1 Department of Kidney Transplantation, Hospital of Nephropathy, The First Affiliated Hospital of Medical College of Xi'an Jiaotong University, 710061 Xi'an, Shaanxi, China
2 Institute of Organ Transplantation, Xi'an Jiaotong University, 710061 Xi'an, Shaanxi, China
3 Department of Pathology, The First Affiliated Hospital of Xi’an Jiaotong University, 710061 Xi'an, Shaanxi, China
Abstract
Background: The use of immature dendritic cells (imDCs) to induce
donor-specific immunotolerance following in vivo stimulation is limited
by their low rate of induction and their tendency to undergo maturation. We
derived imDCs from bone marrow hematopoietic stem cells (HSCs-imDCs). We then
tested the ability of naringenin (Nar) to impede the maturation of HSCs-imDCs for
inducing transplantation immune tolerance. Methods: HSCs derived from
bone marrow were collected and induced to differentiate into imDCs by treating
with Nar (Nar-HSCs-imDCs). Flow cytometry was used to evaluate DC surface
markers, apoptosis, and endocytic ability. The ability of DCs to influence the
in vitro proliferation of T cells and of regulatory T cells (Tregs) was
analyzed by mixed lymphocyte reaction assays. Enzyme-linked immunoassays were
used to quantify cytokine levels in supernatants from co-cultured DCs and Tregs,
as well as in the serum of experimental animals. The level of immunotolerance
induced by Nar-HSCs-imDCs was evaluated by skin grafting in recipient Balb/c
mice, while the Kaplan-Meier method was used to statistically evaluate graft
survival. Results: Compared with HSC-imDCs, Nar-HSCs-imDCs showed higher
expression of cluster of differentiation 11c (CD11c), but lower expression levels
of CD80, CD86, and major histocompatibility complex class II. Nar-HSCs-imDCs also
showed stronger inhibition of T cells and higher Treg cell proliferation.
Interleukin 2 (IL-2) and interferon gamma levels were downregulated in
Nar-HSCs-imDCs, whereas IL-4, IL-10, and transforming growth factor beta levels
were upregulated. The rate of apoptosis and endocytic capacity of Nar-HSCs-DCs
increased significantly after treatment with lipopolysaccharide. HSCs-imDCs or
Nar-HSCs-imDCs were injected into Balb/c mice via the tail vein 7 days before
skin grafting. Significantly reduced donor-specific CD4
Keywords
- naringenin
- hematopoietic stem cells
- immature dendritic cells
- immune tolerance
- skin graft
Organ transplantation is considered the most successful and effective treatment for end-stage organ failure. Advances in medical technology and immunosuppression regimens have to some extent improved the quality of patient life. However, acute and chronic rejection are still major factors that restrict long-term graft survival [1, 2]. It is therefore important to seek new treatment strategies with minimal side effects and with high safety and efficacy for the induction of immune donor-specific hypo-responsiveness, or even tolerance to the transplant.
Dendritic cells (DCs) are specialized antigen-presenting cells that play an essential role in initiating and regulating the immune response to pathogenic microorganisms and to allograft rejection by balancing tolerance and immunity [3]. Immature DCs (imDCs) may prolong allograft survival by inhibiting specific T cells and by enhancing the proliferation of regulatory T cells (Tregs) [4, 5, 6]. However, the application of imDCs for immune tolerance is complicated by their limited induction rate and by their tendency to mature after stimulation.
Numerous studies have reported on the feasibility of deriving imDCs from stem
cells, including the use of cytokines such as granulocyte-macrophage
colony-stimulating factor (GM-CSF), interleukin 10 (IL-10) and transforming
growth factor-
Naringenin (Nar) (5,7,4’-trihydroxyflavanone) is a Chinese medicinal product and
natural citrus flavonoid that has been proposed as a potential immunomodulator
[8]. Nar demonstrates a wide range of anti-inflammatory and neuroprotective
properties [9]. A previous study in a murine model of collagen-induced arthritis
also confirmed that Nar had therapeutic effects by inhibiting the maturation of
DCs [10]. Thus, Nar could potentially prevent the rejection of transplanted
organs [11]. Nar also inhibits T cell proliferation in response to anti-cluster
of differentiation 3 (CD3)/CD28 antibody-stimulated immune cells [12], enhances
the effect of CD4
In the present study we therefore investigated the ability of Nar to maintain HSC-derived imDCs (Nar-HSCs-imDCs) in an immature state, as well as the mechanism by which Nar-HSCs-imDCs can induce immune tolerance in vitro. The findings of this study could offer a new strategy to prevent rejection following organ transplantation.
Balb/c and C57BL/6 mice (6–8 weeks old, 19.9
The induction and culture of imDCs derived from mouse bone marrow HSCs was
performed as described previously [15]. Briefly, bone marrow was obtained from
the femurs of C57BL/6 mice and the erythrocytes were lysed.
Lin-Sca1
HSCs were treated with different concentrations of Nar (purity
Flow cytometry was also used to evaluate the phenotype of HSCs-imDCs,
Nar-HSCs-imDCs, sorted CD3 and CD4 T cells, the effect of HSCs-imDCs and
Nar-HSCs-DCs on Treg cells, apoptosis, and the phagocytic capacity of cells.
HSCs-imDCs and Nar-HSCs-imDCs (1
CD4
Enzyme-linked immunoassay (ELISA; Elabscience, Hubei, China) was used to
quantify the levels of IL-2 (Cat#E-ELM0042c), IL-4 (Cat#E-EL-M0043c), IL-10
(Cat#E-ELM0046c), IFN-
FITC-dextran was used to determine the endocytic ability of HSCs-imDCs and
Nar-HSCs-imDCs before and after stimulation with 5
The Tunel Apoptosis Assay Kit (Cat#1086, Beyotime, Beijing, China) was used to
evaluate the apoptosis of Nar-HSCs-imDCs and Nar-HSCs-DCs cells. Briefly, cells
were treated with protein kinase K and 3% H
C57BL/6 mice served as donors, while Balb/c mice served as the recipients.
Balb/c mice were randomly divided into 8 groups, with 6 mice in each group.
① Sham group; allogeneic mouse skin graft model without treatment;
② phosphate-buffered saline (PBS group); 0.3 mL PBS was infused
intravenously into recipients 7 days before grafting; ③ 10
Spleen cells from each group were collected after mincing. The effect of Nar-HSCs-imDCs on Tregs in the spleen was evaluated by flow cytometry. Unrelated C3H mice were used to evaluate specific reactivity with lymphocytes.
Grafted skin sections (4-mm thickness) were stained with hematoxylin and eosin
(H&E). Images were captured using a BX41 fluorescence microscope (amplification:
200
Quantitative data were shown as the mean
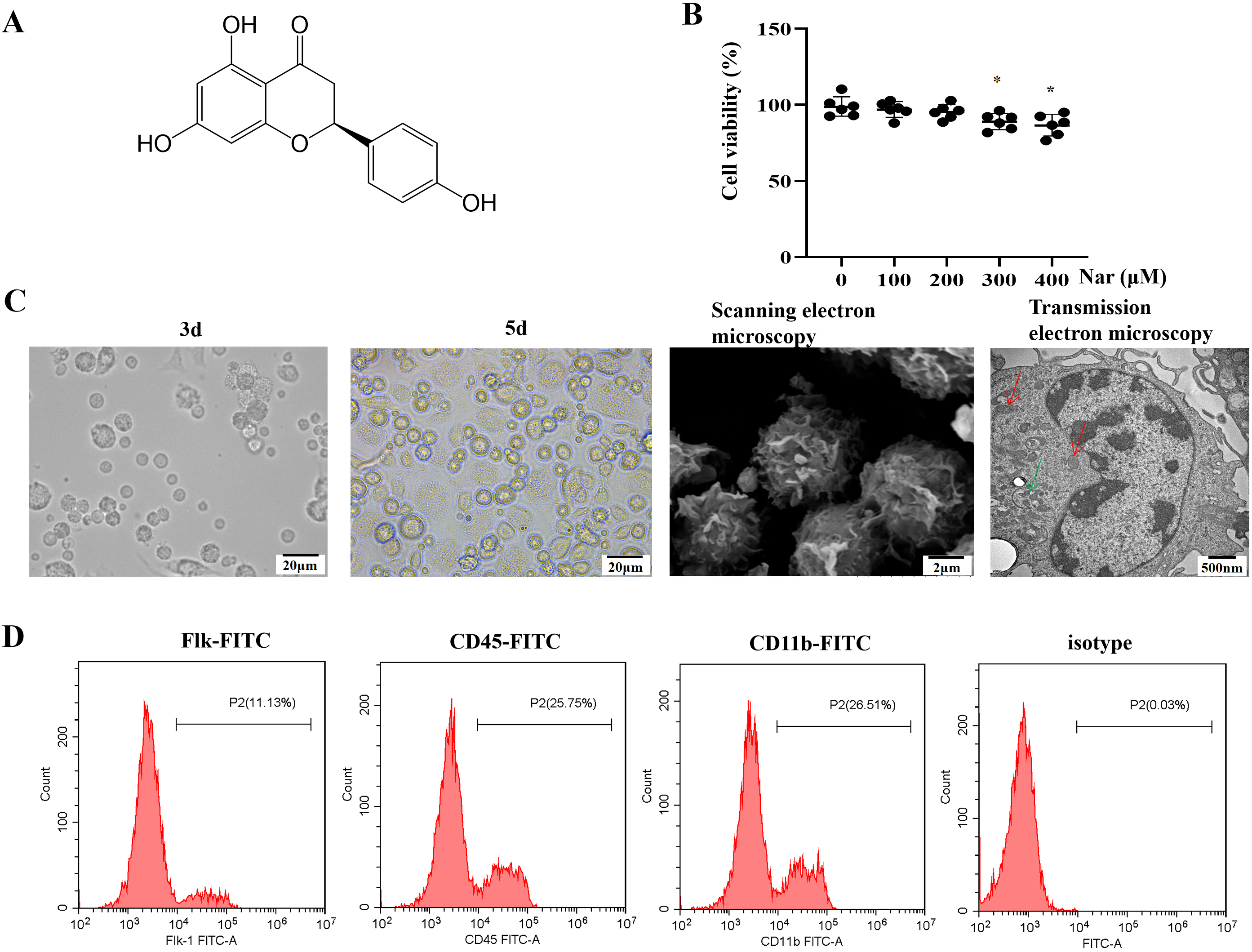
The chemical structure of naringenin is shown in Fig. 1A. Morphological changes
in Nar-HSCs-imDCs were documented in order to assess the role of Nar in promoting
HSC differentiation into imDC, while the cell phenotype during differentiation
was examined by flow cytometry. The optimal concentration of Nar (maximum
concentration at which cell viability was
 Fig. 1.
Fig. 1.Differentiation of HSCs into imDCs under the action of
Nar. (A) Chemical structure of naringenin; molecular formula:
C
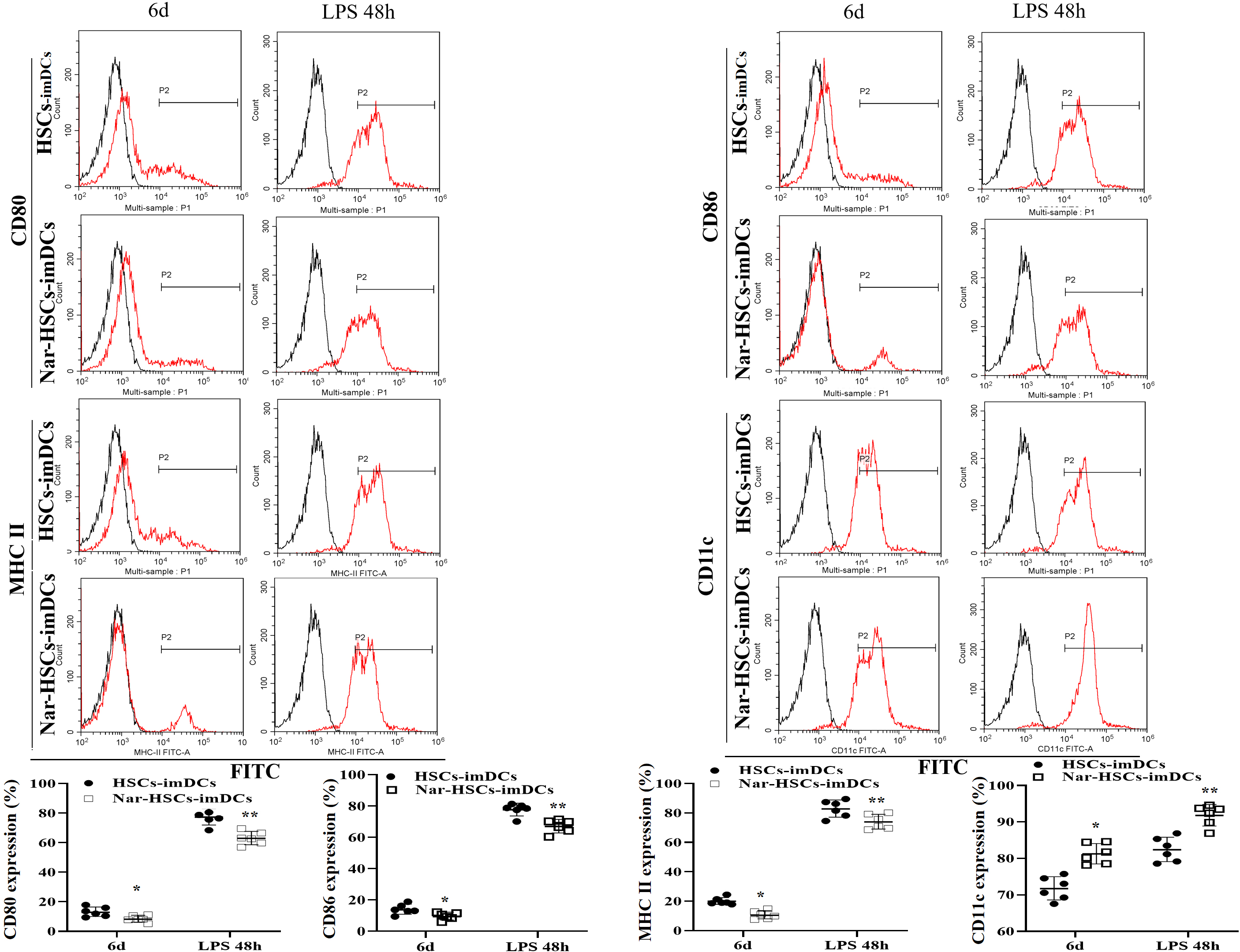
Cell surface phenotypes were analyzed by flow cytometry in order to determine
the effect of Nar on the differentiation of HSCs into imDCs. The results showed
significantly greater downregulation of CD80, CD86, and MHC-II expression on the
surface of Nar-HSCs-imDCs than HSCs-imDCs (p
 Fig. 2.
Fig. 2.DC surface phenotype in differentiation culture. The
Nar-HSCs-imDCs group is compared with the HSCs-imDCs group.
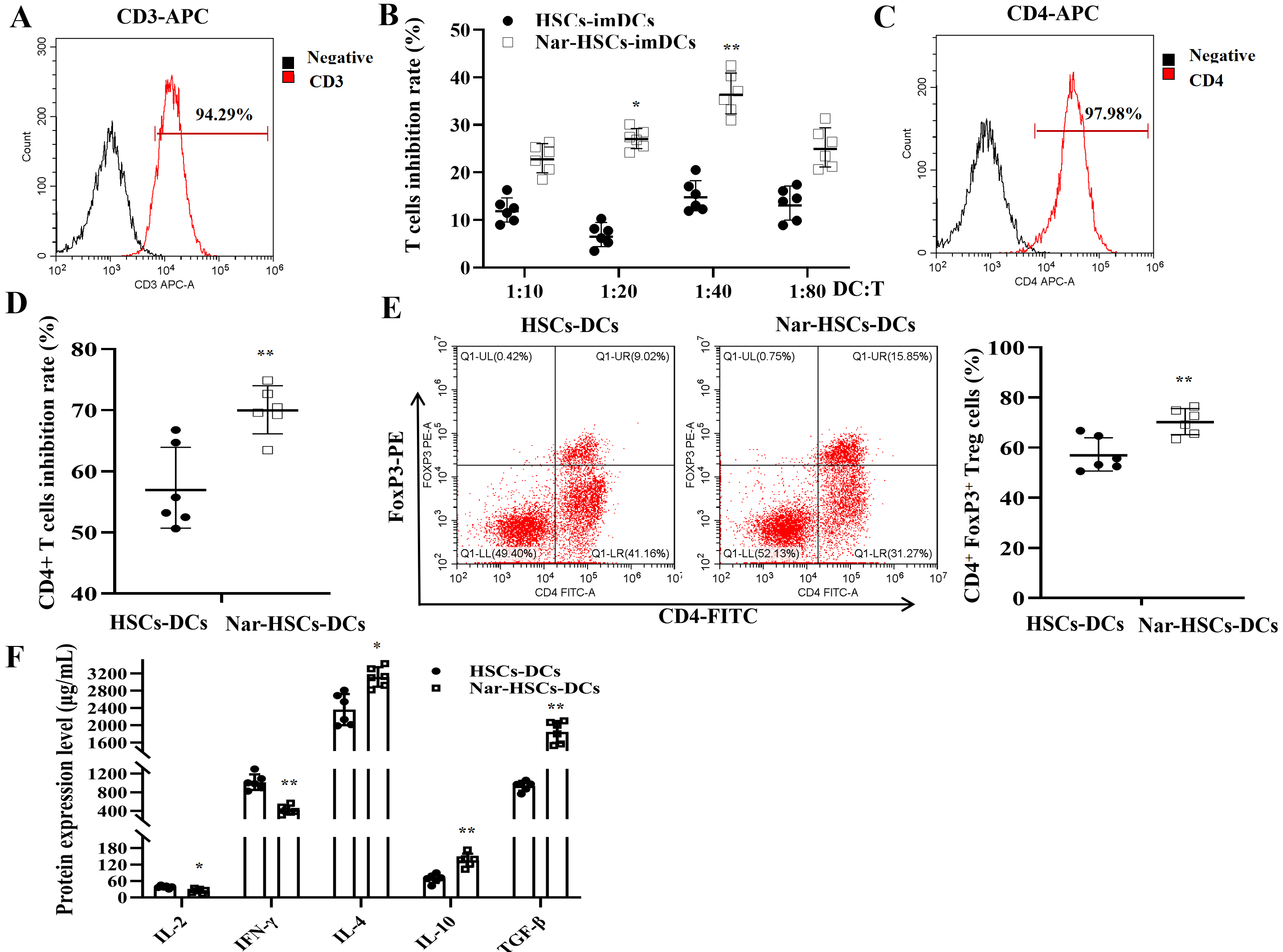
We next investigated the effects of HSCs-imDCs and Nar-HSCs-imDCs on T cells by
isolating CD3
 Fig. 3.
Fig. 3.Nar-HSCs-imDCs suppress T cells and enhance the proliferation of
Treg cells. (A) Purity of CD3
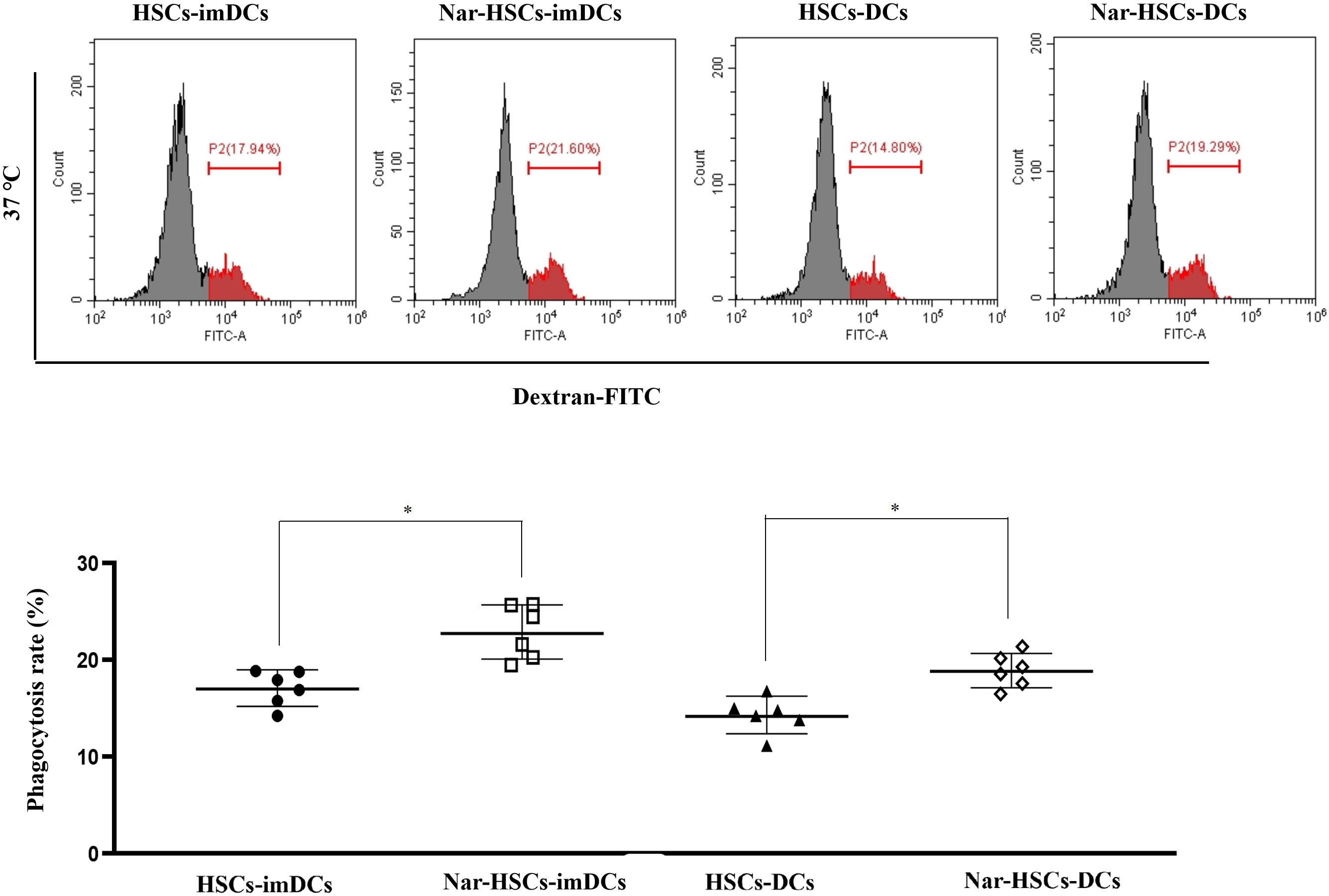
imDCs have been reported to have more vital phagocytosis ability than DCs [6].
The present study found that Nar-HSCs-imDCs and Nar-HSCs-DCs had significantly
higher endocytic capacity for FITC-dextran at 37 °C than HSCs-imDCs and
HSCs-DCs, respectively (Fig. 4) (p
 Fig. 4.
Fig. 4.Endocytic capacity of Nar-HSCs-imDCs and Nar-HSCs-DCs. The
results are compared with the HSCs-imDCs and HSCs-DCs groups, respectively.
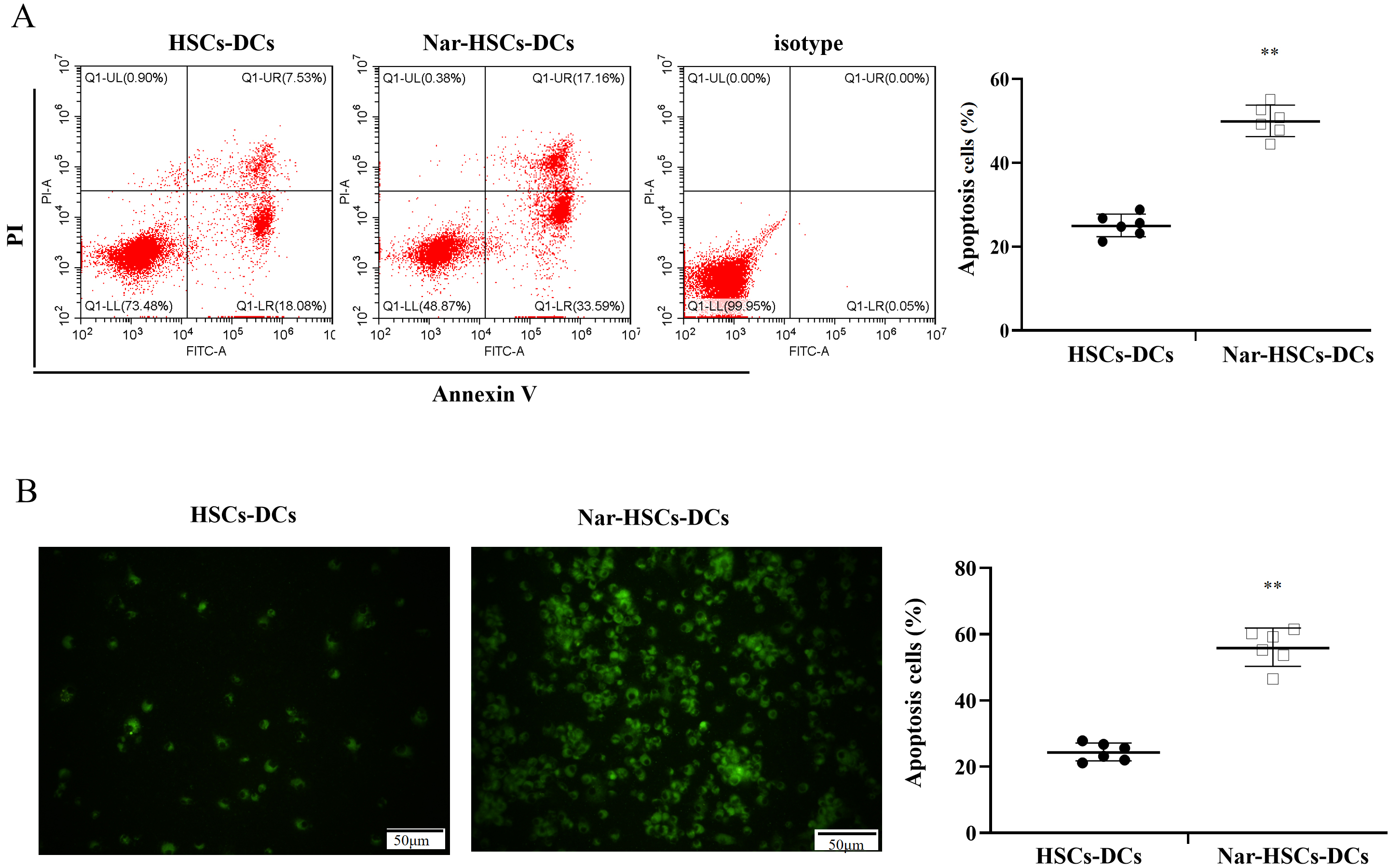
We hypothesized that LPS stimulation of Nar-HSCs-imDC resulted in apoptosis. To test this, flow cytometry was used to quantify the level of apoptosis in Nar-HSCs-DCs. The apoptosis rate of Nar-HSCs-DCs was significantly higher than that of HSCs-DCs (Fig. 5A). TUNEL staining showed similar results (Fig. 5B).
 Fig. 5.
Fig. 5.Apoptosis in HSCs-DCs and Nar-HSCs-DCs. (A) Apoptosis
in HSCs-DCs and Nar-HSCs-DCs was measured by flow cytometry. (B) Apoptosis
calculated by TUNEL staining. Nar-HSCs-DCs was compared with the HSCs-DCs group.
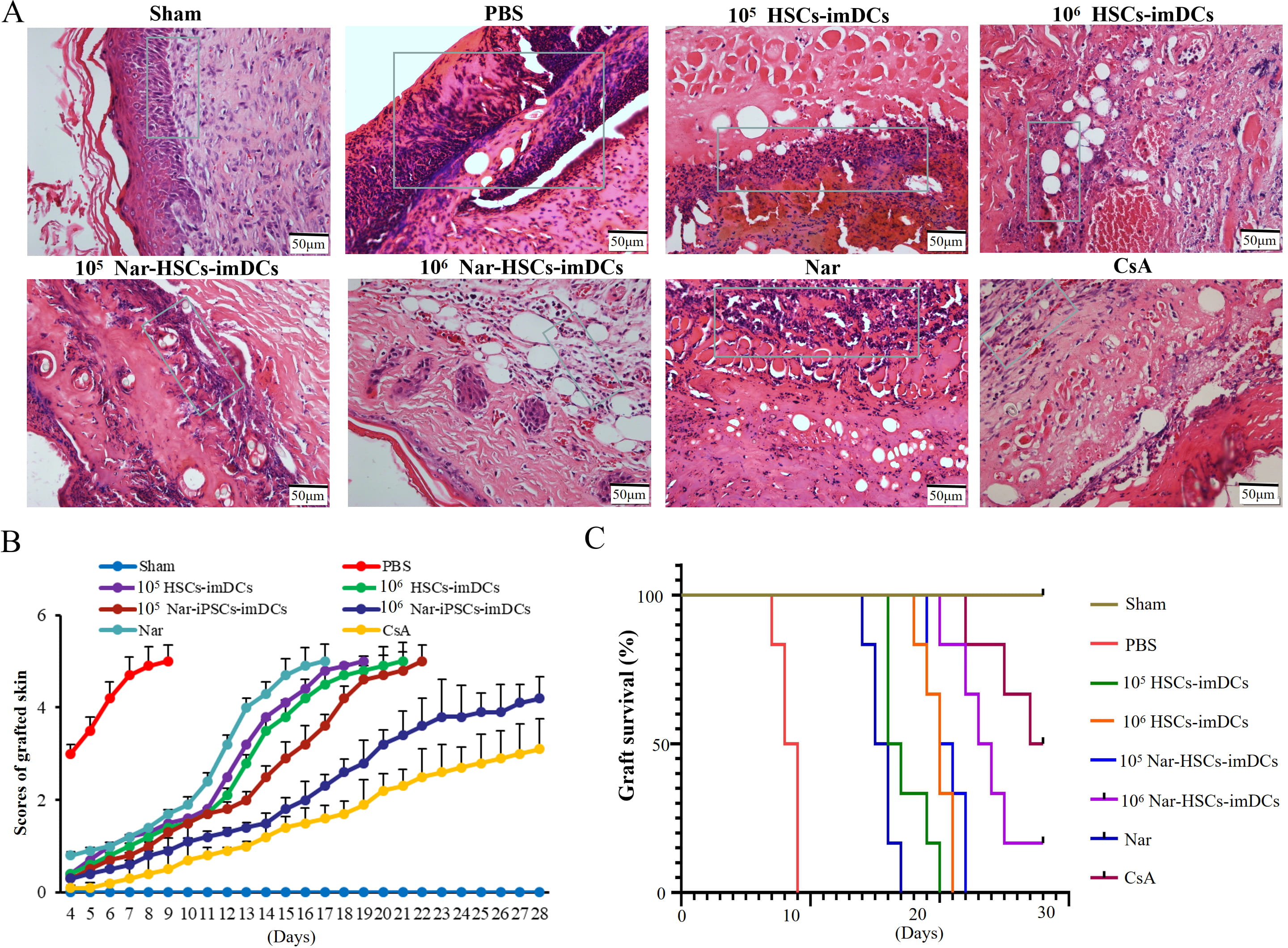
H&E staining showed that lymphocyte infiltration was reduced in the HSCs-imDCs
and Nar-HSCs-imDCs groups compared to the control group, especially in the 10
 Fig. 6.
Fig. 6.Nar-HSCs-imDCs induce immune hypo-responsiveness in the mouse skin transplant model. (A) H&E staining. (B) Graft scores. (C) Graft survival curves. Each experiment contained six biological replicates.
The Nar-HSCs-imDCs group showed a higher percentage of CD4
 Fig. 7.
Fig. 7.Nar-HSCs-imDCs inhibited T cells and promoted Treg cells
proliferation in allografts. (A) The ratios of CD4
Induction of transplantation tolerance could avoid the side effects of long-term
use of immunosuppressants and reduce the risk of immune rejection [17]. With
recent advances in cellular immunotherapy, various cellular solutions for the
induction of immune tolerance have emerged in the transplantation field. imDCs
are increasingly recognized as possible mediators of T cell tolerance [18].
Previous reports showed that CD4 T cell anergy could be induced by the injection
of in vitro-generated imDCs [19]. These imDCs also induced T cells to
differentiate into T helper 2 cells, inhibited the secretion of inflammatory
factors (e.g., IL-2, IFN-
There are currently several ways to impede the maturation of imDCs. These include blocking the expression of imDC surface-specific molecules through gene modification [25, 26, 27], inhibiting the expression of imDCs co-stimulatory molecules with drugs [28], inhibiting imDCs maturation with immunomodulatory factors [29, 30], and preventing the development of imDCs with immunosuppressants [31].
However, these methods have several drawbacks. First, gene modification has potential immunogenicity and biosafety problems, and the transfection efficiency is low. Second, the vast inter-individual differences make it difficult to control the concentration of imDCs in the body using drug intervention. Third, imDCs are prone to mature when stimulated by cytokines, pathogenic microorganisms, grafts, etc., in the body. Therefore, maintaining imDCs in an immature state poses a significant challenge.
In recent years, natural plant-derived ingredients have been
widely used in the medical field due to their low toxicity and wide-ranging
biological activities [32, 33]. Nar is a major flavanone extracted from
grapefruit. It has various pharmacological activities, including antioxidant,
antitumor, anti-atherosclerotic, antibacterial, and neuroprotective effects, as
well as high bioavailability and safety [34]. Niu et al. [35] reported
that Nar can ameliorate experimental autoimmune encephalomyelitis by suppressing
the initiation and proliferation of T lymphocytes and inhibiting production of
the cytokines IL-6, IFN-
Donor immune cell infusion therapy can increase negative immune regulation in recipients, which is the most effective approach for attenuating graft rejection [37, 38]. In the present study we infused Nar-HSCs-imDCs with immunosuppressive properties from donor C57BL/6 mice into recipient Balb/c mice. This was done through the tail vein 7 d before skin grafting. We found that Nar-HSCs-imDCs could maintain immature characteristics for longer, increase Treg cells, and prolong the graft survival time in a donor-specific manner. This is consistent with previous research showing that DCs coordinate the growth and homeostasis of organ-specific Treg cells [39]. An explanation for these findings may be that donor-type Nar-HSCs-imDCs act as a primary vaccine, while the alloantigen acts as a secondary vaccine, thereby promoting the proliferation and sustained activation of donor-specific Treg cells.
We also found that the currently used CsA dose showed the strongest immune-suppressive effect. However, CsA can cause side effects such as increased opportunistic infections and tumorigenicity. In future studies we will therefore use Nar in combination with half or smaller doses of CsA in an attempt to achieve the same induction of immune tolerance as full dose CsA.
This study had several limitations. First, only two groups (high dose
[10
We have shown for the first time that Nar-treated HSCs-imDCs may be an effective therapeutic strategy for inducing immune tolerance in organ transplantation. This study presents a novel approach for the clinical implementation of stem cell biotechnology in combination with traditional Chinese medicine.
All data generated or analyzed during this study are included in this published article.
PT, XH, and CX, designed the research study. KZ, ZJ, YL, XZ, and BZ performed the research. PT, XZ, YF, QF, DL, and JW performed the transplant operations. PT, XZ, and XH analyzed the data. XH and CX wrote the manuscript. PT, XH, and KZ revised the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
All procedures were carried out in accordance with the NIH Guidelines and ethics of animal use were reviewed and approved by the Biomedical Ethics Committee of Xi’an Jiaotong University (No. 2022-198). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Not applicable.
This study was supported by Technology Talent Support Program of Shaanxi Provincial People’s Hospital (2021BJ-07), National Natural Science Foundation of China (81900686), the Key Projects of Shaanxi Provincial Department of Education (21JS038), Science and Technology Incubation Fund Project of Shaanxi Provincial People’s Hospital (2020YXM-08), Shaanxi Province Key R&D Project (2021ZDLSF01-07).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.