounds can be efficiently synthesized from C60Cl6 via regioselective substitution of chlorine atoms with the residues of aminoacids, thioacids, phosphonic acids, and aromatic carboxylic acids [20].
Among the abovementioned types of fullerene-based compounds, water-soluble derivatives with attached residues of amino acid amides are one of the most promising. On one hand, the introduction of aminoacid residues can potentially make fullerene-based compounds more biologically compatible and lead to promising biological activity. On the other hand, aminoacids attached to the fullerene cage directly via C-N bond can be quite easily hydrolyzed in aqueous media, so the introduction of the aromatic amide linker is beneficial to their stability.
Additionally, fullerene derivatives with attached residues of amino acid amides
demonstrated pronounced antiviral and antitumor properties. In our study, we
investigated the effect of the water-soluble fullerene C60 derivatives with
the five residues of potassium salt of 6-(3-phenylpropanamido)hexanoic (F1) or
6-(2-(thiophen-2-yl)acetamido)hexanoic acid (F2) and a chlorine atom attached
directly to the cage (CAS number for F1 is 2408734-09-6, for F2 - 2408734-06-3)
on human cells, which were received by direct arylation from chlorofullerene
C60Cl6 with aromatic amides of
Currently, fullerene derivatives are considered as effective drugs for antiviral
therapy [21, 22, 23, 24, 25], and as delivery systems for antiviral drugs [26, 27]. Fullerene
derivatives, as noted in various studies, protect the organism against the
influenza virus, cytomegalovirus against human immunodeficiency virus (HIV), Ebola virus, and herpes simplex
virus [28, 29, 30, 31]. Fullerene derivative F2 was able to inhibit HIV-1 (isolate BaL) and HIV-2 (isolate ROD)
replication in the low micromolar concentration. Compound F2 was able to
decrease the survival rate of three different types of lung cancer cells (A549,
H460, H1299) down to 27–53% as compared to untreated control [32]. Although
both compounds have five residues of
Electronic absorption and reduction characteristics of fullerene derivatives in biomedical research bring new ideas for oxidative stress treatment in systemic diseases accompanied by oxidative stress, as well as in therapy that stimulates the restoration of tissue defects, in particular, muscle tissue, and peripheral nerves [33, 34]. Although there is great interest in the use of fullerene derivatives in many fields of medicine, there are only limited number of studies devoted to the investigation of fullerenes role in the regulation of the transcriptional activity of genes in various tissues of organism or cell cultures [34, 35].
Despite the interest in the potential toxicological effects of water-soluble derivatives of fullerene C60, little is known about the mechanism of its action on the regulation of gene expression in vitro. The Study is being conducted to evaluate and compare the genotoxic effect and mechanisms of DNA damage when exposed to various types of carbon-based nanocompounds [36]. Several in vivo and in vitro studies have demonstrated the genotoxic potential of carbon nanomaterials in relation to the respiratory system [37], while in other experiments the opposite results were obtained [38]. Discrepancies in the experimental results may indicate that assessment of risk should be carried out for different concentrations of nanocompounds in different cell cultures. For example, it was found that carbon nanomaterials can penetrate the nuclei of respiratory cells, potentially interacting directly with genetic material, enhance ROS production and expression of the TGFB1 gene [36]. In addition, it was shown that for different cell lines, the genotoxicity of carbon-based nanomaterials depended either on the concentration of compounds or on the level of ROS production.
Therefore, additional research is needed to verify the biosafety of newly synthesized fullerene derivatives in human cells before they can be used in medicine. Cytotoxicity of many fullerene derivatives is manifested only when they are used in high concentrations, determining the prospects of their use as independent drugs and for targeted drug delivery [39, 40].
The study of biocompatibility, biodistribution, and biological activity of fullerene derivatives, and their effect on the expression of genes of key signaling pathways in human cells (e.g., regulating antioxidant response and apoptosis) is necessary to determine a therapeutically significant strategy for the further use of newly synthesized fullerene derivatives.
The aim of the present study was to examine the dose-dependent effect of different concentrations of chlorine-containing water-soluble C60 fullerene derivatives (F1 and F2) on the regulation of gene expression in human embryonic lung fibroblasts (HELF), using several techniques to assess the complex biological effects of F1 and F2 on HELF cells.
HELF were chosen as a standard, stable and well described human cell line that can be passaged many times.
Our methods included 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assays to determine cell viability, flow cytometry to analyze protein levels, fluorescence microscopy to visualize cells, and real-time Polymerase chain reaction (PCR) to analyze gene expression.
We suggested, that assessing cell viability alone is not enough to identify the effect of different drug concentrations on the target cells and proposed a complex approach for their investigation including assesement of key signaling pathways genes transcriptional activit. In the present study, we tested such approach, allowing evaluation of compounds effects at the level of gene activity to effectively identify the complex cellular response to the investigated compounds.
Water-soluble fullerene C60 derivatives F1 and F2 (Fig. 1A) have been
synthesized by direct arylation from chlorofullerene C60Cl6 with
aromatic amides of
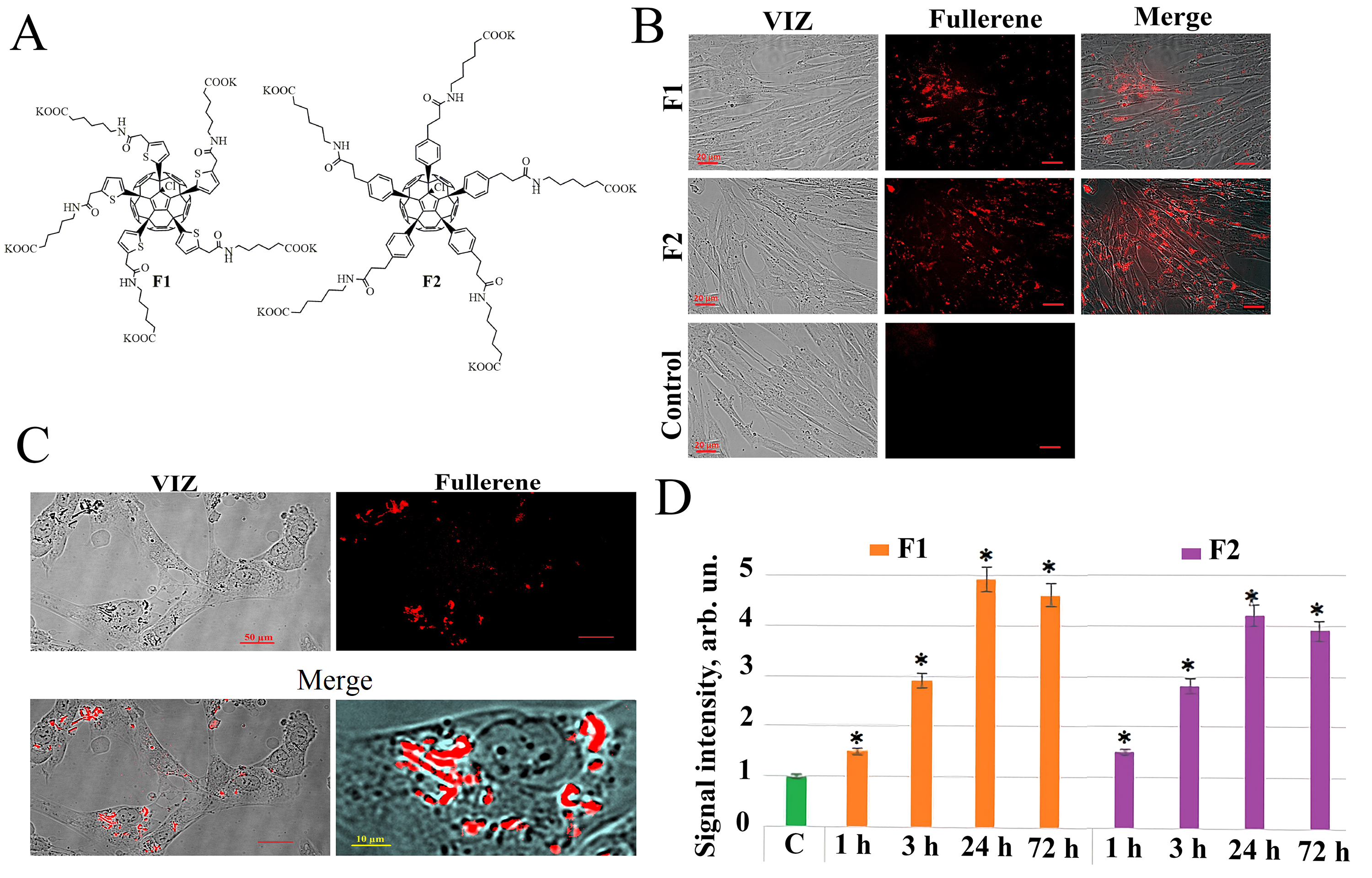 Fig. 1.
Fig. 1.
Fullerenes molecular structures and their penetration into the
HELF. (A) Molecular structure of compounds F1 and F2. (B) Penetration of F1
(concentration 122 µg/mL) and F2 (concentration 122
µg/mL) into HELF after 24 hours of incubation, visualized by
fluorescence microscopy (20
Human embryonic lung fibroblasts (HELF) (the fourth cell passage) provided by the Research Centre for Medical Genetics Biobank were used in experiments. Cell lines were validated by short tandem repeat profiling (STR) profiling and tested negative for mycoplasma.
Cells were cultured at 37 °C in Dulbecco’s Modified Eagle Medium (DMEM) medium (PanEco, Moscow, Russia) with 10% (v/v) fetal calf serum (PAA, Vienna, Austria), 2 mM L-glutamine (PanEco, Moscow, Russia). The medium contained 100 µg/mL of streptomycin, 100 units/mL of penicillin and 10 mg/mL of gentamicin (all reagents were obtained from Sigma-Aldrich, St. Louis, MO, USA). Cells were grown in a humidified atmosphere containing 5% CO2 at 37 °C. After the functionalized fullerenes were added to the medium, the cells were incubated in 96-well plates for time intervals ranging from 1 hour to 72 h.
The study has been granted ethical approval by the Institutional Review Board of the Research Center for Medical Genetics, in accordance with protocol number N.5.
For flow cytometry analyses (FCA) the cells were washed in Versene solution
(Thermo Fisher Scientific, Waltham, MA, USA), and 0.25 % trypsin (Paneco,
Moscow, Russia) under Axio Scope A1 microscope (Carl Zeiss, Oberkochen, Germany) observation. Cells were washed with
culture media, then resuspended in Phosphate-Buffered Saline (PBS) (pH 7.4) (Paneco, Moscow, Russia) and
centrifuged. Paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA) was added at a
final concentration of 2% at 37 °C for 10 min to fix the cells. Then
cells were washed with 0.5% Bovine Serum Albumin (BSA)-PBS (PanEco, Moscow, Russia) (
The following antibodies were used: CY5.5-NADPH oxidase 4 (NOX4) (bs-1091r-cy5-5, Bioss Inc., Woburn, MA, USA);
NF-E2-related factor 2 (NRF2) pSer40 (bs2013, Bioss Inc.); PE-8OHdG (sc-393871 PE, Santa Cruz, Dallas, TX, USA);
DyLight488-
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) tetrazolium reduction assay was used to assess cell viability. The details of the MTT-test are described earlier in [35].
Quantitation of ROS was assessed using 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFH-DA) (10 µM in PBS) (Molecular
Probes/Invitrogen, Carlsbad, CA, USA). After 8
Fluorescence images were obtained using Axio Scope A1 microscope (Carl Zeiss, Oberkochen, Germany) according to the standard procedure as described earlier [35].
Expression levels of the genes BCL2, CCND1, CDKN2A, BRCA1, BAX, NFKB1, NOX4, NRF2, TBP (reference gene) were measured using real-time PCR. The following primers were used (Syntol, Moscow, Russia):
BCL2 (TTTGGAAATCCGACCACTAA; AAAGAAATGCAAGTGAATGA);
CCND1 (TTCGTGGCCTCTAAGATGAAGG; GAGCAGCTCCATTTGCAGC);
CDKN2A (ATGGAGCCTTCGGCTGACT; GTAACTATTCGGTGCGTTGGG);
BRCA1 (GGCTATCCTCTCAGAGTGACATTTTA; GCTTTATCAGGTTATGTTGCATGGT);
BAX (CCCGAGAGGTCTTTTTCCGAG, CCAGCCCATGATGGTTCTGAT);
NFKB1 (CAGATGGCCCATACCTTCAAAT; CGGAAACGAAATCCTCTCTGTT);
NOX4 (TTGGGGCTAGGATTGTGTCTA; GAGTGTTCGGCACATGGGTA);
NRF2 (TCCAGTCAGAAACCAGTGGAT; GAATGTCTGCGCCAAAAGCTG);
TBP (reference gene) (F: GCCCGAAACGCCGAATAT, R: CCGTGGTTCGTGGCTCTCTCT).
RNeasy Mini Kit (Qiagen, Hilden, Germany) was used for isolation of total mRNA. RNA samples were treated with DNase without RNase activity (Silex, Moscow, Russia) to remove the DNA contamination.
RNA concentrations were determined with the help of the dye Quant-iT RiboGreen
RNA reagent (MoBiTec, Goettingen, Germany) using a plate reader (EnSpire equipment, Finland)
(
All reported results were reproduced at least three times as independent
biological replicates. All statistical analyses were performed using StatPlus2007
software (AnalystSoft Inc., Walnut, CA, USA). The significance of the observed
differences was analyzed using non-parametric Mann-Whitney U-tests. All p values
considered statistically significant at p
Cytotoxicity of the studied compounds towards the normal human cells – human embryonic lung fibroblasts (HELF) was estimated using MTT test. We studied effects of water–soluble derivatives of fullerenes F1 and F2 on HELF in concentrations 4.2 ng/mL–2.3 mg/mL. Fullerenes were added to the medium at the beginning of HELF cultivation. The duration of cultivation was 72 hours. Both compounds F1 and F2 did not produce any significant cytotoxic effect on the HELF at a concentration of 4.2 ng/mL–0.5 mg/mL, at a concentration of 0.5 mg/mL–2.3 mg/mL F1 and F2 have a damaging effect on cells, causing the death of more than 20% of cells.
As a result of the analysis of the fluorescent properties of the studied water-soluble fullerene derivatives, it was found that both compounds, F1 and F2 have their own fluorescence in aqueous solutions and cell culture medium. When scanning the excitation spectrum of aqueous solutions of fullerene derivatives (excitation wavelength in the range of 230–600 nm with an emission wavelength of 500 nm), an excitation maximum was detected for the studied compounds at a wavelength of 340 nm. When removing the fluorescence spectrum of the studied compounds at an excitation wavelength of 340 nm, a common emission maximum of 520 nm was also found for these compounds. The fluorescence of the compounds was used to analyze their penetration and localization in cultured human lung embryonic fibroblasts (HELF). Fluorescence signals in cells incubated for 1, 3, 24, and 72 hours with the addition of compounds F1 and F2 were detected during the study of fluorescence in unfixed HELF on a flow cytofluorimeter irradiated with a laser with a wavelength of 370 nm. The fullerene fluorescence signal in cells increases with increase of cultivation time in the presence of fullerene derivatives and reaches a maximum after 24 hours (Fig. 1B). Fluorescence microscopy confirms flow cytofluorometry data obtained in the study of the penetration of F1 and F2 compounds into the cells (Fig. 1B–D).
Thus, we have shown that fluorescence of compounds F1 and F2 allowing the study of their penetration into the cells. It was found that both compounds quickly penetrate through the HELF cytoplasmic membrane—the fluorescence of these compounds in the cells is detected 1–3 hours after addition, in consequent 24 hours the fluorescent signal increases and remains at high level after 72 hours (Fig. 1D).
To study the effects on embryonic fibroblasts, two concentrations of each compound were selected—low, within the range of non–toxic fullerene concentrations—18 ng/mL, and a concentration close to damaging—122 µg/mL, incubation time of cells with compounds—1, 3, 24 and 72 hours. Changes in all values are present in relative units (relative to the control cells cultured without fullerenes).
Most fullerene derivatives demonstrates antioxidant properties in solutions, however, in the living cells interaction of fullerene derivatives with reactive oxygen species (ROS) is more complex. Although most of the water-soluble fullerene compounds can bind ROS, at the same time, the process of free radical synthesis in the cell can start, neutralizing ROS level decrease by fullerene derivatives. In a living cell, ROS are formed in the cytoplasm, on the surface of the cellular membrane and in the nucleus. In addition, cells produce hydrogen peroxide molecules in the intercellular medium. ROS are involved in many cellular processes, such as proliferation, apoptosis and intercellular signaling. On the other hand, excessive ROS synthesis by the cell because of disruption of intracellular processes and/or external exposure can cause cell death or cell damage. The change in the ROS level in cells is the result of the difference in the free radical synthesis activation after exposure to fullerenes and the free radicals binding by the studied compounds.
To assess the level of ROS synthesis occurring in living cells after interaction with compounds F1 and F2 we used H2DCFH-DA (dichlorodihydrofluorescindiacetate), a dye that quickly penetrates cell membranes. In the cytosol, this dye is hydrolyzed to non-permeable 2′,7′-dichlorodihydrofluorescein (DCFH) by cell hydrolases. Non-fluorescent DCFH is a sensitive intracellular marker of oxidative stress, oxidized to intensely fluorescent 2′,7′-dichlorofluorescein (DCF) in the presence of ROS.
When fullerene derivatives F1 and F2 were added to cells at concentration 122 µg/mL, a slight ROS level decrease was observed after 1 hour, ROS level increase (by about 10–20%) after 3 hours (which remained elevated during incubation of cells with F1 and F2 compounds for 24 hours), and significant ROS level increase 72 hours after F1 and F2 additions to the cells (Fig. 2A). Probably, fullerene derivatives F1 and F2 added to the cell culture medium at high concentrations work as free radical acceptors, but over time, free radical synthesis activates in the cells, leading to the ROS level increase after 3–72 hours of cultivation in the presence of these compounds.
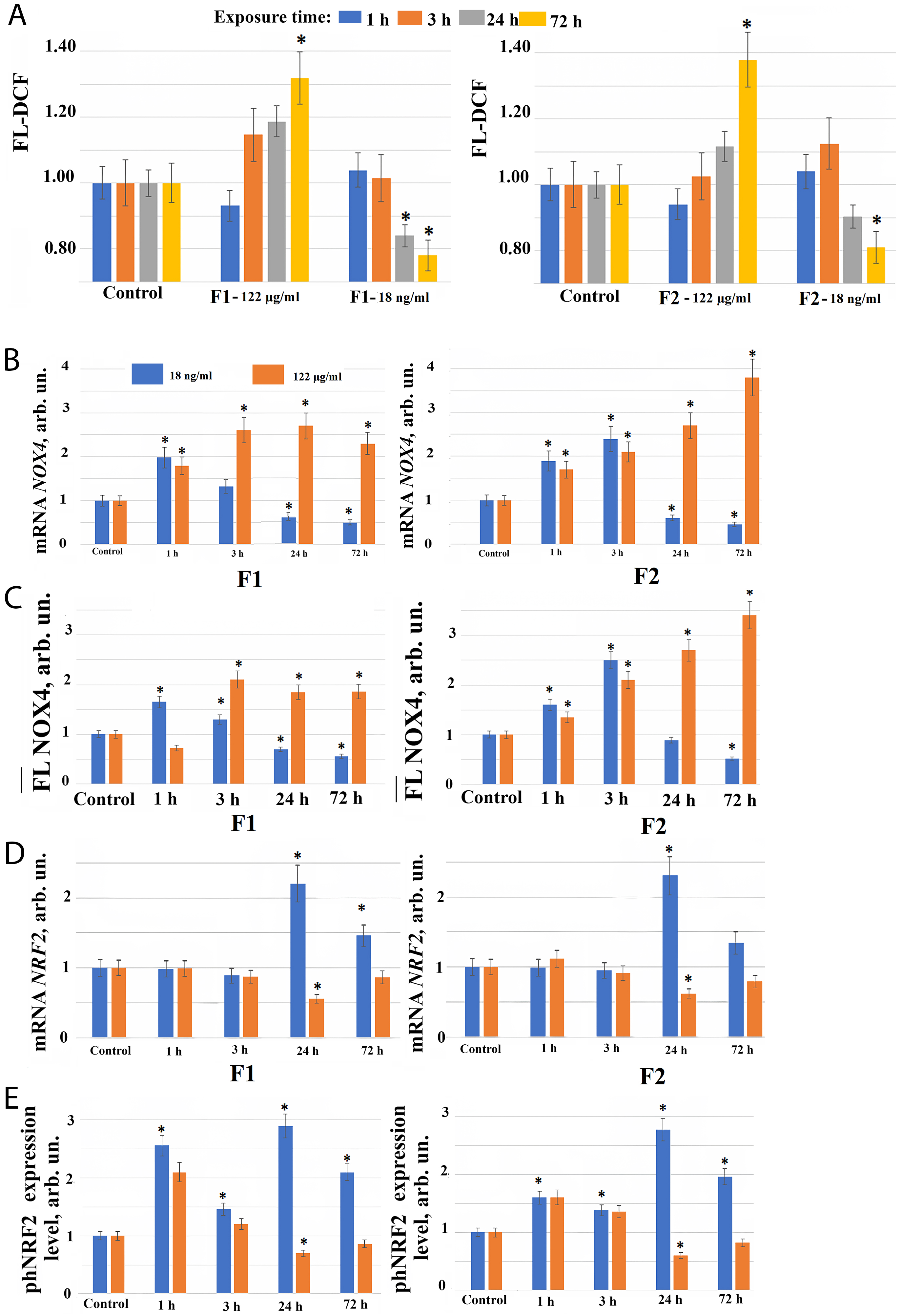 Fig. 2.
Fig. 2.
Reactive Oxygen Species (ROS) synthesis level and the expression of genes in
HELF following the addition of fullerenes. (A) 2′,7′-dichlorofluorescein (DCF) fluorescence in cells during
incubation with F1 (left), and F2 (right) fullerenes at concentration 122
µg/mL and 18 ng/mL (control cells were cultured without fullerenes)
after 1, 3, 24, 72 hours of incubation. (*) – significant differences compared to
control cells, p
However, low fullerene derivatives F1 and F2 concentrations have the opposite effect on the ROS level compared to high concentrations of these compounds. When F2 is added to the cells at a concentration of 18 ng/mL, there is a tendency to increase of the ROS level by 5–12% after 1–3 hours, after 24 hours the ROS level decreases below the control level, and after 72 hours this decrease becomes significant. When F1 is added to cells at a concentration of 18 ng/mL, there is a tendency to increase of the ROS level by 5–7% after 1 hour, and after 24–72 hours ROS level significantly decreases (Fig. 2A).
Possibly ROS level increase by high fullerenes concentration can be explained by the ROS synthesis stimulation, neutralizing the free radicals binding by fullerene derivatives. The ROS generation by mitochondria occurs during ATP synthesis as a byproduct of oxidative phosphorylation. However, neither compound F1 nor F2 induced an increase in mitochondrial potential in the cells examined by flow cytofluorimetry and fluorescence microscopy.
NADPH oxidases also produce physiological amounts of ROS in the cells. NOX4 is one of NADPH oxidases expressed in fibroblasts; its expression is regulated at the gene level. We studied NOX4 expression at the level of gene and protein after exposure of the cells to F1 and F2.
When fullerene derivatives F1 and F2 are applied to the cells at a concentration of 122 µg/mL, NOX4 expression in cells increases 1.5–3.7 fold at the gene level after 1–72 hours, and at the protein level 3–24 hours after the start of incubation with compounds (Fig. 2B,C). A significant NOX4 protein expression increase during incubation of cells with the studied compounds in concentration 122 µg/mL may explain the ROS level increase in cells 24–72 hours after the addition of these compounds to the cells.
Thus, after 1–72 hours of cultivation in presence of high F1 and F2 concentrations, an increase in the NOX4 gene and protein expression is observed, leading to increased ROS synthesis and possible damaging effect development in the cells. An addition of NOX4 inhibitors to the fullerenes during incubation may possibly prolongate their antioxidant effect.
The transcription factor NRF2 participates in the regulation of the antioxidant response of cells. It was found that NRF2 gene expression increases 2–2.5 fold only 24–72 hours after F1 and F2 fullerene derivatives addition at a concentration of 18 ng/mL to the cell culture medium. When fullerene derivatives F1 and F2 are used in concentration 122 µg/mL, the expression of NRF2 gene, on the contrary, decreases after 24 hours of incubation (Fig. 2D).
The expression of phosphorylated form of NRF2 protein (phNRF2) increases 24 hours after the addition of F1 and F2 fullerene derivatives to the cell culture medium at a concentration of 18 ng/mL, apparently due to the translation amplification. We also observed a 2-fold increase in the phosphorylated NRF2 protein level already 1 hour after F1 and F2 fullerene derivatives addition to the cell culture medium at concentration 18 ng/mL (Fig. 2E). It is most likely due to the NRF2 release from NRF2 deposited in cells in association with KEAP1.
When fullerene derivatives F1 and F2 are added to the cells at a concentration of 122 µg/mL, the phosphorylated NRF2 also increases 1.5–2.5 fold after 1 hour (Fig. 2E), confirming that NRF2 factor activity in cells rapidly after fullerenes addition is most probably associated with the release from protein complexes and by phosphorylation of NRF2 deposited in cells. When fullerene derivatives F1 and F2 are added to the cells at a concentration of 122 µg/mL, NRF2 gene expression decreased after 24 hours of incubation, which is proportional to the change in the expression of the phNRF2 protein (Fig. 2D,E).
Thus, the antioxidant effect of fullerenes on cells is a result of the combined effect of the free radicals binding, ROS synthesis induction, and the transcription factor NRF2 modulation, which regulates the expression of antioxidant defense genes in cells. Observed ROS level decrease in the presence of low fullerenes concentrations 24 hours after an early ROS levels increase is most likely explained by NRF2 gene expression increase. At the same time, an increase in ROS level in the presence of high fullerene derivatives concentrations found after 24 hours incubation is associated with the absence of NRF2 gene transcription activation.
The change in the transcriptional activity of the NFR2 gene is often
inversely proportional to the activation of the NFKB1 gene
transcription. Transcription factor NF-
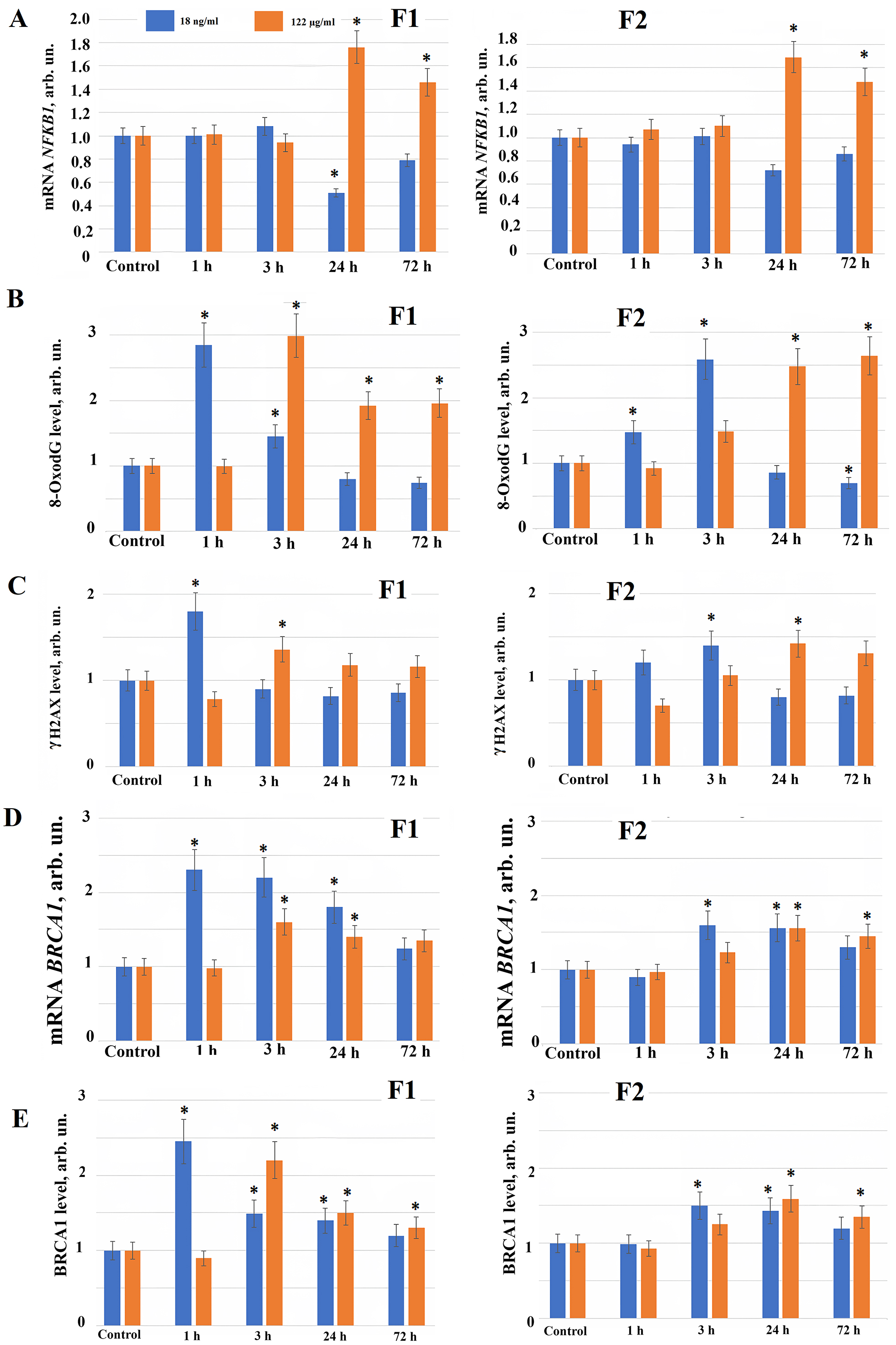 Fig. 3.
Fig. 3.
The expression of genes in HELF following the addition
of fullerenes. (A) The expression of NFKB1 gene in cells
(HELF) incubated with F1 (left) and F2 (right) at the concentrations indicated in
the figure. The amount of NFKB1 RNA is the average value (for three
experiments) of the NFKB1 gene expression in the presence of fullerene
derivatives relative to the expression of the NFKB1 gene in the control
cells. We used TBP as the internal standard gene. (*) – significant
differences with control cells (cultured in the absence of fullerenes),
p
Since we found the ROS synthesis after adding new water-soluble fullerene derivatives to the HELF, possibly, free radicals produce a damage to the cells. The water-soluble fullerene derivatives damaging effect on the cells can be detected as oxidative modifications and as the nuclear DNA breaks. We studied oxidative damage produced by compounds F1 and F2 to the DNA of fibroblast nuclei. 1 hour after fullerene F1 derivative is applied to the cells at concentration 18 ng/mL, the level of oxidative DNA modifications (8-Oxo-2′-deoxyguanosine or 8oxodG) increases 2.5–3 fold compared to the control, after 3–72 hours the level of the DNA oxidation marker (8oxodG) in the cells decreases (Fig. 3B).
When fullerene derivative F1 is applied to the cells at concentration 122 µg/mL, 8oxodG increases 3 fold after 3 hours and remains elevated 1.5–2 fold for 72 hours (Fig. 3B). F2 at concentration 18 ng/mL 3 and 24 hours after its addition to the cells increases 8oxodG 1.5–2.5 fold, after 72 hours oxidative DNA modifications decreases below the control values (Fig. 3B). When fullerene derivative F2 is added to the cells at concentration 122 µg/mL, the level of 8-oxodG increases after 3 hours, and after 24–72 hours it increases 2–2.5 fold (Fig. 3B).
An increase in the level of 8oxodG can cause the formation of DNA breaks. One of
the methods for double-strand DNA breaks detection is based on the
phosphorylation of the serine 139 residue in the conservative histone protein
involved in DNA chromatin packaging (H2AX) with the participation of ATM, ATR,
and DNA-PK kinases. Phosphorylated histones
Using the flow cytofluorometry method (comparing median distributions), it was shown that 1 hour after fullerene F1 derivative addition to the cells at concentration 18 ng/mL, the level of double-strand DNA breaks increased 1.7–1.8 fold compared to the control, correlating with an increased level of oxidative DNA modifications. After 3, 24 and 72 hours we observed a tendency of double-strand DNA breaks decrease below control values (Fig. 3C). 3 hours after addition to the cells in concentration 122 µg/mL F1 induces 1.4–1.5-fold increase of double-strand DNA breaks level compared to the control, that saves a tendency to be elevated after 24 and 72 hours. Compound F2 at concentration of 18 ng/mL increases the level of double-strand DNA breaks 3 hours after its addition to the cells, after 24–72 hours - reduces the double-strand breaks level; F2 at concentration of 122 µg/mL increase in the level of double-strand DNA breaks 24 hours after addition to the cells culture medium (Fig. 3C).
In response to DNA damage we found an activation of genes involved in the DNA repair processes. Among them BRCA1 gene plays a pivotal role. Using real-time PCR and flow cytofluorimetry (comparing median distributions), it was shown that when fullerene F1 derivative was applied in concentration 18 ng/mL 1 hour after its addition BRCA1 gene and protein expression increased 2–2.5 fold compared to the control. Increased BRCA1 gene and protein expression persisted until the end of observation (up to 72 hours), explaining the decrease in double-strand breaks 24–72 hours after F1 fullerene addition at concentration of 18 ng/ml to the cells (Fig. 3D,E). When the fullerene F1 derivative is used in concentration 122 µg/mL, BRCA1 gene and protein expression also increases 1.5–2 fold 3–24 hours after its addition to the cells (Fig. 3D,E).
Compound F2 in concentrations 18 ng/mL and 122 µg/mL 3–24 hours after its addition to the cells causes an increase in the expression of the BRCA1 gene and protein 1.5 fold after 3–72 hours (Fig. 3D,E). Possibly, an increase in the level of expression of genes and proteins involved in the repair processes in response to DNA breaks produced in the cells neutralizes the negative impact of compounds after their addition to the cells.
DNA breaks in the cells can be repaired by the cell cycle arrest. In addition, cells can activate a program of programmed cell death (apoptosis). Using the MTT test, we did not find an increase in the proliferative activity of HELF in the presence of F1 and F2 compounds at concentrations of 18 ng/mL. In concentrations 122 µg/mL these compounds reduced the number of cells in the population by about 20%.
We investigated the effect of compounds F1 and F2 on the genes regulating the cell cycle. With the cell cycle progression, the cyclin D1 protein synthesis is activated, encoded by the CCND1 gene. Cyclin D1 plays a key role, regulating the transition of cells from the G1 phase of the cell cycle to the S phase. Inhibitors of cyclin-dependent kinases belong to the regulators of the cell cycle. The p21 kinase inhibitor has a negative effect on the activity of cyclin-dependent kinase (CDK) complexes in the G1 phase of the cell cycle, an increase in the expression of the p21 gene (CDKN1A gene) can lead to G1 arrest. A complex system of regulation of the genes of the cell cycle leads either to its progression or to its inhibition.
1 hour after fullerene F1 derivative addition to the cell culture medium in concentration 18 ng/mL, we observed a short–term decrease of CCND1 gene expression and an increase in CDKN1A and CDKN2 gene expression. It may reflect short-term G1 arrest of cells observed during the activation of repair genes, and contribute to a decrease in the level of double-strand DNA breaks (Fig. 4A–C). 3–24 hours after exposure of the cells to the fullerene F1 derivative in concentration 18 ng/mL, the cell cycle progression continues.
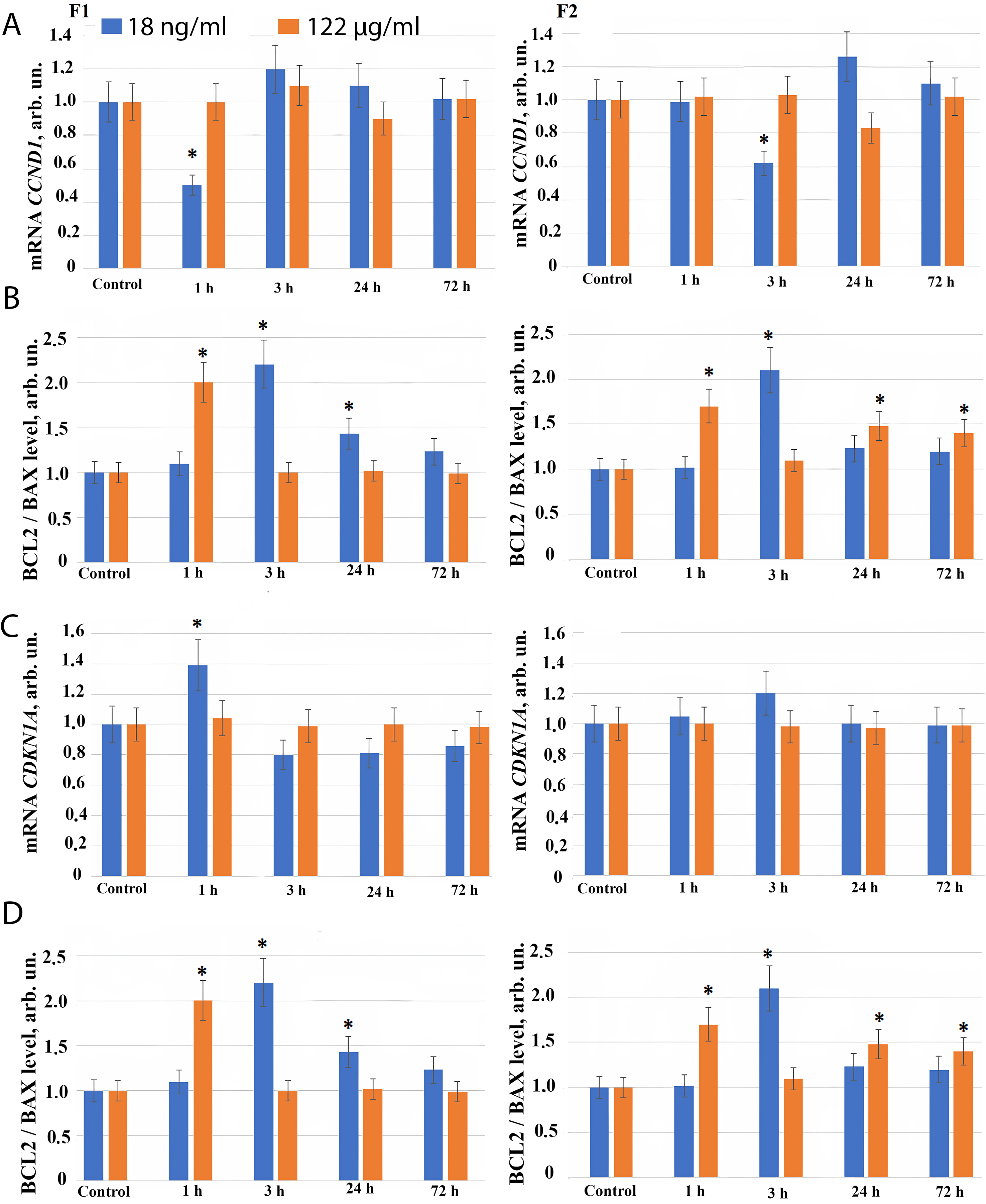 Fig. 4.
Fig. 4.
The expression of genes in HELF following the addition
of fullerenes. (A) Expression of CCND1 gene in cells (HELF) after
addition of F1 (left), and F2 (right), at the concentrations indicated in the
figure. The amount of CCND1 RNA is the average value (for three
experiments) of CCND1 gene expression in the presence of fullerene
derivatives relative to CCND1 gene expression in the control. The
TBP gene was used as the internal standard gene. (*) – significant
differences with control cells, p
Similarly, when the fullerene derivative F2 is applied at concentration of 18 ng/mL, a short-term decrease in CCND1 gene expression and an increase in CDKN1 and CDKN2 genes expression is found 3 hours after addition of the compound to the cell culture medium, possibly indicating a short-term G1 arrest observed during repair genes activation (Fig. 4A–C).
When F1 and F2 compounds are added to the cells at concentration of 122 µg/mL for 72 hours, we observed no changes in the RNA level of CCND1, CDKN1 and CDKN2 genes (Fig. 4A–C).
The change in the number of cells in a population depends both on the progression of the cell cycle and the apoptosis. We investigated the effect of F1 and F2 compounds on the expression of pro-apoptotic (BAX) and anti-apoptotic (BCL2) genes. The most adequate and frequently used ratio of the expression of the anti-apoptotic protein BCL2 to the pro-apoptotic BAX indicates a decrease in the level of apoptosis in the cell population.
3 hours after fullerene derivatives F1 and F2 are added in concentration 18 ng/mL, an increase in the expression of the anti-apoptotic protein BCL2 is observed compared to BAX, while the ratio BCL2/BAX increases 2–2.5 fold (Fig. 4D). It indicates a short-term inhibition of apoptosis and reflects an increase in the number of cells in the early cultivation stages produced by these compounds in nanomolar concentration.
1 hour after F1 and F2 compounds are added to the cells at concentration 122 µg/mL, the BCL2/BAX ratio increases 1.5–2 fold, and after 3–72 hours the BCL2/BAX ratio returns back to the control level.
The present study reveals the genotoxic and cytotoxic effects of various doses of Cl-containing water-soluble derivatives of fullerene C60, which also affect the expression of genes regulating the antioxidant response of cells. The study shows that increase in the dose and period of exposure to Cl-containing fullerene derivatives can lead to dysregulation of the cellular antioxidant response and consequent oxidative DNA damage in cultured HELF.
One of important scientific tasks of the last decade is to investigate the adverse effects of nanomaterials on human cells. Nevertheless, there is limited information about genes expression in cells exposed to fullerene derivatives [34, 35].
The aim of the present study was to analyze the dynamics of the early and late reactions of cultured HELF to different concentrations of Cl-containing water-soluble derivatives of fullerene C60. We used the same concentrations of F1 and F2—18 ng/mL (low, within the range of non-toxic concentrations) and 122 µg/mL (close to damaging concentration) for four different time points (1, 3, 24, and 72 hours). Embryonic fibroblasts were selected to analyze the biological activity of fullerenes. Fullerene derivatives F1 and F2, when added to the culture medium, penetrate into the cells, localizing in the cytoplasm. Earlier, studying the biological response of cells to fullerene derivatives, we also observed their penetration through the cytoplasmic membrane with localization in the cytoplasm of cells [35]. In our previous studies, we have already noted that many fullerene derivatives, after a short antioxidant effect, increase the level of reactive oxygen species in cells, accompanied by damage to the genetic material and often lead to apoptosis if the antioxidant protection in the cells is not activated [35]. At the same time, as a rule, the effects of low and high doses of fullerene derivatives were identical and unidirectional, but differed in strength of their effect on the expression of genes regulating the level of oxidative stress in cells and apoptosis.
Our study for the first time showed that after preliminary rise in ROS level consequent incubation (for 24–72 hours) with low concentrations of newly synthesized compounds decreases ROS levels in cells, which may be due to selective regulation of gene expression in cells, depending on the concentration of added compounds.
The penetration of F1 and F2 in low concentrations led to a short-term increase
in the expression of NOX4 gene (1 hour after addition of F1 and 3 hours
after F2, Fig. 2B,C). NADPH oxidase (NOX) complexes are the main sources of
endogenous non-mitochondrial ROS production in normal and cancer cells [42]. The
expression of NOX4, which produces ROS, is regulated by various transcription
factors (TF), including NRF2 and NF-
The ROS level reduction in the presence of low concentrations of F1 and F2 compounds after 24 hours is most probably explained by NRF2 gene expression and its functional activity increase (Fig. 2D). Therefore, the studied compounds in low concentrations can work as antioxidants with delayed and prolonged action. Putatively, at early stage of oxidative stress F1 and F2 induce NRF2 dissociation from KEAP1, moving to the nucleus, and binding to the antioxidant response element (ARE), which contributes to the subsequent secondary expression of the antioxidant protein, which we observe in the cells (Fig. 2E) [45].
F1 and F2 in close to toxic concentrations increase NOX4 gene and
protein expression in cultured HELF after 24–72 hours, which induce ROS synthesis
in cells and the delayed damaging effect development of these compounds:
oxidative modifications and double-strand DNA breaks of cell nuclei after 24
hours of incubations (Fig. 3B,C). At the same time, an increase in the ROS level
in the presence of high concentrations of fullerene derivatives studied after 24
hours is possibly associated with the absence of NRF2 gene transcription
activation (Fig. 2D,E). It was shown that NFKB1 gene expression increases 24
hours after addition of fullerene derivatives F1 and F2 to the cell culture
medium in concentration 122 µg/mL, which is inversely proportional
to the change in the NRF2 gene expression. NOX4 enzyme translation is controlled
by redox-sensitive transcription factors, such as NF-
In the present study, we investigated molecular mechanisms of toxicity
development, defined as signaling pathways of cellular response; combined
activation or inhibition of these processes leads to a damaging effect on human
cells. We propose an approach to assess the toxic effects of compounds based on
the study of the relationship between toxicity and altered expression of
interconnected genes, which co-expression or inhibition determine the complex
cellular response to such agents. For the first time, we found diametrically
opposite activation of key signaling pathways in HELF that regulate the level of
oxidative stress and apoptosis under high and low F1 and F2 doses. High F1 and F2
concentration (122 µg/mL) cause long term (after 24–72 hours)
activation of NOX4 expression, induction of ROS synthesis, DNA damage, and
breaks, activation of NF-
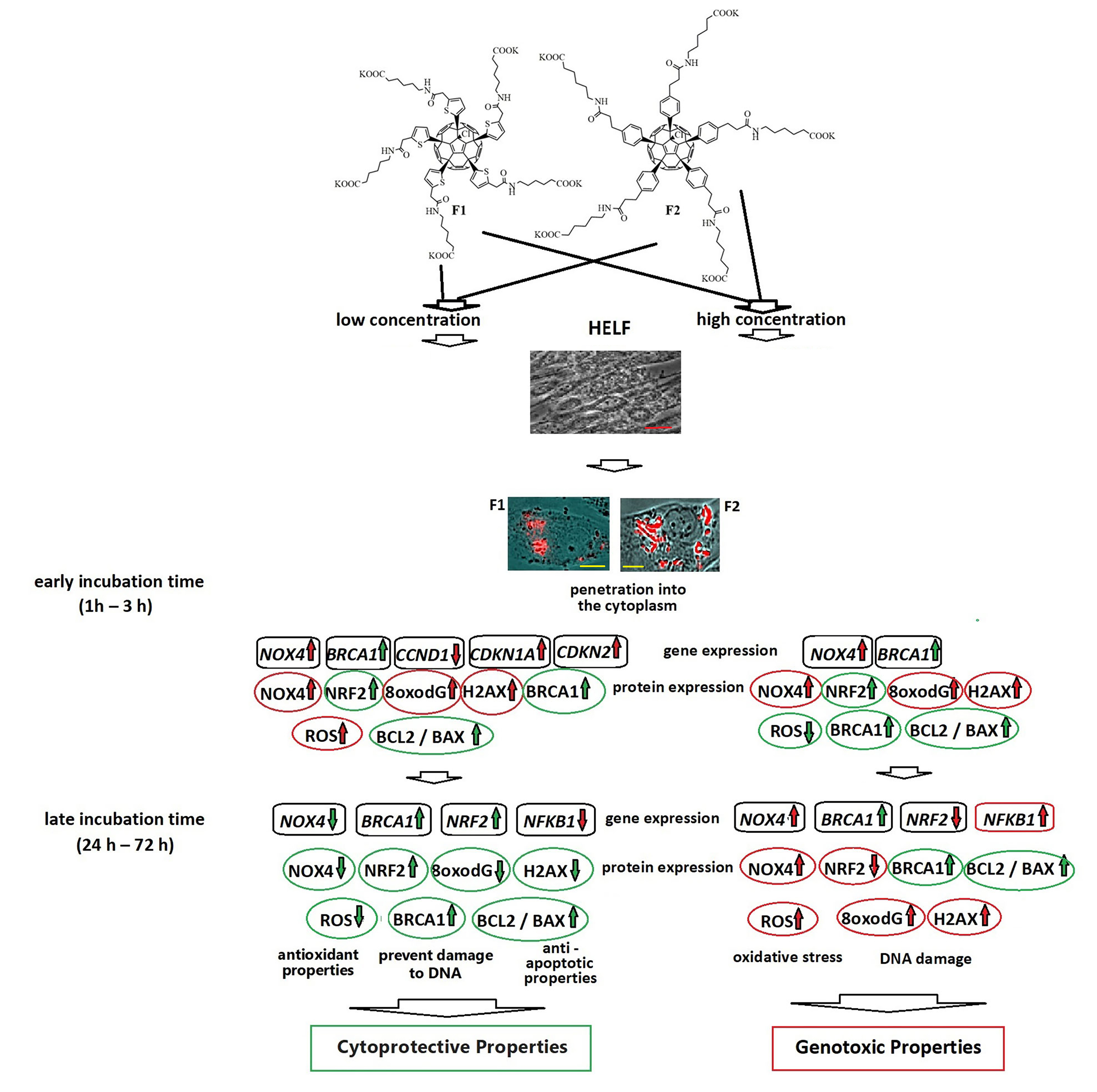 Fig. 5.
Fig. 5.
The final scheme combining the effects of low and high doses of Cl-containing fullerene derivatives on HELF. Red scale bars are 20 µm, yellow scale bars are 10 µm. NOX4, NADPH oxidase 4; BRCA1, breast cancer 1; CCND1, cyclin D1; CDKN1A, cyclin-dependent kinase inhibitor 1A (p21); CDKN2, cyclin-dependent kinase inhibitor 2; NRF2, nuclear factor erythroid 2-related factor 2; BCL2, B-cell lymphoma 2; NFKB1, nuclear factor kappa B subunit 1; 8oxodG, 8-Oxo-2′-deoxyguanosine; BAX, Bcl-2 associated X protein; H2AX, H2A histone family member X; ROS, reactive oxygen species.
Antioxidant characteristics of F1 and F2 in low concentrations are shown and these compounds may have a wide range of applications, including prevention of aging [49], treatment of socially significant diseases accompanied by oxidative stress [50], as cytoprotectors against radiation [34], e.g., during radiation therapy [50].
In the present study, that is a logical continuation of our previous study [51], we show a complex effect of chlorinated water-soluble derivatives of C60 fullerenes on the selective regulation of gene expression in human embryonic lung fibroblasts.
As conclusion, we found a previously unobserved effect of ROS levels reduction in the presence of low concentrations of newly synthesized C60 fullerenes 24 hours after an early rise in ROS levels. This effect may be explained by the selective regulation of gene expression in cells, depending on the concentration of added compounds. The observed reduction of ROS levels in the presence of low concentrations of newly synthesized compounds after 24 hours is most likely due to an increase in the expression of the NRF2 gene and an increase in its functional activity, which allows us to consider the studied compounds in low concentrations as antioxidants of delayed and prolonged action. The antioxidant properties of the studied water-soluble fullerene derivatives in low concentrations may determine the potential antiviral activity of these compounds, since oxidative stress induced by viruses not only interfere with metabolic processes of the body, but also regulates viral replication, and a wide range of antiviral activity is shown for many antioxidants. Water-soluble derivatives of fullerenes F1 and F2 at a concentration of 18 ng/mL 24 hours after addition to cells reduce the level of oxidative DNA damage and reduces the number of double-strand breaks in the HELF population, which makes it possible to consider these compounds in low concentrations as DNA protectors against the damaging genotoxic factors.
Compounds F1 and F2 in concentrations close to toxic caused an increase in the
expression of the NOX4 gene and protein in cultured HELF, which induced synthesis
of ROS in cells and the development of the damaging effect in cells: oxidative
modifications and double-stranded DNA breaks of cell nuclei after 24 incubations.
At the same time, an increase in the level of ROS in the presence of high
concentrations of fullerene derivatives studied after 24 hours is associated with
the lack of activation of the NRF2 gene transcription. It was found that the
expression of the NFKB1 gene increases 24 hours after the addition of fullerene
derivatives F1 and F2 to the cell culture medium at a concentration of 122
µg/mL, which is inversely proportional to the change of NRF2 gene
expression. An increase in the expression of the NF-
Dose-effect assessment is becoming increasingly important in determining the risk of drug effects on biological objects. The increasing number of in vitro methods is developing for evaluation of well-defined control points of the dose-effect models, with concentrations traditionally used corresponding to 10% or 50% of the maximum effect. In the present study, we determined that even minor fluctuations in such an important index as the viability score (only by 10%) may indicate a multidirectional effect of different concentrations on key signaling pathways in cells, which leads either to a cytoprotective effect or causes a cytotoxic response. This is important because we have shown that assessing cell viability alone is not enough to identify the effect of different drug concentrations on the regulation of transcriptional activity of signaling pathway genes in cells, which can be further used as a method of prioritizing research in the development of pharmaceuticals. This approach, allowing evaluation of compounds effects at the level of gene activity, makes it possible to most effectively identify the complex cellular response of the investigated compounds.
Obtained results show that in low doses these compounds may serve as perspective DNA protectors against the damaging genotoxic factors. Further research of dose-dependent effects of the studied fullerenes should be conducted for better understanding of their role in regulation of oxidative stress and apoptosis.
The data that support the findings of this study are available from the corresponding author upon request.
Conceptualization, SVK and NNV; methodology, SVK, EMM and SIK; validation, SEK, ESE, PEU and PAT; investigation, EAS, ESE, OAK, PEU, SVK, LVK, TAS and IVR; resources, SIK; data curation, SEK, ENM and NNV; writing — preparation of the original project, SVK, EMM and PEU; writing — reviewing and editing, SVK, EMM and PEU; visualization, NNV; supervision, SVK, PEU. All authors have read and agreed with the published version of the manuscript. All authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to its accuracy or integrity. All authors contributed to editorial changes in the manuscript.
The study has been granted ethical approval by the Institutional Review Board of the Research Center for Medical Genetics, in accordance with protocol number N.5.
Not applicable.
The synthesis of water-soluble fullerene derivatives was supported by the Russian Science Foundation, project No. 22-43-08005, and by state assignment of the Ministry of Science and Higher Education (studies of the effect of fullerene derivatives in concentrations close to toxic: experiments on the dynamics of cytotoxicity and antioxidant properties of tested fullerene derivatives).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.





