1 Department of Biochemistry, College of Science, King Saud University, 11451 Riyadh, Saudi Arabia
2 Department of Biochemistry and Biotechnology, Faculty of Sciences, Annamalai University, 608002 Annamalai Nagar, India
3 Department of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, 11451 Riyadh, Saudi Arabia
4 Department of Botany and Microbiology, College of Science, King Saud University, 11451 Riyadh, Saudi Arabia
Abstract
Background: Recent studies suggest that numerous naturally occurring
agents have the potential to kill cancer cells via mitochondrial dysfunction.
Solanum nigrum is a herb widely used in alternative medical
systems. This study aimed to investigate the cytotoxic effect of Solanum nigrum water extract (SNWE) against Michigan Cancer Foundation-7
(MCF-7) and MD Anderson-Metastatic Breast Cancer-231 (MDA-MB-231) cells.
Methods: We used an MTT reduction assay for cytotoxicity analysis. To
explore the mode of action, the cellular adenosine triphosphate (ATP) levels and
mitochondrial membrane potential were analyzed using a colorimetric ATP assay and
Rhodamine-123 fluorescent staining, respectively, during SNWE treatment for 72 h.
Results: The cytotoxic effect was significant in both cell lines, with
IC
Keywords
- Solanum nigrum
- mitocans
- mitochondrial dysfunction
- mitochondrial membrane potential
- energy metabolism
- breast cancer
Targeting mitochondria is emerging as a successful therapeutic approach for cancer because of the involvement of mitochondria in redox signaling, cellular metabolism, and cellular homeostasis [1]. The mitochondria are the site of adenosine triphosphate (ATP) synthesis from glucose and fatty acids through a series of biochemical reactions known as oxidative phosphorylation. The essential requirement for oxidative phosphorylation is the electron transport chain (ETC) that is associated with the production of reactive oxygen species (ROS), as well as adenosine triphosphate (ATP) synthesis [2]. Thus, an efficient redox balance during cellular ATP synthesis is necessary for cellular proliferation and viability. However, dysfunction of mitochondria has a significant negative impact on overall ROS generation and cellular energy metabolism, which ultimately adversely affects the fate of cells. Nevertheless, in cancer cells, the shifting of energy metabolism from the usual oxidative phosphorylation to active glycolysis impedes mitochondrial activity, leading to increased levels of ROS generation, which can ultimately be linked to numerous oncogenic signals [2].
Mitochondrial membrane potential (abbreviated to
Many recent reports showed that herbal extracts and active isolated compounds can cause the disruption of mitochondrial membrane potential in different cancer cell lines [11, 12, 13]. Solanum nigrum (black nightshade) is a commonly used medicinal herb in alternative systems of herbal medicine. The crude extract from Solanum nigrum has shown anticancer potential in different types of cancer including breast cancer, cervical cancer, endometrial cancer, colorectal cancer, and human melanoma [14, 15, 16, 17]. The Solanum nigrum water extract from leaves is significantly cytotoxic in breast cancer cell lines due to apoptosis and epithelial-mesenchymal transition [18]. The leaf extract of Solanum nigrum possesses antioxidant [19] and anti-inflammatory [20] properties that can efficiently cope with oxidative stress and inflammation, the primary hallmarks of cancer. Furthermore, the leaf extract of Solanum nigrum also proved capable of promoting mitochondrial fission and, hence, reducing the normal function of mitochondria in breast cancer cell lines [18]. These reports suggest that Solanum nigrum works mechanistically by targeting mitochondria membrane potential and hampering ATP generation in breast cancer. We, therefore, attempted to investigate the effect of Solanum nigrum on mitochondria membrane potential and ATP generation in breast cancer cells.
The Solanum nigrum plants were collected from regions in South India and air-dried for 7 days to remove the moisture content (Fig. 1). The leaf of Solanum nigrum was powdered, and 50 g of the powdered plant material was immersed in 250 mL of distilled water. The mixture was transferred into a 500 mL conical flask, plugged with sterile cotton, and then incubated in a boiling water bath for 60 minutes. Next, the solution was filtered through Whatman filter paper and syringe filters (Fig. 1). The solution was then incubated in a shaking incubator for 24 h at 60 °C. Finally, a clear Solanum nigrum water extract (SNWE) was obtained and stored in the refrigerator at –20 °C for later use. The required amount of SNWE was weighed and a stock solution was prepared in dimethyl sulfoxide (DMSO) (Sigma Aldrich, St. Louis, MO, USA) [21]. The final concentration of DMSO in the solution was 0.1%.
 Fig. 1.
Fig. 1.Preparation of Solanum nigrum water extract (SNWE). The leaves of Solanum nigrum plants were air-dried for one week to remove the moisture content. The dried leaves were soaked in distilled water and incubated in a boiling water bath for 1 h. The mixture was then filtered through Whatman filter paper and syringe filters and incubated in a shaker incubator for 24 h at 60 °C. The final aqueous extract of Solanum nigrum was dissolved in 0.1% dimethyl sulfoxide (DMSO) for experimental use.
Breast carcinoma cell lines including MDA-MB-231 (Triple-negative) and MCF-7
(Luminal A) were obtained from National Centre for Cell Science (NCCS), Pune, MH,
India. Both the cell lines were authenticated using the AmpFISTR Identifier Plus
PCR amplification Kit from Applied Biosystems (Waltham, MA, USA) and sixteen
short tandem repeats (STR) loci were amplified using this kit. The commercial
supplier stated that no mycoplasma contaminations were detected in these cell
lines. The cells were grown in Dulbecco’s modified Eagle medium (DMEM) media
supplemented with 10% fetal bovine serum (FBS) and 50 units/mL of
penicillin-streptomycin at 37 °C in a 5% CO
The MCF-7 and MDA-MB-231 cells were seeded in a 96-well plate at a density of
10,000 cells/well and incubated for 24 h at 37 °C in 5% CO
The intracellular ATP measurement is the most sensitive method available for evaluating cell viability [23]. The MCF-7 and MDA-MB-231 breast cancer cells were seeded in 96-well plates and allowed to reach confluence, upon which different concentrations of SNWE treatment were added to the cells. The cellular ATP levels were determined after the treatment of SNWE for 24 h, 48 h, and 72 h using a colorimetric ATP assay kit (Sigma-Aldrich, St. Louis, MO, USA), according to the manufacturer’s instructions. To calculate the unknown concentrations of test values, a standard curve was plotted with the concentration ranges from 0–12 nmoL and analyzed using GraphPad Prism 9.0 (GraphPad Software Inc., San Diego, CA, USA) software.
Mitochondrial membrane potential was analyzed using Rhodamine-123 fluorescent
staining [24]. The MCF-7 and MDA-MB-231 cells (1
We conducted one-way ANOVA (analysis of variance) on the resulting data using
the GraphPad Prism 9.0.0 software package. The results are reported as
mean
The MCF-7 and MDA-MB-231 cells were treated with different concentrations of
SNWE. We observed that SNWE induces cytotoxicity in both the breast cancer cell
lines in a concentration-dependent manner (Fig. 2). We noticed that the
antiproliferative effect of SNWE was more prominent on MCF-7 cell lines than on
MDA-MB-231 cells. The IC
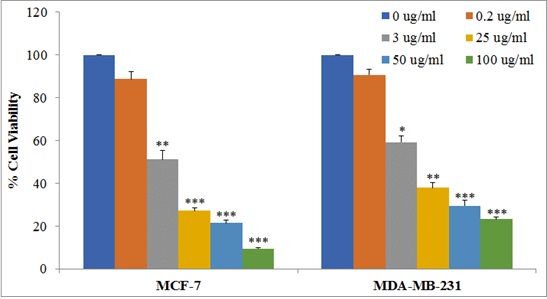 Fig. 2.
Fig. 2.Effect of SNWE on cytotoxicity in MCF-7 and MDA-MB-231 cells.
Cells (10,000 cells/well) were treated with increasing concentrations of SNWE
ranging from 0.20–100 µg/mL for 72 h, and then assessed for cell
viability using MTT assay. Data are presented as mean
The high energy metabolism rate in cancer cells results in the production of
significant amounts of ATP. As shown in Fig. 3, the untreated control group had a
high ATP concentration which was significantly reduced by SNWE treatment in both
MCF-7 and MDA-MB-231 cells. Furthermore, we observed that the cells showed
decreased ATP concentration in a concentration-dependent manner that was directly
proportional to the decrease in the viability of cells (Fig. 3). The incubation
of 24 h, 48 h, and 72 h treatment of 100 µg/mL SNWE showed 0.85
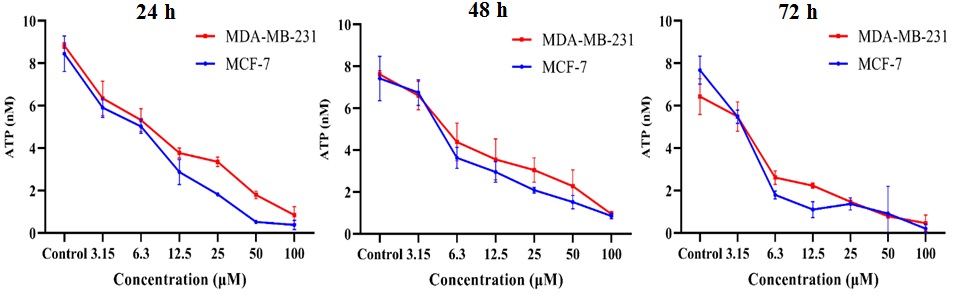 Fig. 3.
Fig. 3.Effect of SNWE on adenosine triphosphate (ATP) levels in the
breast cancer cells at different time intervals (24 h, 48 h, and 72 h). There
was a concentration-dependent decrease in cellular ATP levels after SNWE
treatment. The data are presented as mean
Rhodamine-123 (Rh-123) is a membrane-permeable cationic dye that stains the mitochondria in living cells. There was significant retention of Rh-123 fluorescent dye in untreated MCF-7 and MDA-MB-231 cells, indicating functional mitochondria in these cells (Fig. 4). However, this green fluorescence was gradually reduced with increasing concentrations of SNWE exposure in both the cancer cell types (Fig. 4).
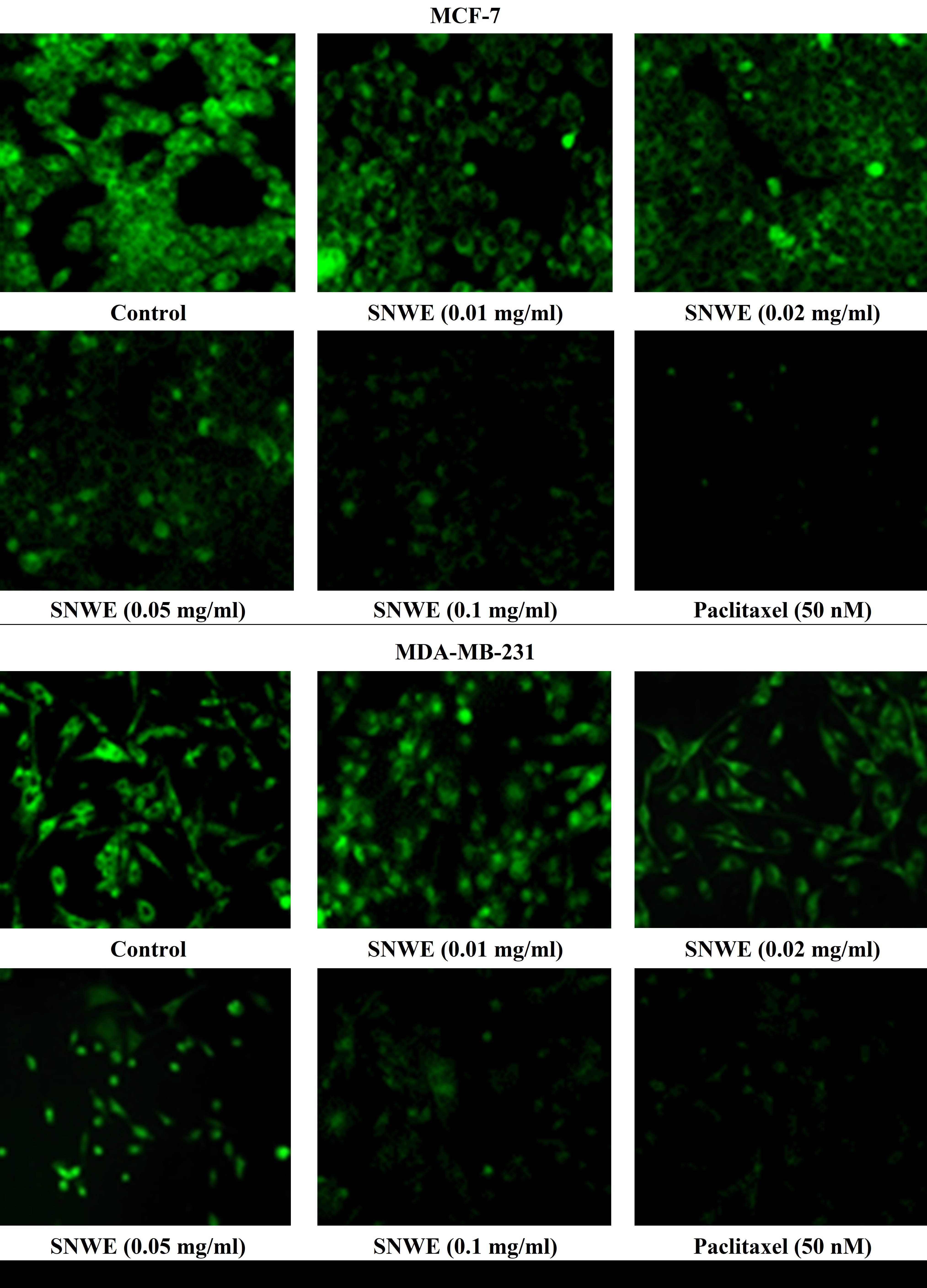 Fig. 4.
Fig. 4.Effect of different concentrations of SNWE on Rhodamine-123
fluorescence dye retention in MCF-7 (upper panel) and MDA-MB-231 cells (lower
panel). Control cells showed a massive accumulation of Rhodamine-123 which was
gradually decreased after exposure to different concentrations of SNWE. The
anticancer drug, paclitaxel, was used as a positive control (Original
magnification, 20
The SNWE treatment altered the mitochondrial membrane potential
(
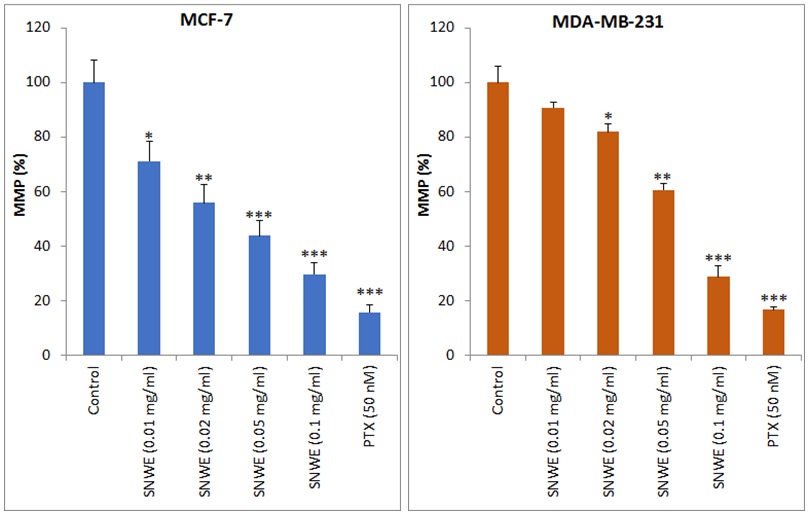 Fig. 5.
Fig. 5.Effect of SNWE on the loss of mitochondrial membrane potential
(MMP) in MCF-7 (left panel) and MDA-MB-231 cells (right panel). SNWE treatment
significantly decreased mitochondrial membrane potential in a
concentration-dependent manner. Bar graphs show MMP in terms of % fluorescence
intensity of Rhodamine-123 at excitation and emission wavelengths of 450 nm and
490 nm, respectively. Paclitaxel (PTX) was used as a positive control. Values are
mean
Solanum nigrum is used in various medicinal systems due to its numerous valuable pharmacological properties [25, 26, 27, 28]. Our results showed that Solanum nigrum water extract (SNWE) inhibited the growth of both estrogen receptor-positive as well as estrogen receptor-negative breast cancer cell lines (MCF-7 and MDA-MB-231, respectively) in a concentration-dependent manner (Fig. 2). Several studies showed the cytotoxic effect of Solanum nigrum water extract in breast cancer cell line AU565; cervical cancer cell line U14; hepatoma cell line HepG2; endometrial cancer cell lines HEC1A, HEC1B, and KLE; and colorectal cancer cell lines DLD-1 and HT-29 [14, 15, 16, 29, 30]. Furthermore, SNWE induces apoptosis and autophagy in cancer cells [31, 32]. Similarly, lower concentrations of SNWE caused autophagy and no apoptosis, whereas high concentrations inhibited p-Akt and resulted in apoptosis- and autophagy-induced cellular damage [16]. Moreover, adjuvant therapy with SNWE can also augment the cellular toxicity of known cancer drugs such as docetaxel, doxorubicin, and cisplatin [32, 33, 34].
In our study, we found that SNWE significantly diminished intracellular ATP levels in a dose-dependent manner (Fig. 3). The intracellular ATP levels are markers for cell viability and can be used to evaluate the efficacy of anticancer drugs in cancer cells. Various concentrations of SNWE significantly reduced the ATP levels in the breast cancer cells in a dose-dependent manner, while the untreated control cells showed higher ATP levels. These results reveal the failure of the mitochondria to produce ATP for cellular proliferation in both MCF-7 and MDA-MB-231 breast cancer cells. Similarly, Hernández et al. [35] observed that Petiveria alliacea L leaf extracts reduced the cellular ATP concentrations in breast cancer cells and breast cancer murine models.
Our results show that SNWE treatment significantly disrupts mitochondrial membrane potential (MMP) in breast cancer cells (Fig. 5). Mitochondria are the central regulators of the intrinsic apoptosis pathway as they maintain the balance between pro-apoptotic and anti-apoptotic events while controlling programmed cell death [36]. The recent literature proposes targeting mitochondria as an attractive strategy to combat cancer [37]. The various concentrations of SNWE caused the dose-dependent reduction in oxidative phosphorylation and electron transport through the disruption of MMP, which leads to the induction of caspase-dependent apoptosis. Rhodamine-123 is a fluorescent chemical probe widely used for its ability to accumulate in the mitochondria and thus acts as an indicator for mitochondria membrane potential [38]. High retention of rhodamine-123 fluorescence was detected in both untreated MCF-7 and MDA-MB-231 cells (Fig. 4). Further, the fluorescence was significantly reduced after SNWE treatment in both types of breast cancer cells. This mechanism of action suggests that SNWE induces mitochondrial damage and thereby leads to ATP depletion that, in turn, causes cell death. Several other evidence-based studies have shown that a significant number of naturally occurring agents and herbs, including withanone from Withania somniferum, can induce mitochondria-mediated cancer cell death by elevating oxidative stress, disturbing the cellular redox system, reducing ATP levels, and causing loss of mitochondrial membrane potential [39, 40]. The concept of mitochondria-targeted anticancer agents is attracting increased attention and, in the literature, such agents have been termed “mitocans”, mitochondria-targeting anticancer drugs, with various modes of action [41]. Naturally occurring products act as mitocans by targeting the mitochondrial membrane potential [42].
Mechanistically, several bioactive ingredients found in Solanum nigrum,
including uttroside B, solanine, solamargine, and physalins, were tested for
their anticancer effects in both in-vitro and in-vivo cancer
models [43].
Reprogramming of energy metabolism is one of the emerging hallmarks of cancers because uncontrolled cell division demands an increase in fuel and biosynthetic precursors by adjusting energy metabolism in cancerous cells. Our findings showed that SNWE significantly interferes with energy metabolism in cancer cells by disrupting mitochondrial membrane potential that results in the depletion of cellular ATP levels. However, the involvement of other potential targets behind the anticancer effects of SNWE may not be ruled out. For instance, solanine, the main ingredient of Solanum nigrum, showed antitumor ability against different tumors by targeting different proteins [48].
Thus, the use of SNWE alone or as an adjuvant with low doses of known cancer drugs could serve as an economical therapeutic modality with fewer side effects.
The SNWE treatment induced the loss of mitochondrial membrane potential, which resulted in reduced cellular ATP levels and subsequent apoptotic cell death in the MCF-7 and MDA-MB-231 breast cancer cells. Since this study was conducted in breast cancer cells, further studies are required to confirm the implications of our findings for the treatment of human breast cancer. In the early stages, breast cancer is treatable with high success rates. Pharmacotherapies based on traditional medicine are not only cost-effective but mostly free from side effects. Further studies are warranted to explore the therapeutic and prophylactic anticancer effects of Solanum nigrum using animal models of cancer.
Datasets used and/or analyzed for this study are available from the corresponding author upon appropriate request.
Conceptualization, HAK; methodology, HAK, NRP, AAA, SHA, BSA, AAKH; formal analysis, NRP, AAA, BSA, AAKH; investigation, HAK, NRP, AAA, SHA; resources, HAK, SHA, NRP; data curation, HAK, NRP, AAA, BSA, AAKH; original draft preparation, HAK, AAA; writing, review and editing, HAK, AAA, NRP; supervision, HAK, AAA; project administration, HAK; funding acquisition, HAK. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to its accuracy or integrity.
The study protocol was approved by the Institutional Review Board of King Saud University, Riyadh, Saudi Arabia (Approval No. KSU-SE-22-18, dated 24/03/2022). This study was conducted in rats and did not involve human subjects.
We acknowledge the technical support from the laboratory staff.
This research was funded by the National Plan for Science, Technology, and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number (2-17-03-001-0044).
The authors declare no conflict of interest. HAK is serving as one of the Guest Editors and Editorial Board Members of this journal. We declare that HAK had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to SN.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.





