1 Department of Clinical Sciences and Translational Medicine, University of Tor Vergata, 00133 Rome, Italy
†These authors contributed equally.
Endocrine disrupting chemicals (EDCs) represent a risk for human health and the environment worldwide [1]. The U.S. Environmental Protection Agency (EPA) defines an endocrine disruptor as “an exogenous agent that interferes with synthesis, secretion, transport, metabolism, binding action, or elimination of natural blood-borne hormones that are present in the body and are responsible for homeostasis, reproduction, and developmental process”. Accordingly, many in vitro, in vivo and cohort studies have shown that EDCs can potentially cause adverse health effects, including reproductive disorders, metabolic syndromes, and cancer by altering homeostasis of the endocrine system [1, 2].
EDCs are classified based on their origin and their use and include natural and synthetic substances that interfere with hormonal function. Synthetic EDCs can be divided into several categories: industrial chemicals like polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs), plastics such as bisphenol A (BPA), plasticizers like phthalates, pesticides including dichlorodiphenyltrichloroethane (DDT), fungicides like vinclozolin, and pharmaceutical agents such as diethylstilbestrol (DES). Natural EDCs, including phytoestrogens, are found in human and animal food. Furthermore, EDCs are categorized based on their occurrence into four main groups: natural and artificial hormones (e.g., phytoestrogens and contraceptive pills), drugs with hormonal side effects (e.g., naproxen and clofibrate), industrial and household chemicals (e.g., phthalates, fire retardants, solvents), and byproducts of industrial and household processes (e.g., polycyclic aromatic hydrocarbons (PAHs) and dioxins). This last classification helps in understanding the diverse nature of these chemicals and their widespread presence in various products and environments [1, 2]. Additionally, EDCs are found in everyday products such as children’s objects, electronics, personal care products, and building materials. The primary route of EDCs exposure is through the diet, particularly via processed foods and food packaging materials. This pervasive presence causes significant exposure risks, especially for vulnerable subjects like children. Certain pesticides like DDT and organophosphorus compounds are notable EDCs due to their historical and ongoing use and their significant impact on human health. The health concerns associated with EDCs range from reproductive system alterations to metabolic disorders, including obesity and diabetes [1, 2]. Initially, EDCs were believed to primarily interact with nuclear hormone receptors such as estrogen (ER), androgen (AR), progesterone (PR), thyroid, and retinoid receptors. However, recent studies have revealed that the mechanisms through which EDCs exert their effects is additionally via nuclear receptors, non-nuclear steroid hormone receptors such as membrane ERs, and nonsteroid receptors including neurotransmitter receptors like serotonin, dopamine, and norepinephrine receptors. Additionally, orphan receptors like the aryl hydrocarbon receptor (AhR), and various enzymatic pathways involved in steroid biosynthesis and metabolism are involved. These various mechanisms underscore the wide-ranging impact of EDCs on endocrine and reproductive systems [1].
Among EDCs, BPA exhibits estrogen-like activity, which can disrupt normal hormonal functions even at low concentrations. BPA has been linked to obesity, diabetes, cancer and cardiovascular diseases through its impact on reactive oxygen species (ROS) production, inflammatory pathways, insulin action and lipid metabolism [3]. Phthalates can produce biological toxic effects including genotoxicity, endocrine and reproductive disorders, developmental and metabolic toxicities [3]. Moreover, cadmium, another notable EDC, has been associated with kidney damage, osteoporosis, and increased risk of cancers by inducing oxidative damage [4].
As evidence mounts regarding the detrimental health effects of EDCs on the reproductive system, prostate, breast, lung, liver, thyroid, and metabolism, attributed to their estrogenic and obesogenic activities, there is a growing interest in identifying natural compounds that might mitigate these adverse outcomes [1, 3]. Polyphenols, found abundantly in fruits, vegetables, tea, wine, and supplements, have emerged as potential candidates to counteract the adverse effects of EDCs due to their antioxidant and anti-inflammatory properties [3, 5]. However, the interaction between polyphenols and EDCs is quite complex since some polyphenols potentially enhance the detrimental effects of EDCs because of their pro-oxidant and phytoestrogenic activities [6] (Fig. 1).
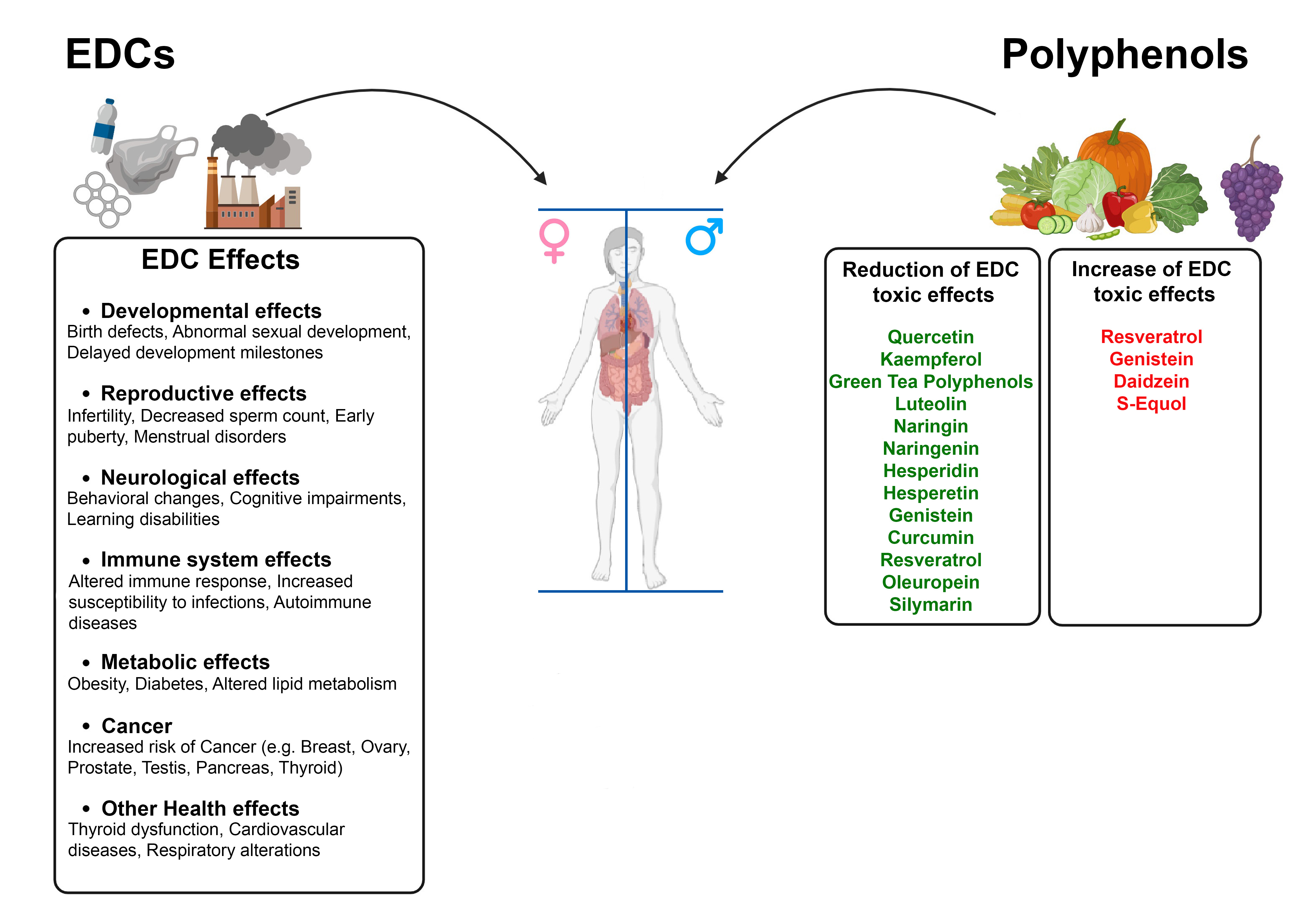 Fig. 1.
Fig. 1.
The impact of polyphenols on the toxic effects of Endocrine-Disrupting Chemicals (EDCs). The figure illustrates the detrimental health effects of Endocrine-Disrupting Chemicals (EDCs). On the right, polyphenols are shown to potentially reduce the toxic effects of EDCs due to their antioxidant and anti-inflammatory properties. However, some polyphenols may enhance the toxic effects of EDCs due to their pro-oxidant and phytoestrogenic activities. The figure was created with BioRender.com.
However, several studies have highlighted the protective effects of several classes of polyphenols in reducing the toxic effects of different synthetic EDCs. Efficacy of polyphenols is primarily attributed to their ability to scavenge ROS, enhance antioxidant enzyme activities, and modulate critical signaling transduction pathways. These abilities are a result of their chemical structure, which includes multiple functional groups, double bonds, and an aromatic ring. For instance, quercetin, kaempferol, luteolin, naringin, genistein, oleuropein, silymarin and curcumin have shown substantial protective effects against BPA-induced oxidative stress and tissue damage [3, 7]. Similarly, polyphenols like quercetin, curcumin, naringenin, hesperidin, hesperetin, through their anti-inflammatory, antioxidant, free radical scavenging and metal sequestration actions, mitigate toxicity induced by cadmium and other heavy metals [4, 8]. In a recent paper, curcumin showed protective effects against the cardiotoxicity induced by atrazine, a water-soluble herbicide commonly used to control broad-leaf and monocotyledonous weeds [9]. Similarly, green tea polyphenols are able to alleviate liver disfunction induced by Di-(2-ethylhexyl) phthalate (DEHP) or Mono-2-ethylhexyl phthalate (MEHP) or heavy metals [10].
Oppositely, polyphenols also possess pro-oxidant properties and the ability to act as phytoestrogens due to their chemical structure which resemble endogenous estradiol, modulating androgen and estrogen receptors in a tissue-specific manner [6]. In this regard, resveratrol and genistein, found in grapes, berries, and soy products respectively, exhibit a complex dual role in their interaction with EDCs by both mitigating or enhancing their detrimental effects, depending on concentration, context, and biological environment. Resveratrol has shown significant protective effects against EDCs such as BPA, cadmium and phthalates, by inhibiting cancer cell proliferation and ameliorating metabolic disruptions and organ toxicity induced by these chemicals [3, 5, 8]. However, resveratrol also modulates estrogenic activity, acting as both an agonist and antagonist of estrogen receptors. Indeed, resveratrol enhances the growth of estrogen-dependent cancer cells at low concentrations and disrupts thyroid function by acting as a goitrogen, thus raising concerns about its safety and efficacy [6, 11].
Similarly, genistein, known for its phytoestrogenic properties, protects against neurotoxicity induced by atrazine and mitigates the adverse effects of phthalates on testicular development, while its antioxidant properties counteract oxidative stress and inflammation induced by BPA [7, 12, 13]. However, genistein’s estrogenic activity can also promote adverse consequences in hormone-sensitive cancers, with long-term exposure potentially leading to the overexpression of Human Epidermal Growth Factor Receptor 2 (HER2) and supporting hormone-independent cancer growth [14]. A recent study highlights the importance of considering not just the individual effect of a given compound but also their interactions, which can have significantly different biological impacts when they co-occur in food products. In particular, soy isoflavones (genistein, daidzein and S-equol), combined with mycoestrogens, like mycotoxin zearalenone, significantly amplify estrogenic responses [15].
In conclusion, although polyphenols offer promising protective effects against the adverse activities of EDCs, their multifaceted nature and potential side effects, at certain doses, highlight the need for a deep knowledge related to their interaction with EDCs before their use. In addition, several evidence underscore the complex role of phytoestrogens in managing androgen- and estrogen-related conditions. Thus, encouraging a diet rich in natural polyphenols through fruits, vegetables, and whole foods can be a proactive measure to counteract EDC exposure. However, supplementation should be approached with caution and regulatory bodies should take into account the potential harmful effects in particular conditions when evaluating the safety and efficacy of polyphenol supplements and substitutes. Future studies are needed to investigate the long-term health impacts of polyphenol consumption and to explore the synergistic effects of combining different polyphenols with dietary sources. Indeed, the effects of polyphenols depend not only on the amount consumed, but also on their bioavailability at the site of physiological activity. Dietary variables, endogenous factors and gut microbiota can all affect the bioavailability. By advancing our understanding of the interaction between EDCs and polyphenols, we can develop more effective strategies to protect public health from the risk of EDCs.
MB, CF, LC and RB wrote the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
The authors wish to thank Dr. Raffaele Carrano (recipient of the Tor Vergata PhD program in Tissue Engineering and Remodeling Biotechnologies for Body Functions) for Fig.
This research was funded by a grant from the Ministero dell’Università e della Ricerca, PRIN 2020 grant (Prot. 20205HZBP8 to R.B.).
The authors declare no conflict of interest. Given Roberto Bei’s role as the Editorial Board Member, he had no involvement in the peer-review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Jen-Tsung Chen.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

