1 Occupational Medicine Department, “Carol Davila'' University of Medicine and Pharmacy, 050474 Bucharest, Romania
2 Occupational Medicine Department, “Gr. T. Popa'' University of Medicine and Pharmacy, 700115 Iasi, Romania
3 Faculty of Pharmacy, Titu Maiorescu University, 040441 Bucharest, Romania
4 Pharmacology and Pharmacotherapy Department, “Carol Davila” University of Medicine and Pharmacy, 050474 Bucharest, Romania
†These authors contributed equally.
Abstract
Silicosis, an occupational lung disease that can be prevented, is still a significant public health concern in many countries, despite its considerably decreased incidence over the years. The latency period for silicosis ranges from a few years to several decades, depending on the duration and intensity of exposure to silica dust. The complex pathogenic mechanisms of the disease are not fully understood, but it is known to be characterized by inflammation, the formation of silicotic nodules, and progressive and irreversible fibrosis. The aim of this paper was to present the current sources of exposure to silica dust and summarize the updates on risk factors (e.g., socioeconomic status, genetic susceptibility) and sex differences, silico-tuberculosis, prognostic markers including 16-kDa Clara cell secretory protein, antifibrotic treatment, and other therapeutic possibilities with promising results. There are no effective treatment options for silicosis, and prevention remains the primary tool to significantly reduce the risk of disease. There are promising new treatments under investigation including antifibrotic, cellular, and immunomodulatory therapies, but further research is needed to demonstrate the efficacy and safety of these therapies in adequately powered clinical trials.
Keywords
- silicosis
- inflammation
- fibrosis
- current treatment
- antifibrotic treatment
Silicosis, one of the oldest occupational diseases, is a pneumoconiosis caused
by the long-term inhalation of inorganic dust with high concentrations (
The latency period for silicosis ranges from a few years to several decades, depending on the duration and intensity of exposure to silica dust. In some occupational pulmonary diseases, such as silicosis, mesothelioma, and asbestosis, the diagnosis can be delayed due to the long latency period, leading to unfavorable disease outcomes [2]. Thus, it is important to increase awareness of the disease, impose strict safety guidelines and regulations, and follow them in order to minimize workers’ exposure to silica dust. Apart from prevention measures, the long-term health monitoring of individuals who have been exposed to silica dust is also important for the early identification of any potential health problems.
Awareness of the health problems related to silica dust exposure and a rigorous occupational history are essential tools for an early diagnosis. This is especially important, as an accurate diagnosis might be associated with a better prognosis. The migration of workers globally may result in a lack of adequate health monitoring or failure to account for prior exposure to silica dust.
The International Labour Organization (ILO) and the World Health Organization set a goal in 1995 to eliminate silicosis from workplaces by 2030 through the Global Program to Eliminate Silicosis [3]. This goal cannot be easily reached since new sources of silica dust are identified in new technological processes, and the effectiveness and feasibility of dust control methods and technologies vary in different countries.
Silicon dioxide, also known as silica, is a widespread mineral that makes up part of the structure of the Earth’s crust. It is formed from silicon and oxygen under increased pressure and temperature conditions. There are two forms of silica: crystalline and amorphous. The crystalline form is very aggressive in lung tissue [4]. Occupational exposure to silica can occur in many workplaces or industries such as mining, metallurgical and car manufacturing industries, abrasive materials, glass, porcelain, or the tile industry [5]. In addition, occupational exposure to silica particles has also been identified in the case of new technological processes such as jewelry manufacturing, denim sandblasting or manufacturing, and processing of artificial stones [6, 7, 8], which are associated with accelerated forms of silicosis and the rapid degradation of lung function in young people [9]. The results of a study by Hua et al. [9] identified a possible significant emerging population of young stoneworkers who have severe and irreversible silicosis. The artificial stones have a content of 85–93% silica; thus, processing such stones is currently the major cause of the increase in the number of silicosis cases, especially in Spain, Australia, and Israel [10].
During the early 2000s, artificial stones were introduced in Australia, which were processed by dry-cutting methods [11]. A study conducted in Israel on 68 non-smoking workers handling artificial stone showed that ultrafine particles accumulated in their airways, causing sustained neutrophilic inflammation [12]. Another report from Rose et al. [13] described 18 cases of silicosis in Hispanic stone mill workers from four US states. In 2018, the government of Australia launched in Queensland a screening program for workers in artificial stone factories, which diagnosed 98 individuals with silicosis out of the 799 screened [13]. Furthermore, Quan et al. [14] discovered that patients with silicosis linked to artificial stone processing have a more than 5-fold higher risk of experiencing progression with a significant decline in lung function compared to patients with non-artificial stone-related silicosis.
There are three clinical forms of silicosis: chronic (classic), accelerated, and acute (silicoproteinosis). The chronic form of silicosis is the most common and occurs after 15–20 years of exposure to free crystalline silica dioxide particles. Accelerated silicosis can occur after 5–10 years of exposure to increased concentrations of silica dust, and the acute form usually occurs after very high exposure to silica in a period of a few months to 2 years [15, 16]. Individual susceptibility is an important element that could explain the different reactions to silica exposure observed in workers from the same working environment who are exposed to similar concentrations of silica dust. Genetic and epigenetic components play important roles in individual susceptibility [17].
Several valuable reviews on silicosis have been published in the last several years focusing on certain aspects of silicosis such as epidemiology, pathogenic mechanisms, diagnosis, and treatment [9, 11, 18, 19, 20]. Since there is a great deal of interest in silicosis, new data are constantly emerging. The aim of this paper is to integrate all available data, present the current sources of exposure to silica dust, and provide updated information on risk factors (e.g., socioeconomic status, genetic susceptibility), sex differences, silico-tuberculosis (TB), prognostic markers including 16-kDa Clara cell secretory protein (CC16), antifibrotic treatment, and other therapeutic possibilities with promising results. Additionally, the aim of this paper is to raise awareness of the impact of this preventable disease on public health, and to call for continued action to address this important occupational health issue. It is important to ensure that workers are adequately protected from the harmful effects of silica dust exposure and that the incidence of silicosis is reduced. Even though the link between occupational exposures and respiratory diseases is well known, there is often a lack of recognition and awareness of this association among clinicians and workers, and education is an essential tool to overcome unfortunate outcomes.
The epidemiology of silicosis is complex and multifactorial and includes several risk factors, such as sex differences, socioeconomic status. The most important risk factors for silicosis include prolonged exposure to silica dust, the duration and intensity of exposure to silica dust, and the shape and size of silica particles. New information on sex differences in silicosis has emerged. Initially, silicosis was considered a disease that predominantly affected males because exposure to silica dust was mostly occupational for men and epidemiological studies have mainly been conducted in heavy male-dominated industries. However, there is an increasing number of women working as dental laboratory technicians, artists, and ceramic technicians, as well as in mining or foundry work, and recent studies have shown that women are also at risk of developing silicosis. A cohort study carried out in the car manufacturing industry in China, which included 2009 subjects (1405 men [70%] and 603 women [30%]), proved that the incidence of silicosis was higher in men and increased with duration of the exposure period [21]. This conclusion was supported by studies carried out in workers in pottery factories [22]. According to Ndlovu [23], the employment of women in gold mining in South Africa has significantly increased, from less than 1% before 2002 to more than 10% in 2013. The development of new technological processes, such as jeans sandblasting or jewelry polishing, can cause silicosis equally in men and women.
Socioeconomic status also plays a role in the development of silicosis. Lack of protective equipment and lack of safety measures in the workplace that might be found in nonregulated countries, are associated with a higher risk of exposure to silica dust. The additional exposure to high levels of air pollution may also lead to an increased risk of developing silicosis [24, 25, 26, 27]. Other factors that may contribute to the epidemiology of silicosis include genetic factors, other co-existing medical conditions, and environmental factors. Individual susceptibility is an important factor that can explain different reactions in workers exposed to silica dust, even though they share the same working environment and are exposed to silica dust in similar concentrations. This is explained by individual genetic factors. According to Zhou et al. [17], susceptibility to silicosis is associated with the presence of single nucleotide polymorphism rs1814521 in long non-coding RNA ADGRG3. Co-existing medical conditions that contribute to developing silicosis are TB or human immunodeficiency virus [4, 16]. As aforementioned, environmental factors such as high levels of air pollution or additional irritants in the air may impact the inhalation and deposition of silica particles in the lungs.
Although efforts have been made to prevent workers from being exposed to silica dust in recent decades, silicosis is still a public health issue with a higher prevalence in developing countries. Even though its incidence has decreased considerably, it is a disease with poor clinical prognosis, which does not yet have an effective treatment. Each year, more than 230 million workers worldwide are exposed to free crystalline silica particles [28], with more than 40.5 million people employed in artisanal and small-scale mining, which involves unmechanized mining methods that result in high exposure to silica dust [29]. In Brazil, exposure to silica dust affects more than 6 million workers daily [30], with the prevalence of silicosis being higher in people aged 60 years and older, followed by adults aged 40–59 years [11]. Similarly, recent literature data suggest that in India, more than 10 million workers are exposed to silica particles [30]. In South Africa, silicosis is a significant public health concern, particularly in the gold mining industry where miners are exposed to high silica dust content (ranging from 9% to 39%). The social conditions associated with mining involve migration and dormitory-style housing [31]. The United States and Europe each have more than 2 million people working with silica [10], whereas in the United Kingdom, about 600,000 workers are considered at risk of illness [7]. In southern Spain, a total of 106 patients were were diagnosed with artificial stone silicosis between 2009 and 2018 [32]. In Romania, there were 29,046 workers exposed to silica dust in 2007, representing 0.98% of all those exposed to occupational hazards [33], with 3066 new cases of silicosis diagnosed between 2007 and 2021 [34]. Statistical data have shown that 4.2% of deaths reported for Chinese workers are caused by exposure to breathable particles of silica [35], with more than 6000 new cases of silicosis reported annually [36]. Globally, the incidence of silicosis is more than 20,000 cases per year [8] and over 12,900 deaths are recorded, with an estimated 0.65 million disability-adjusted life years in 2019 [37].
Inhalation of respirable silica particles leads to formation of mineral deposits in the terminal bronchioles and alveoli and induces pulmonary tissue reactions of the inflammatory type and proliferation of fibroblasts by complex pathogenic mechanisms, causing fibrosis as presented in Fig. 1 [38]. Disease severity and pathogenicity depend on the quantity of inhaled dust and the time of exposure. Under normal conditions, lung epithelial cells can replace damaged cells, due to exposure to silica dust through cell proliferation and differentiation. However, chronic exposure to silica dust can lead to repetitive damage and repair of airway epithelia, resulting in depletion of airway epithelial stem cells in pulmonary silicosis [39].
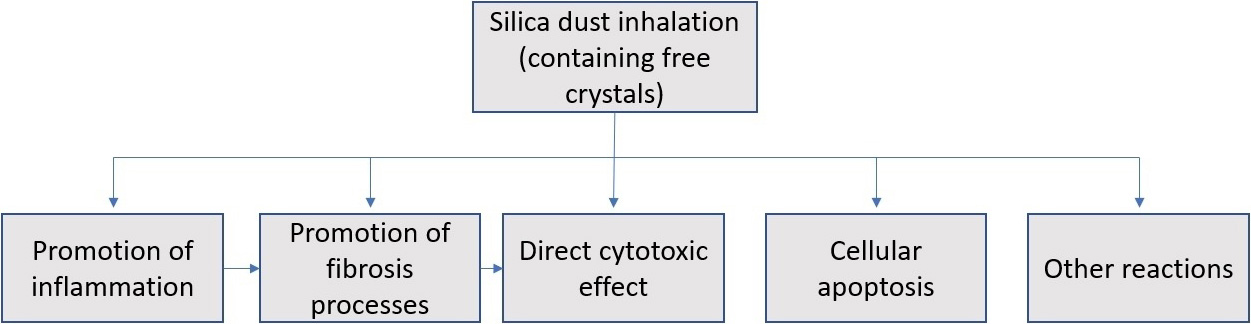 Fig. 1.
Fig. 1.Schematic representation of the pathogenetic mechanisms of silicosis.
Lung tissue reactions result from the joint action of several mechanisms, such as the direct cytotoxic effects of silica particles on macrophages, activation of the surface of receptors of the macrophages, rupture of lysosomes, production of free radicals, activation of inflammasomes, production of cytokines and growth factors, and cellular apoptosis, which finally lead to fibrosis [19]. Most of the studies investigating the pathogenesis of silicosis have focused on the roles of alveolar macrophages and alveolar epithelial cells, which secrete pro-inflammatory and profibrotic mediators secondary to exposure to silica [40].
Alveolar macrophages, alveolar epithelial cells, and fibroblasts are involved in the complex pathogenesis of silicosis. Alveolar macrophages are the first cells to react against inhaled silica particles, as they are found in the alveolar surfactant. They have a protective role as they recognize, phagocytose, and degrade the silica dust. During this process, alveolar macrophages can undergo disintegration, necrosis, and apoptosis. The apoptosis of alveolar macrophages leads to pulmonary fibrosis. In addition, apoptotic alveolar macrophages release a significant amount of inflammatory factors, increasing inflammation [41, 42]. Alveolar epithelial cells are also affected by silica exposure. One study showed that silica exposure increases the permeability of alveolar epithelial cells [43]. Another study identified the activation of autophagy in alveolar epithelial cells and concluded that blockage of autophagic flux in alveolar epithelial cells is crucial in silica-induced pulmonary fibrosis [44]. Fibroblast activation and myofibroblast differentiation are also part of the pathogenetic mechanism of pulmonary fibrosis in silicosis. Following exposure to silica dust, fibroblasts are activated and differentiate into myofibroblasts, secreting substantial amounts of extracellular matrix (ECM) proteins. According to Li et al. [45], silicosis is associated with the deposition of excessive ECM produced by activated fibroblasts.
As aforementioned, silica particles can activate alveolar macrophages and also
directly damage epithelial cells, which leads to the release of a large number of
profibrotic factors (e.g., tumor transformation and growth factor beta
[TGF-
Oxidative stress induces cellular apoptosis. During apoptosis, the cells release chemotactic factors that recruit new inflammatory cells, thereby increasing inflammation. In addition, following phagocytosis, macrophages release silica particles back to the lung parenchyma, where they are phagocytosed by other macrophages, perpetuating the vicious cycle of tissue destruction [30]. The increased production of profibrotic substances and the role of cytokines in differentiation into fibroblasts, cause excessive accumulation of collagen and fibronectin fibers, leading to the formation of silicotic nodules, tissue fibrosis, and the reduction of gaseous exchange surfaces [4, 40]. The process of pulmonary fibrosis is irreversible and the impairment of lung function is progressive and continues even after the cessation of exposure to silica dust affecting all areas of the respiratory system, including lung parenchyma and central and small airways [52].
The pathogenesis of silicosis may also be associated with an immunological mechanism, an idea supported by the identification of serum immunoglobulins, anti-DNA, and antinuclear factor antibodies in the serum of patients with silicosis [51]. After inhalation, the silica particles that are deposited in the lung tissue or lymph nodes can modulate the activity of immune cells such as natural killer cells, B cells, regulatory T cells, CD4 T helper cells, and cytotoxic T lymphocytes, thereby influencing immune tolerance. A study by Tomokuni et al. [53] reported that soluble Fas levels (a molecule that inhibits Fas-mediated apoptosis of responder T lymphocytes) were significantly higher in silicosis patients than in healthy volunteers. Additional studies are needed to clarify the cellular and molecular pathogenetic mechanisms of silicosis. These could also be useful for the prevention of immune diseases associated with silicosis (e.g., rheumatoid arthritis, systemic lupus erythematosus, scleroderma, and vasculitis) [54].
Silicosis is a disease with progressive evolution to respiratory failure, the main cause of death. In 1997, the International Agency for Research on Cancer introduced crystalline free silica into group 1 of carcinogens. There is strong and consistent evidence of a dose-response relationship between silica exposure and the risk of lung cancer [55]. The prognosis of the disease can be severe, especially when silicosis is associated with comorbidities such as pulmonary TB, autoimmune diseases, fungal or bacterial infections, pulmonary cancer, chronic obstructive pulmonary disease (COPD), or kidney disease [4, 16].
Since there is a long time between silica exposure and the pulmonary tissue
reactions becoming radiologically visible, biomarkers that can be found in the
initial stages of silicosis would be particularly useful for the screening or
health evaluation of workers with occupational exposure to free silica particles.
The serum level of the following inflammatory mediators could be a prognostic
biomarker in silica-exposed workers: IL-6, IL2R, IL-1b, IL-8, TNF-
Several researchers have shown increased serum levels of angiotensin convertase in pulmonary granulomatous diseases such as silicosis and sarcoidosis. Increased serum values of copper and ceruloplasmin can be associated with pathological changes, such as fibrosis and the proliferation of collagen tissue in the silicotic lung [51]. Furthermore, studies have shown a correlation between an increase in the neutrophil/lymphocyte ratio and the radiological evolution from simple silicosis to massive progressive fibrosis, given that no other factors that influence the pulmonary inflammatory process are associated such as smoking or other acute or chronic respiratory diseases [59, 60]. However, none of these factors is conclusively a specific biomarker with clinical application or the potential for early diagnosis in silicosis. Thus, further research on the pathogenic and biological mechanisms of the disease is needed.
Pulmonary TB is one of the most common comorbidities associated with silicosis, with an increased incidence in less developed countries. Silico-TB is active TB superimposed on silicosis. The relative risk of patients with silicosis developing pulmonary TB is estimated to be 2.8 [61]. Approximately one-quarter of patients with silicosis and coal workers’ pneumoconiosis, commonly affected by peripheral lymph node calcification known as “eggshell calcification”, have silico-TB [62]. The risk of TB increases with the severity of silicosis, and exposure to silica dust increases the risk of TB even without silicosis [18].
The diagnosis of silico-TB is often difficult, especially in the early stages of disease when clinical manifestations may not be indicative and radiological changes may be indistinguishable from those due to pre-existing silicosis. The suspicion of silico-TB should be raised when there are radiological findings of the rapid appearance of new opacities and pleural effusion or excavations. Generally, active disease is difficult to detect clinically in patients with silicosis [61]. Silicosis can be a contributing factor of TB mortality due to diagnostic confusion and consequent delayed TB treatment [36].
According to Rupani’s study [37], which included 138 patients with silico-TB and 2610 patients with TB without silicosis, patients with silico-TB were 2.3 times more likely to have unfavorable TB treatment outcomes compared to patients with TB without silicosis, perhaps due to impairment of macrophage function by silica dust and/or poor drug penetration into fibrotic nodules [61]. Furthermore, Rupani suggested undertaking collaborative TB-silicosis activities to improve the outcomes of silico-TB treatment [37].
The fatality rate among patients treated for TB is three times higher in patients with silicosis than in those with non-silicosis due to difficulties in diagnosis, impaired lung function, or reduced effectiveness of tuberculostatic treatment due to pre-existing pulmonary fibrosis [36].
CXR and high-resolution CT (HRCT) are the main tools used for diagnosis. The diagnosis of silicosis typically involves the use of three international criteria: a history of silica exposure that is sufficient to cause the disease, the presence of chest radiograph features consistent with silicosis, and the absence of other illnesses that mimic silicosis.
It is accepted that prolonged exposure to respirable free silica at levels exceeding standards is needed for the development of silicosis. However, there are individual differences in susceptibility, with some workers developing more severe disease than others, even when employed in the same environment. When assessing exposure, factors such as the length of employment, exposure measurements, and whether or not the worker was provided effective respiratory protection should be taken into consideration.
The ILO recommends using CXRs to classify and record radiographic abnormalities caused by dust exposure. Simple silicosis is characterized by rounded opacities that are less than 10 mm in diameter, whereas progressive massive fibrosis results from the conglomeration of small rounded opacities [63]. HRCT is more specific and sensitive than CXRs in the early evaluation of silicosis [64]. Pulmonary function tests are used to assess disease severity and distinguish between restrictive and obstructive disorders, although there is often no correlation between radiological changes in silicosis and lung function impairment [65]. Lung biopsy is the only way to achieve an accurate diagnosis when there is no history of occupational exposure, there is disagreement between CXR and HRCT imaging results, or there are atypical presentations that cause physicians to consider other differential diagnoses. The presence of silicotic nodules confirms the diagnosis [20].
It is important to exclude other medical conditions that may show similar X-ray images as silicosis such as rheumatoid nodules, tumors, infections, other pneumoconiosis, or sarcoidosis. This helps to ensure that the correct diagnosis is made and appropriate treatment is given.
Currently, there is no effective treatment for silicosis, and prevention is the only tool to decrease the risk of disease. Numerous experimental studies and several clinical studies have focused on several therapeutic options that could slow down the progression of silicosis such as antifibrotic drugs, cell therapies, antibiotics, and immunomodulatory agents. However, the small number of patients with silicosis is a big obstacle to attaining an adequate sample size for clinical trials. Table 1 summarizes the treatment options (in use or under investigation).
| Current treatment | |
| Smoking cessation | |
| Psychological support | |
| Anti-inflammatory treatment (corticosteroids) | |
| Pulmonary rehabilitation | |
| Symptomatic treatment (bronchodilators, oxygen) | |
| Prevention of infection | |
| Whole lung lavage | |
| Lung transplantation | |
| Treatments under investigation | |
| Antifibrotic treatments (e.g., pirfenidone, nintedanib) | |
| Stem cell therapies (e.g., mesenchymal stem cells) | |
| Immunomodulatory agents (e.g., thymalfasin) | |
| Monoclonal antibodies (e.g., infliximab) | |
| Antibiotics (e.g., azithromycin) | |
| Other experimental treatments (e.g., interferon gamma, N- acetylcysteine, gene therapy) | |
| Alternative treatments | |
| Tetrandrine (natural alkaloid calcium channel blocker) | |
| Polyvinyl-pyridine-N-oxide (polymer) thrombomodulin (protein) | |
Aluminum-based compounds have been extensively studied for their ability to coat silica particles, reducing crystal reactivity and thereby protecting lung tissue. Between 1943 and 1979, aluminum powder (McIntyre Powder) was used by miners for the prophylaxis of silicosis, but subsequent research did not show significant differences in mortality due to silicosis between the groups exposed and not exposed to aluminum powder. Instead, cognitive function impairment, correlated with exposure time, was observed in workers who inhaled McIntyre powder [66]. This observation was supported by subsequent research carried out in subjects exposed to aluminum, which, in addition to the decline of cognitive function, also showed that the exposed group also presented with memory disorders, anxiety, depression, and personality disorders [67].
The therapeutic options for silicosis are currently limited; therefore, effective prevention measures are essential to significantly decrease the risk of disease. There is no curative treatment available—only supportive and symptomatic treatment that include the use of bronchodilators and the administration of oxygen to improve the respiratory symptomatology and prevent infections, but with no influence on the progression of the disease. In the initial stages of silicosis, the therapeutic interventions consist of smoking cessation, improving cardiovascular and respiratory conditions, psychological support, and whole lung lavage (used for pulmonary alveolar proteinosis). Whole lung lavage is an invasive procedure that, unfortunately, is not associated with long-term benefits for evolution of the disease [68]. For the common forms of the disease, treatment recommendations include anti-inflammatory treatment, pulmonary rehabilitation, symptomatic treatment, and treatment for complications. Despite the frequent use of corticosteroids in silicosis, there is no evidence to support their benefit in treating the disease. During short-term use, corticosteroids can relieve symptomatology; however, long-term use of corticosteroids is associated with an increased risk of infection [30].
Pulmonary rehabilitation has recognized benefits in patients with lung conditions such as COPD [69]. Rehabilitation programs combine a series of physical exercises (e.g., gymnastics, walking, muscle-toning exercises), respiratory gymnastics, relaxation techniques, and nutritional advice. Several studies have shown a significant improvement in standard test results in patients with interstitial lung disease such as the 6-min walk test, pulmonary function (maximum rate of oxygen consumption), and quality of life immediately after completion of the rehabilitation program [70].
Lung transplantation is the only treatment option for severe forms of silicosis [71]. Even though there are few studies due to the small number of patients with compatible donors, the results have shown improved lung function and increased survival rate in transplanted patients [72]. Even if the 3-year survival rate of transplanted patients is 76%, the complexity of the intervention, the increased costs, and the high operative risk greatly limit the use of lung transplantation in silicosis cases [71].
Antifibrotic treatment, the therapy with the most advanced results, is used for the treatment of various interstitial diffuse lung diseases. Silica dust induces inflammatory reactions in pulmonary tissue and the proliferation of fibroblasts, causing pulmonary fibrosis [38]. Antifibrotics have anti-inflammatory effects and inhibit the proliferation of fibroblasts, making them good candidates for silicosis treatment. Studies on animal models of diffuse interstitial pulmonary diseases, systemic sclerosis, rheumatoid arthritis, and silicosis have shown that antifibrotics significantly decrease inflammation and pulmonary fibrosis. Clinical studies have shown that pirfenidone and nintedanib, two antifibrotics approved for the treatment of diffuse interstitial lung diseases, are well tolerated by patients and reduce the rate of decline of pulmonary function. These two drugs downregulate growth factors involved in fibrosis development and progression [73].
Pirfenidone, a pyridine compound, is an immunosuppressant with antifibrotic and
anti-inflammatory effects. Pirfenidone can inhibit the mechanism of fibrosis by
regulating or suppressing fibroblast growth factor, connective tissue growth
factor, TGF-
Nintedanib, a tyrosine kinase inhibitor, is clinically used for treating fibrotic lung diseases including idiopathic pulmonary fibrosis and chronic interstitial lung diseases, to mitigate lung function decline and risk of pulmonary exacerbation. The results of the studies in which nintedanib was administered, improved the rate of pulmonary decline both in patients with moderate or severe impairment of lung function as well as in those with relatively preserved lung function [77].
An in vivo study in mice with silica-induced pulmonary inflammation treated daily with nintendinab showed a significant decrease in neutrophils, lymphocytes, and cytokines, effects that support the slowing of fibrosis progression [78]. An experimental study in which nintedanib was administered intratracheally in the form of a nanosuspension, provided remarkable antifibrotic activity in a mouse model of silicosis without incurring local and systemic safety concerns [79].
In a double-blind, placebo-controlled, phase 3 clinical trial (INBUILD) that
enrolled a total of 663 patients with progressive massive fibrosis, excluding
idiopathic pulmonary fibrosis, patients received nintedanib 150 mg twice a day
for 2 years. The treated patients showed a significant decrease in the rate of
lung function decline compared to the control group [80]. Another study of
nintedanib, an open-label, randomized study (INJOURNEY), was conducted in
patients with interstitial pulmonary diseases and forced vital capacity
The efficacy of antifibrotic drugs in patients with silicosis is under investigation. A clinical study conducted in 100 patients with the most common types of pneumoconiosis (silicosis, asbestosis, and coal miner’s lung), treated with nintedanib 150 mg twice daily for 3 years, will be completed in 2025 [82]. Two other antifibrotic drugs are in phase 1 clinical trials. One of them is CRV43, a cyclosporine derivative, which is a selective inhibitor of cyclophilin proteins and plays a role in formation of the ECM [83]. The second drug, caveolin-1 scaffolding domain peptide, has been shown to reverse pulmonary fibrosis in experimental studies in mice [84].
Stem cell therapies can repair damaged pulmonary tissues by replacing the endogenous damaged cells through cell regeneration and changing the microenvironment, thus opening up new avenues of research for the treatment of interstitial lung diseases including silicosis [85]. Mesenchymal stem cells derived from lung tissue, adipose tissue, blood, bone marrow, or the umbilical cord have the ability to differentiate into alveolar epithelial cells. Experimental studies in mice with silicosis treated with cells derived from the bone marrow, which have tropism for inflammation areas, have shown reduced inflammation and fibrosis [86]. The results of a pilot prospective study in five patients with chronic and accelerated silicosis showed the effectiveness of cell therapy, which slowed disease progression without significant side effects. Furthermore, another study showed the early and sustained increase of perfusion at the base of both lungs after bone marrow-derived mononuclear cell administration [87]. Although the results of experimental research are promising, the mechanisms of action are not yet well understood and more work is needed to establish the correct dose, treatment duration, and route of administration [80].
Immunomodulatory agents are also a useful treatment option that needs further
investigation. Free crystalline silica particles act at the level of the
pulmonary interstitial tissue and through immunological mechanisms. A study
conducted by Xiong et al. [88] showed a significant decrease of T
lymphocytes (CD3
Antibiotics are under investigation as some have anti-inflammatory and antifibrotic effects such as azithromycin. In vivo studies have shown that azithromycin acts selectively on fibroblasts involved in pulmonary fibrosis, without destroying normal fibroblasts [90]. Clinical trials are needed to confirm the benefits of prophylactic treatment with azithromycin in patients with frequent respiratory infections during the evolution of interstitial lung disease. Recurrent infections commonly found in patients with interstitial lung diseases can be considered a predictive factor for disease progression [91]. Other experimental treatments with promising results have been used including IFN-g, suppressive oligodeoxynucleotides, N-acetylcysteine, methyl palmitate, gene therapy, and chemotherapy drugs (e.g., dasatinib).
Tetrandrine, a natural alkaloid and calcium channel blocker, was shown to inhibit and limit inflammatory processes in experimental silicosis by reducing the production of free radicals and reducing the incorporation of silica particles by macrophages [92]. Even though the mechanism of action of tetrandrine is not fully known, experimental data have shown that it intervenes in the pathogenesis of silicosis by inhibiting the NALP3 inflammasome. Tetrandrine was approved in China for the treatment of patients with silicosis [93].
Polyvinyl-pyridine-N-oxide (PVNO) interacts with silica to create a polymer film on the silica surface and then changes the silicic acid’s hydrogen transfer effect [94]. PVNO has shown encouraging results in experimental studies, but no similar results have been reported in clinical studies [30].
Thrombomodulin, a protein with anticoagulant and anti-inflammatory properties, was shown to improve the survival rate in patients with diffuse interstitial lung disease with frequent exacerbations. However, recent studies showed that there were no significant differences in the survival rate compared to the control group, and it also led to serious side effects such as hemorrhage [95]. Other natural extracts, such as Plantago leaf extract, that showed anti-inflammatory effects [96] have wound healing properties on respiratory track [97].
There are currently no ways to eliminate silicosis and no drug treatments available to stop the progression of fibrosis or improve the disease. Thus, it is necessary to implement effective safety measures to prevent the disease. Accordingly, prevention strategies are being sought worldwide. In 2019, a collaborative statement was released by the American Thoracic Society and European Respiratory Society, asking for more stringent occupational exposure limits regarding silica dust. In addition, the statement highlighted the need for enhanced clinical recognition and increased public awareness of the role of occupational factors in the development of various nonmalignant respiratory diseases including silicosis [98].
Primary prevention methods aim to reduce or eliminate exposure to silica dust by implementing proper ventilation using dust collectors, wetting techniques, and substituting materials that do not contain quartz. Secondary prevention methods such as respiratory protection and medical monitoring aim to limit exposure and detect the disease early. It is important for employers to provide appropriate training and education to workers on the hazards of silica dust and the proper use of personal protective equipment. Considering the latency period for silica, it is essential to monitor individuals who have been exposed to silica dust, even after exposure has ceased, to detect any potential health problems early. In addition, a rigorous occupational history is the key factor for an early, accurate diagnosis that might be associated with a better prognosis.
CXR and spirometry are the primary methods used to monitor the respiratory health of workers exposed to silicon dust. Once an individual is diagnosed with silicosis, that person should be immediately removed from further exposure to silica [20]. Early detection and removal from further exposure to silica are critical measures to prevent the progression of silicosis and reduce the risk of developing other respiratory diseases such as TB. Implementing prevention measures can effectively reduce exposure to silica dust and prevent silicosis in the workplace (Table 2).
| Primary prevention | |
| Engineering controls (e.g., equipment and technologies to control dust at its source). | |
| Personal protective equipment (e.g., respiratory protection such as N95 respirators, gloves, eye protection). | |
| Workplace practices (e.g., frequent cleaning, proper disposal of dust, reducing the amount of silica-containing materials used). | |
| Secondary prevention | |
| Education and training: providing education/training to workers with information on the hazards of silica dust, proper use of personal protective equipment, and workplace safety practices. | |
| Regular monitoring workers’ exposure to silica dust and implementing controls to reduce exposure when necessary. | |
| Regulatory compliance with occupational health and safety regulations and standards. | |
Silicosis is a progressive and incurable disease. Although efforts have been made to prevent workers from being exposed to silica dust in recent decades, silicosis is still a public health issue with a higher prevalence in developing countries, and new data regarding various aspects of this disease are constantly emerging. Currently, there is no treatment for silicosis and prevention remains the most effective way to decrease the risk of developing the disease. There are ongoing studies investigating potential treatments for silicosis including antifibrotic, cellular, and immunomodulatory therapies; however, further research should be done to show the clinical safety and efficacy of such treatments in adequately powered studies. In parallel with research aiming for the development or identification of an effective treatment for silicosis, efforts should be made to develop effective prevention strategies adapted to the current sources of silica dust exposure.
All authors (CMH, MC, ILG and IG) have contributed to the conception and design of the manuscript. CMH has been involved in drafting the manuscript and all authors (CMH, MC, ILG and IG) have been involved in revising it critically for important intellectual content. All authors (CMH, MC, ILG and IG) contributed to editorial changes in the manuscript. All authors (CMH, MC, ILG and IG) have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to its accuracy or integrity. All authors (CMH, MC, ILG and IG) read and approved the final manuscript.
Not applicable.
Publication of this paper was supported by the University of Medicine and Pharmacy Carol Davila, through the institutional program Publish not Perish.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

