1 Dobney Hypertension Centre, School of Biomedical Sciences – Royal Perth Hospital Unit, University of Western Australia, Crawley, WA 6009, Australia
2 Department of Molecular Ophthalmology, University of Western Australia, Crawley, WA 6009, Australia
3 Dobney Hypertension Centre, Medical School – Royal Perth Hospital Unit, University of Western Australia, Crawley, WA 6009, Australia
4 Department of Cardiology and Department of Nephrology, Royal Perth Hospital, Perth, WA 6000, Australia
†These authors contributed equally.
Abstract
Background: Diabetic retinopathy (DR) is a leading cause of end-stage blindness globally and is arguably one of the most disabling complications of both Type 1 and Type 2 diabetes. Sodium Glucose Cotransporter-2 (SGLT2) inhibitors have now been successfully introduced to clinical medicine and exert multiple beneficial effects in diabetic patients. Given the broad therapeutic application of SGLT2 inhibitors, we hypothesised that SGLT2 inhibition may alleviate the progression of DR. Therefore, we aimed to compare the effectiveness of two clinically available SGLT2 inhibitors, Empagliflozin and Canagliflozin, on the progression of Retinopathy and DR using well-characterised mouse models, Kimba and Akimba, respectively. Methods: Empagliflozin, Canagliflozin (25 mg/kg/day) or vehicle was administered to 10-week-old mice via drinking water for 8-weeks. Urine glucose levels were measured to ascertain SGLT2 inhibition promoted glucose excretion. Weekly body weight and water intake measurements were obtained. After 8-weeks of treatment, body weight, daily water intake, fasting blood glucose levels were measured and eye tissue was harvested. The retinal vasculature was assessed using immunofluorescence. Results: Empagliflozin treated Akimba mice exhibited metabolic benefits suggested by healthy body weight gain and significantly reduced fasting blood glucose levels. Treatment with Empagliflozin reduced retinal vascular lesions in both Kimba and Akimba mice. Canagliflozin improved body weight gain, reduced blood glucose levels in Akimba mice, and reduced the development of retinal vascular lesions in Kimba mice. Conclusions: Our data demonstrates that Empagliflozin has future potential as a therapeutic for Retinopathy and DR and should now be considered for human trials.
Keywords
- diabetic retinopathy
- diabetes
- Akimba
- Kimba
- mouse models
- sodium glucose cotransporters
- SGLT2 inhibitors
- empagliflozin
- canagliflozin
- retinal vasculature
Diabetic retinopathy (DR) is recognised as one of the most prevalent chronic microvascular complications of both Type 1 [1, 2] and Type 2 diabetes [3, 4] and is one of the leading causes of vision impairment globally [5, 6]. The classification of DR is primarily based on the changes that occur in the pre-existing retinal microvasculature and presence or absence of retinal neovascularisation [7, 8]. Diabetic retinopathy is divided into two principal stages. The early Non-Proliferative Diabetic Retinopathy (NPDR) stage is characterized by the presence of microaneurysms and the more advanced stage Proliferative Diabetic Retinopathy (PDR) is characterised by neovascularisation which may eventually lead to vision loss [7, 8].
It is shown that intensive therapy leading to near-normal blood glucose levels reduces the likelihood of developing retinopathy [9]. Beyond glycemic control, most treatment options such as anti-vascular endothelial growth factor (VEGF) injections, panretinal laser photocoagulation and vitrectomy largely target the late PDR stage where vision loss occurs [10]. Therefore, therapies targeting the potentially reversible early pathogenic stage of DR is urgently needed to prevent the onset or slow the progression of DR and eventual vision loss.
The kidneys play an important role in maintaining glucose homeostasis. Sodium glucose co-transporter 2 (SGLT2) is a high-capacity, low affinity glucose co-transport protein which helps to reabsorb about 90–95% of glucose in the S1 and S2 segments of the renal proximal tubular epithelial cells [11]. Interestingly, a new class of anti-diabetic drugs act by inhibition of SGLT2, thereby decreasing the reabsorption of glucose from the renal proximal tubules. Hence, specific SGLT2 inhibition facilitates the excretion of glucose via urine (glucosuria), resulting in reduced plasma glucose levels and improved glycaemic parameters [11]. The SGLT2 inhibitors have been shown to reduce HbA1c, fasting plasma glucose levels, blood pressure, and body weight [12, 13]. Additionally, it has been shown that SGLT2 inhibitors have protective effects in cardiovascular and kidney disease, which are two of the main complications of diabetes [14]. Nevertheless, systematic studies in both pre-clinical and clinical settings, to determine the effect of SGLT2 inhibition on retinal vascular disorders such as DR is limited [15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25]. With respect to the eye, retinal pericytes are identified as a novel source of SGLT2 expression [17, 26]. Furthermore, we have shown elevated levels of Sglt2 mRNA in the whole eye and SGLT2 protein expression in retinal ganglion cells [25]. Leley et al. [27] has also shown a statistically significant increase in Sglt2 mRNA levels in the retina of diabetic mice. In an attempt to elucidate the effect of SGLT2 inhibition on the development of DR, we have shown for the first time that the treatment with Empagliflozin (EMPA) profoundly reduced retinal vascular abnormalities such as capillary dropout, vessel tortuosity, microaneurysms, intraretinal microvascular abnormalities (IRMA) and neovascular tufts in young diabetic Akimba mice (4–5 weeks postnatal) [25]. However, thus far, the effect of SGLT2 inhibition on the progression of DR has not been examined. In addition, to the best of our knowledge there is no data comparing individual SGLT2 inhibitors in the context of DR, and therefore, it remains unclear whether a class effect of SGLT2 inhibitors on DR pathogenesis is present. It is reported that the possible difference in pharmacological effects and outcomes between different SGLT2 inhibitors is particularly due to the degree of SGLT2 selectivity [28, 29, 30]. Given the extensive use of SGLT2 inhibitors in clinical practice, particularly for the treatment of cardiovascular and kidney disease, it is important to compare the effect of different SGLT2 inhibitors on features of DR.
Here we report the first in vivo study comparing the two independent SGLT2 inhibitors, EMPA and Canagliflozin (CANA), on the progression of retinopathy and DR in the following well-established mouse models: (1) Kimba and (2) Akimba. Importantly, these models capture an array of hallmark features seen in human DR and therefore our findings are likely to have clinical relevance [31, 32, 33, 34, 35, 36].
Specific pathogen free 10-wk-old male Kimba and Akimba mice [32, 35, 36, 37] were bred and obtained from the Animal Resources Centre (ARC, Perth, Australia). These models are highly relevant for the study of retinal vascular disease [15, 18, 31, 32, 33]. In our study, we investigated 10-week-old animals with the aim of evaluating the effect of EMPA and CANA on the progression of retinopathy (Kimba model) and DR (Akimba model). Only male mice were used for these studies as disease progression in females is slower and inconsistent [32, 37]. Genotyping of Kimba and Akimba mice were performed as published previously [33, 37, 38].
The animal handling and experimental procedures were conducted at the animal holding facility of the Harry Perkins Institute for Medical Research in Perth, Western Australia, following the guidelines of the Institutional Animal Care and Use Committee. The study was approved by the Harry Perkins Institute for Medical Research Animal Ethics Committee (AE141/2019 approved: 12/02/19). All procedures were carried out in compliance with the Association for Research in Vision and Ophthalmology (ARVO) statement for the use of animals in ophthalmic and vision research.
All animals were acclimatised for one week. Urine glucose levels were obtained
(Keta-Diabur-Test 5000, Roche Diagnostics, Leverkusen, Germany) prior to
treatment to confirm the diabetic status of Akimba mice and the non-diabetic status of
Kimba mice. The 10-week-old mice were housed individually under a 12-hour
light/dark cycle and maintained at a temperature of 21
Body weights, fluid intake and urine glucose levels (Keta-Diabur-Test 5000, Roche Diagnostics, Leverkusen, Germany) were recorded weekly. At the end of experiment, mice were fasted for 5 hours with free access to treated drinking water. Fasting blood glucose levels were measured using Accu-Chek Performa blood glucose monitoring system (Roche Diagnostics, North Ryde, Australia) with a range of 0.6 to 33.3 mmol/L. Readings over 33.3 mmol/L were treated as 33.3 mmol/L during data analysis.
At the experimental endpoint, all animals were deeply anesthetised with isoflurane inhalation and were euthanised by cervical dislocation. For retinal vascular assessment, eyes were enucleated, cornea was penetrated with a 27G insulin needle and was fixed in ice-cold 10% buffered paraformaldehyde (PFA; Sigma-Aldrich, Sydney, Australia) for 2 hours at 4 °C.
Retinal whole-mounts were prepared and analysed as previously published by our
team [15, 25]. In brief, fixed eyes were washed in ice cold 1
Whole-mounted retinas were imaged with an inverted fluorescent microscope (Nikon
Eclipse Ti, Nikon, Tokyo, Japan) equipped with a digital camera (CoolSNAP HQ2,
Photometrics, Tucson, AZ, USA) linked to a computer running the image analysis
software ‘NIS-Elements Advanced Research’ (Nikon, Tokyo, Japan). Serially
overlapping high-resolution images of the whole retina were captured using the
4
All morphometric data were analyzed using two-tailed Student’s t-test
or one-way analysis of variance (ANOVA) and graphs were produced using GraphPad
Prism 9 (GraphPad Software Inc., San Diego, CA, USA). Quantitative data is
presented as mean
Testing of urine glucose levels during the acclimatisation period confirmed the
genotype of each strain. The non-diabetic Kimba mice did not show the presence of
glucose in their urine while the diabetic Akimba mice showed the presence of
glucose indicated by the brown colour (
The Kimba model lacks the hyperglycemic background. Therefore, Kimba mice act as
an internal control for the determination of the effectiveness of the SGLT2
inhibitors EMPA and CANA used in our study. As anticipated, the consumption of
EMPA (Fig. 1A) and CANA (Fig. 1B) in drinking water at a
concentration of 25 mg/kg/day, showed marked glucose excretion in the urine of
the non-diabetic Kimba mice as indicated by the brown colour (
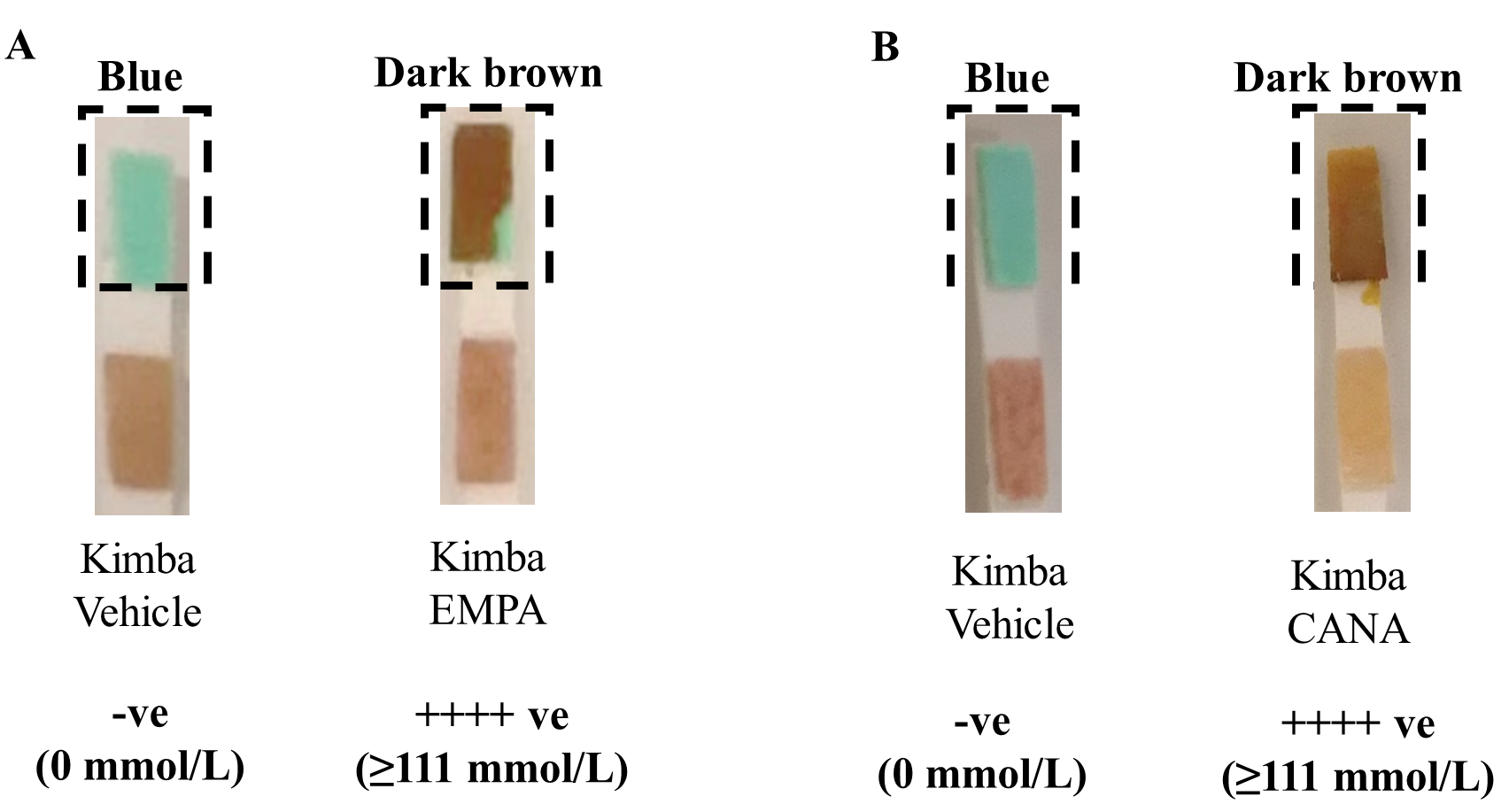 Fig. 1.
Fig. 1.SGLT2 inhibition promotes glucosuria in Non-Diabetic Kimba
mice. Representative image of urine glucose levels in (A) EMPA (25 mg/kg) and
(B) CANA (25 mg/kg) treated mice. Blue = 0 mmol/L glucose; Dark brown
At the end of treatment (after 8 weeks), relative to vehicle treated mice, EMPA
or CANA did not significantly alter fasting blood glucose levels in Kimba mice
(Fig. 2A,C, respectively). However, fasting blood glucose levels in diabetic
Akimba mice were significantly reduced after 8 weeks of EMPA (Fig. 2B) or CANA
(Fig. 2D) administration. Interestingly, when compared to EMPA (Vehicle 32.67
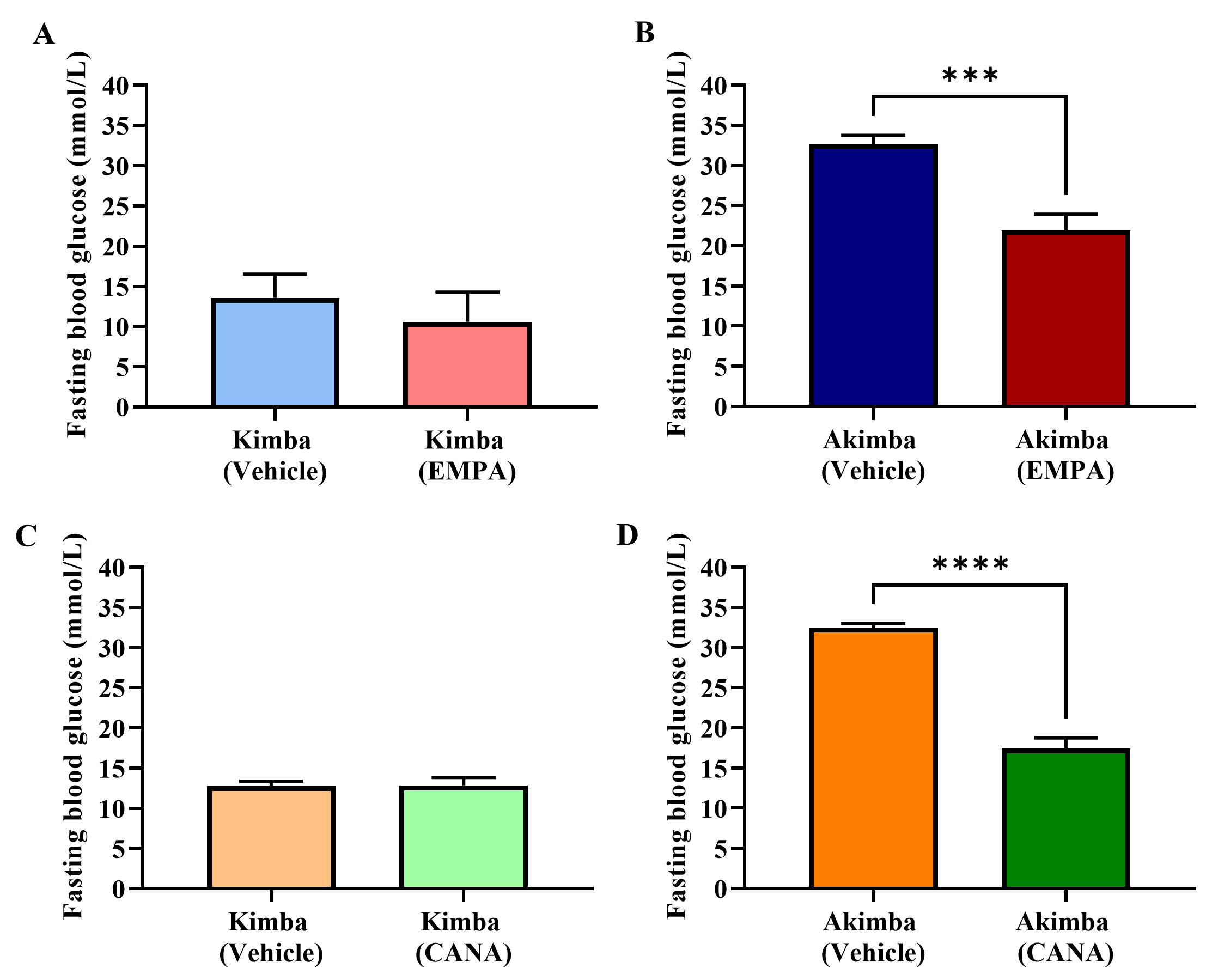 Fig. 2.
Fig. 2.Role of SGLT2 inhibition on fasting blood glucose in Kimba and
Akimba mice treated with EMPA or CANA. Graphs show fasting blood glucose levels
after 8 weeks of treatment with EMPA in (A) Kimba and (B) Akimba mice and
treatment with CANA in (C) Kimba and (D) Akimba mice. ***p
Firstly, we compared the starting (day 0) and final body weight (day 49; 7 weeks post-treatment) of Kimba and Akimba mice treated with vehicle. After 7 weeks non-diabetic Kimba mice showed a significant increase in body weight compared to the starting body weight (Fig. 3). However, diabetic Akimba mice showed lack of weight gain (Fig. 3), a hallmark of the failure to thrive phenotype in these animals.
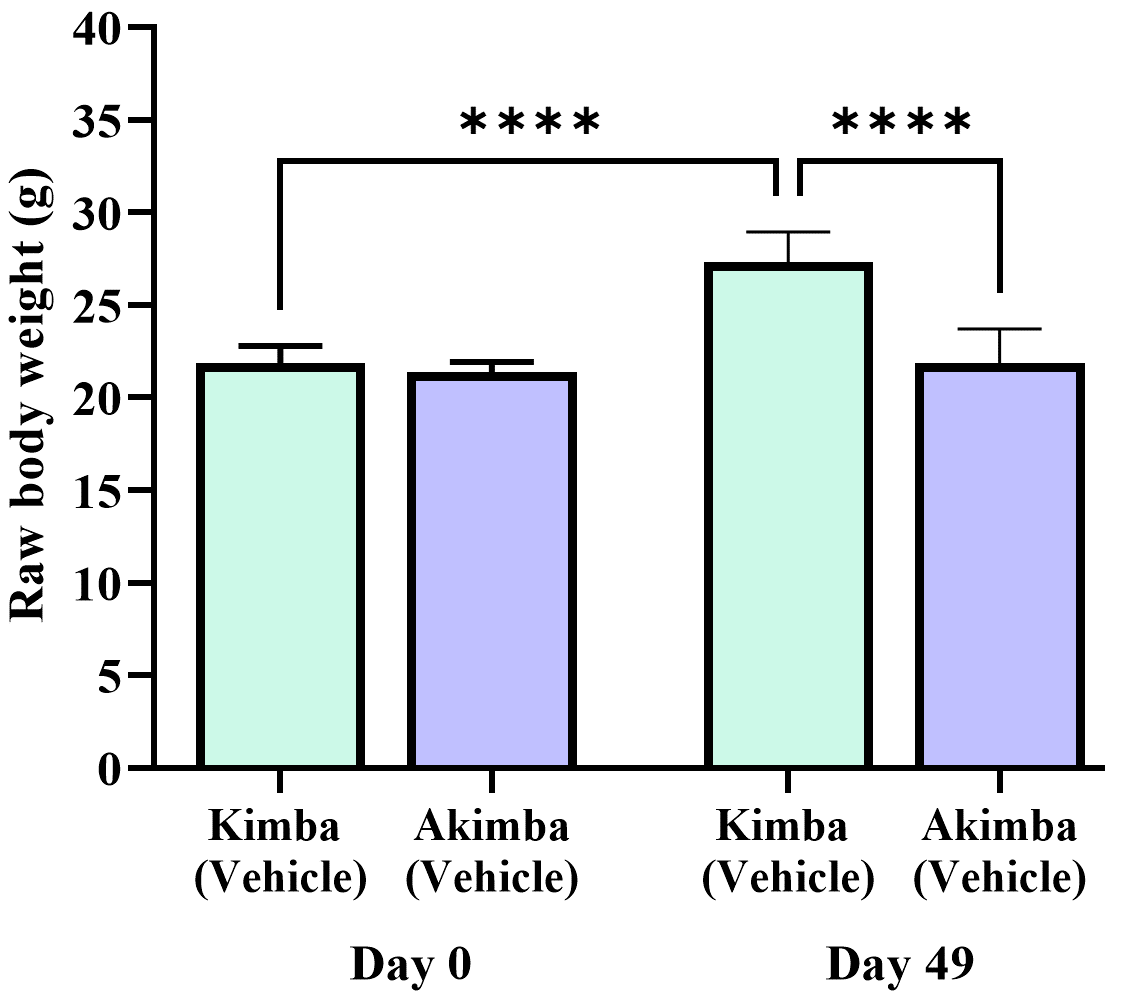 Fig. 3.
Fig. 3.Failure to thrive phenotype in diabetic Akimba mice. Graph
depicts the raw body weight of non-diabetic Kimba and diabetic Akimba mice
without treatments at the start of the experiment (day 0) and 7 weeks post (49
days). ****p
As shown in Fig. 4, EMPA treatment showed a reduction in percentage body weight gain in non-diabetic Kimba (Fig. 4A) mice throughout the treatment period from week 1 to 7. Interestingly, the percentage weight reduction was significant from week 2–7. In contrast, throughout the experiment period, the weight gain was increased in diabetic Akimba mice after treatment with EMPA (Fig. 4B). The CANA treatment also resulted in a reduction in percentage body weight gain in Kimba mice compared to vehicle treated mice (Fig. 4C). The percentage weight reduction was significant only at week 7. Similar to EMPA, treatment with CANA progressively increased body weight in diabetic Akimba mice (Fig. 4D).
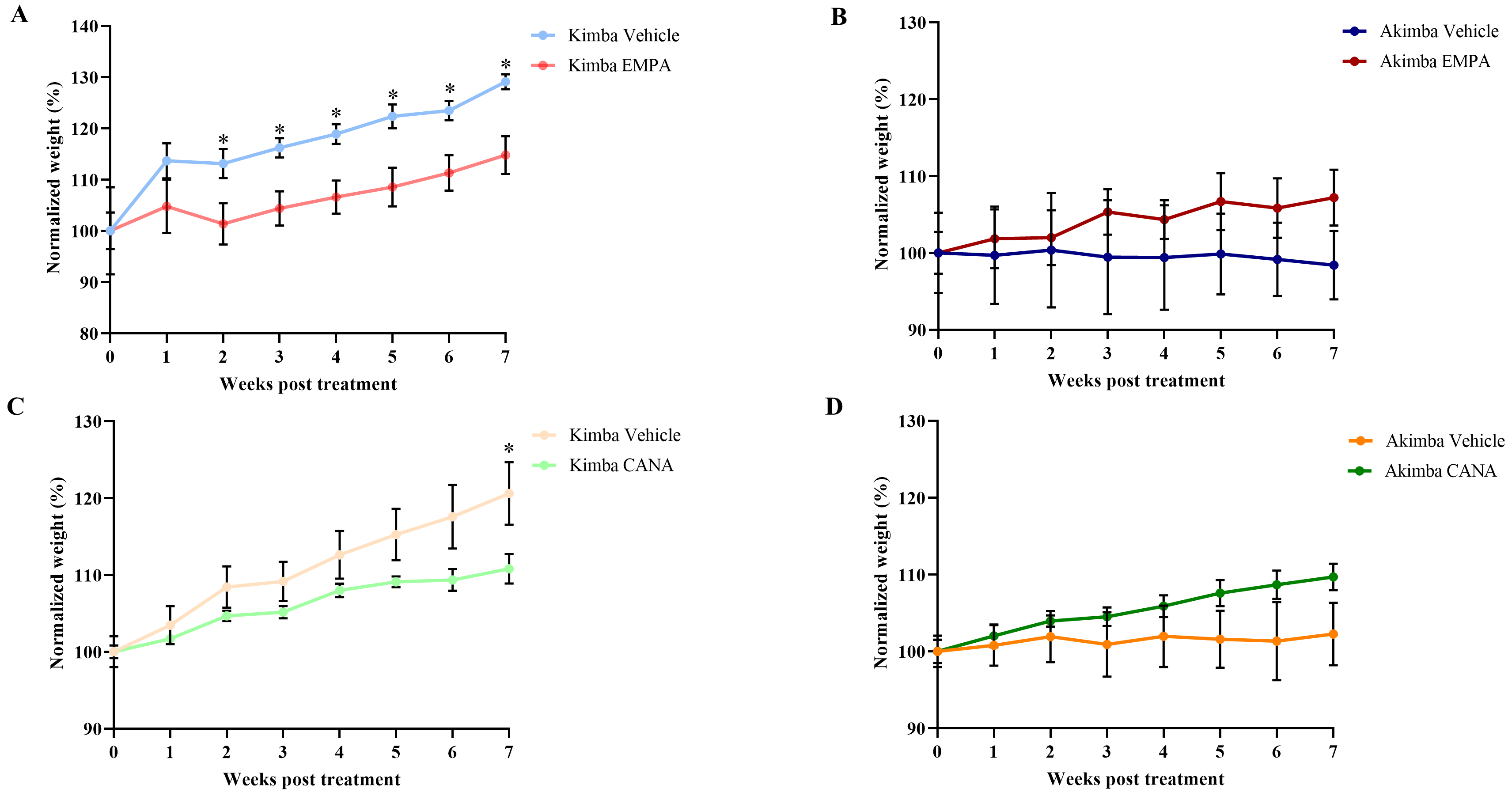 Fig. 4.
Fig. 4.Effect of SGLT2 inhibition on body weight in
Kimba and Akimba mice treated with EMPA or CANA. Graphs show the normalized body
weight percentage during 7 weeks of treatment with EMPA in (A) Kimba and (B)
Akimba mice and treatment with CANA in (C) Kimba and (D) Akimba mice. *p
At the age of 10 weeks, non-diabetic Kimba mice had a water intake of less than 10 mL per day, which was considerably lower than that of the diabetic Akimba mice. In addition, diabetic Akimba mice showed a significant increase in water intake from day 3 to day 31 of the experiment (Fig. 5) suggestive of excessive thirst or polydipsia associated with the progression of diabetes.
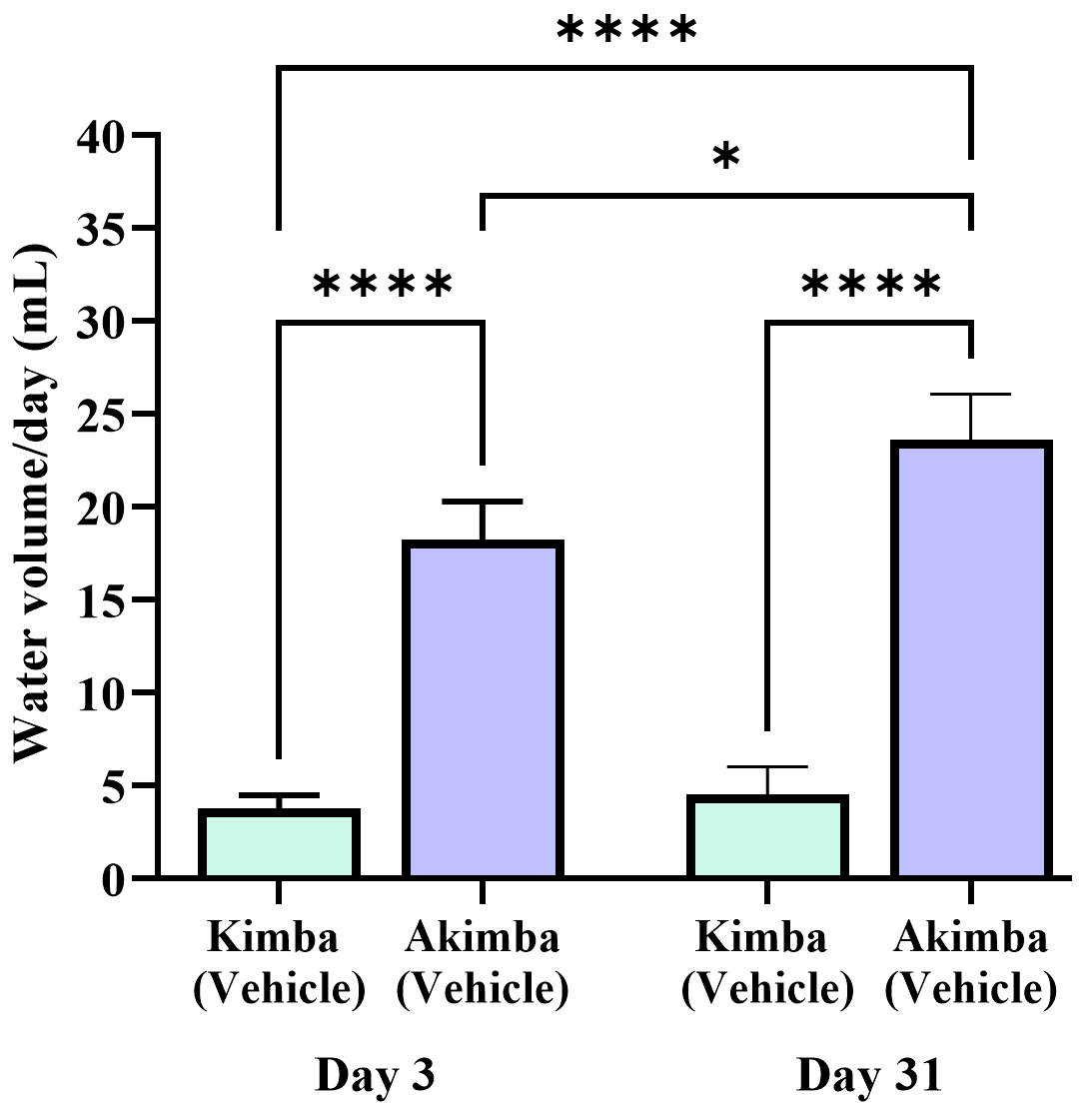 Fig. 5.
Fig. 5.Increased water intake in diabetic Akimba mice. Graph shows
water intake in non-diabetic Kimba and diabetic Akimba mice after 3 days of
treatment (day 3) and 4 weeks (31 days) post treatment. *p
In Kimba mice treated with EMPA or CANA (Fig. 6A,C), water intake from day 0 to day 31 post-treatment was slightly increased when compared to vehicle treated mice. There was no significant effect of EMPA on water intake in Akimba mice (Fig. 6B). Interestingly, CANA promoted reductions in water intake in treated mice at days 24- and 31-days post-treatment (Fig. 6D).
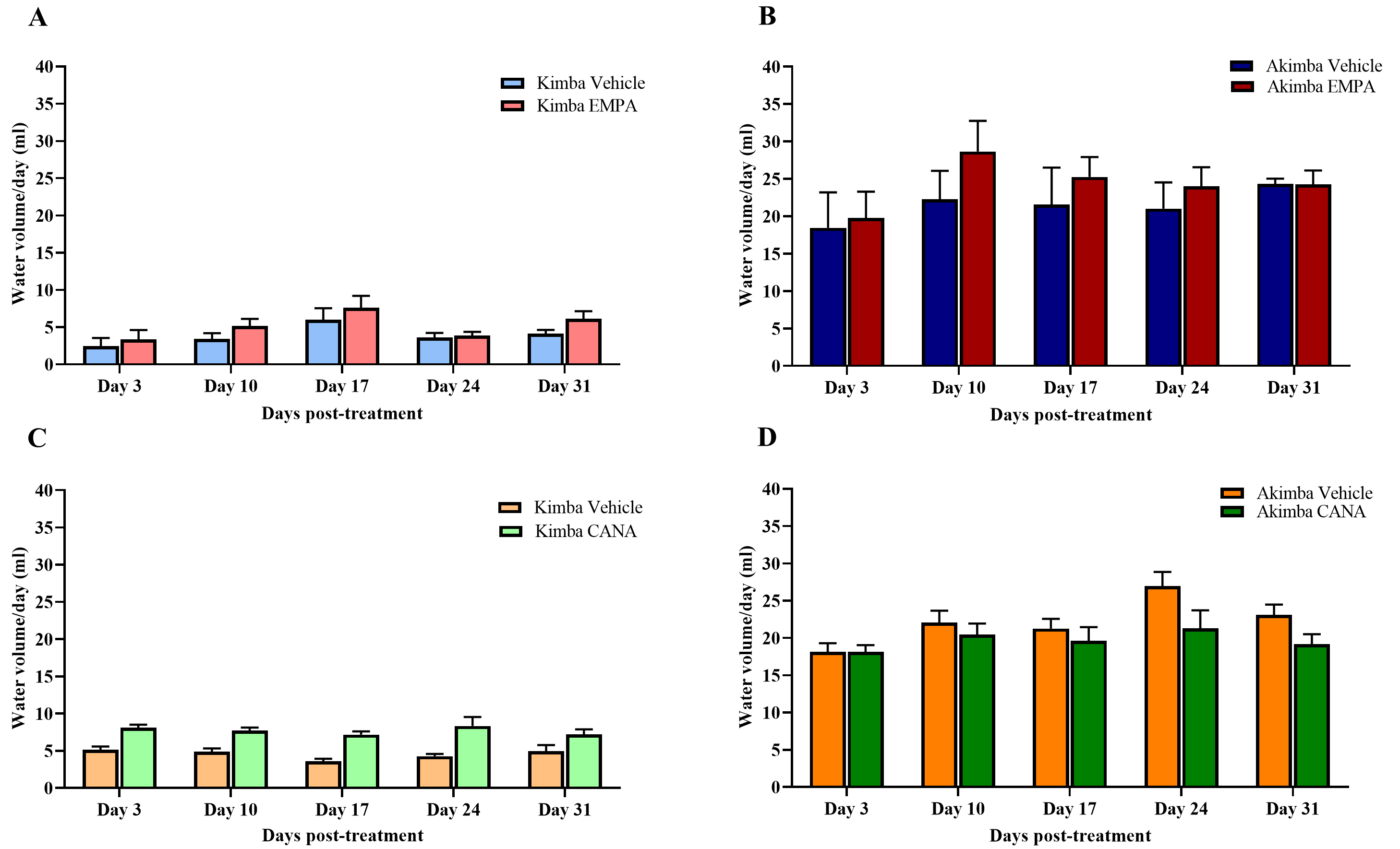 Fig. 6.
Fig. 6.Effect of SGLT2 inhibition on water volume consumed by Kimba and
Akimba mice treated with EMPA or CANA. Graphs depict the volume of water
consumed at day 3, 10, 17, 24 and 31 by (A) Kimba and (B) Akimba mice treated
with EMPA and (C) Kimba and (D) Akimba mice treated with CANA. Data represented
as mean
The objective of our study was to investigate whether inhibition of SGLT2 can delay the advancement of retinopathy and DR. To achieve this, the severity of retinal vascular lesions was evaluated after 8 weeks of treatment in Kimba and Akimba mice administered with either vehicle, EMPA or CANA. The number of vascular lesions in Kimba mice treated with EMPA was noticeably reduced when compared to vehicle treated mice (Fig. 7A–C). Interestingly, when compared to vehicle treatment, CANA significantly reduced retinal vascular lesions in Kimba mice (Fig. 7D–F). Similar to Kimba mice treated with EMPA, Akimba mice also showed a noteworthy reduction in retinal vascular lesions (Fig. 8A–C). However, there was no appreciable difference between the number of lesions in Akimba mice receiving vehicle or CANA (Fig. 8D–F).
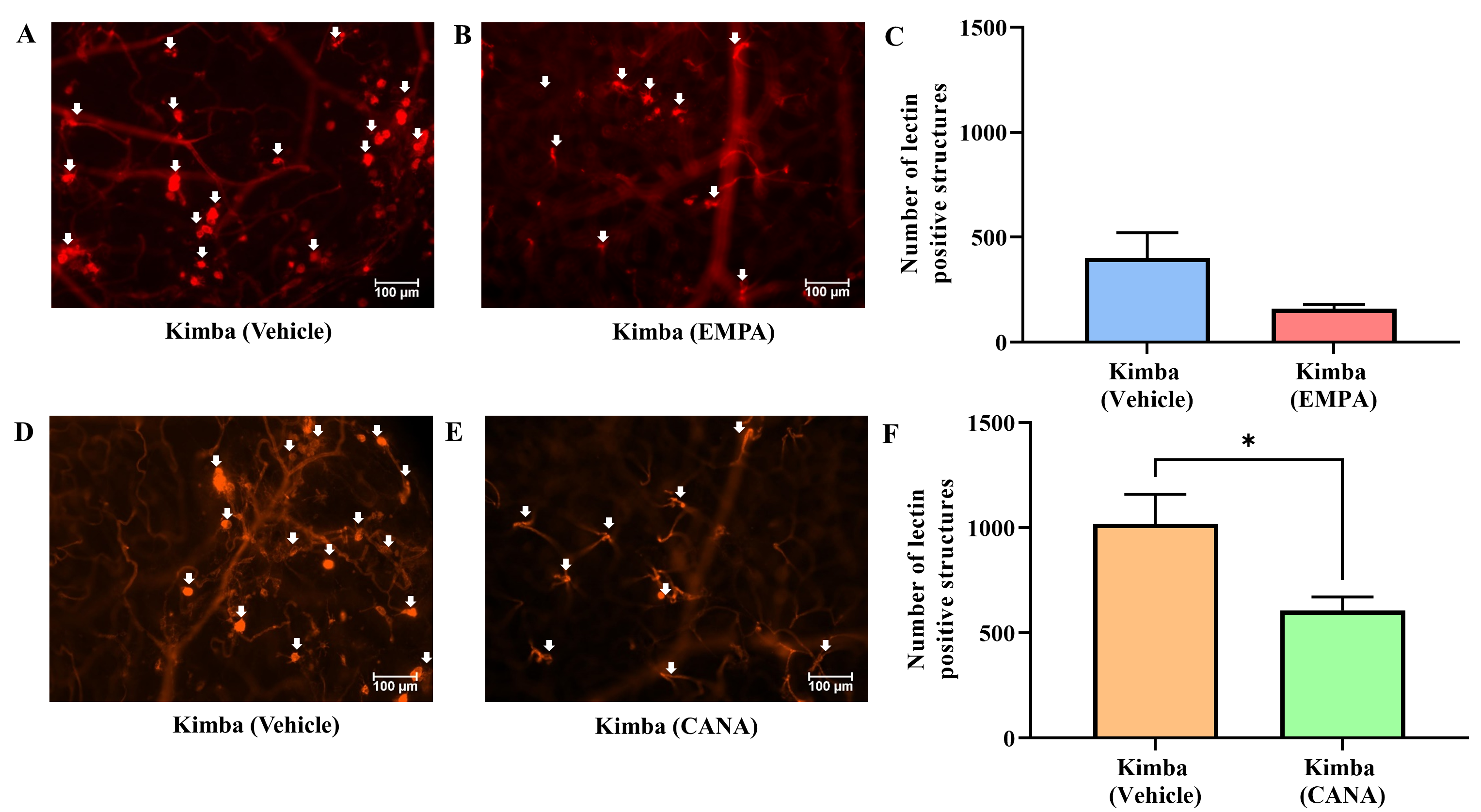 Fig. 7.
Fig. 7.The effect of SGLT2 inhibition on retinal microvascular lesions
in non-diabetic Kimba mice. Representative areas of isolectin-B4 and Cy-3
stained vasculature from Kimba mice treated with (A and D) vehicle or (B) EMPA or
(E) CANA and (C and F) quantification of retinal vascular lesions. *p
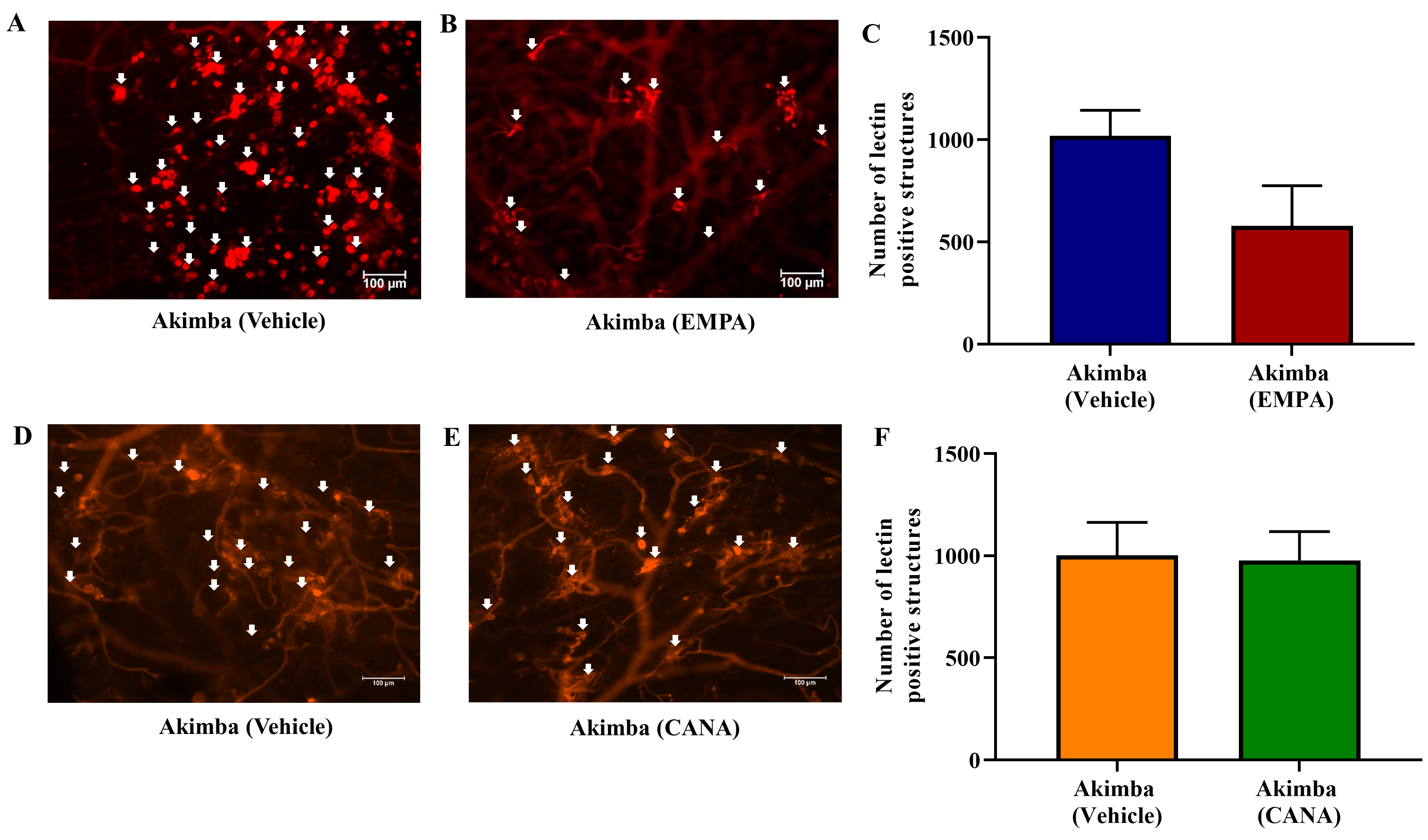 Fig. 8.
Fig. 8.The effect of SGLT2 inhibition on retinal microvascular lesions
in diabetic Akimba mice. Representative areas of isolectin-B4 and Cy-3 stained
vasculature from Akimba mice treated with (A and D) vehicle or (B) EMPA or (E)
CANA and (C and F) quantification of retinal vascular lesions. Data represented
as mean
An in-depth analysis of the retinal vasculature was carried out with the semi-quantitative grading of the retinal vascular features in EMPA or CANA treated Kimba and Akimba mice. These results are summarised in Tables 1,2 (Kimba and Akimba, respectively). When compared to vehicle treated mice, EMPA treated Kimba mice showed a marked reduction in capillary non-profusion, loss of vessel integrity and vascular leakage (Table 1 Panel A; symbol $) as well as vessel tortuosity (Table 1; Panel A; symbols &, $ and ^, respectively). The vascular leakage in EMPA treated Kimba mice was often confined to the areas with large IRMA. In CANA treated Kimba mice, capillary non-profusion, and vessel tortuosity (Table 1 Panel B; symbols & and ^, respectively) was not improved with SGLT2 inhibitor treatment. However, vascular leakage was markedly reduced in CANA treated Kimba mice when compared to vehicles (Table 1 Panel B; symbol $). In vehicle treated Kimba mice, although the number of microaneurysms/IRMA/vessel tufts appeared reduced these structures were often large and convoluted (Table 1 Panel A; symbol #) spanning into the mid and deep capillary beds of the retina. In EMPA treated mice, this phenomenon was noted only in 25% of retinas, whereas 75% of the retinas appeared to have minimal microaneurysms/IRMA/vessel tufts. The number of infiltrating macrophages and monocytes were also decreased with EMPA (Table 1 Panel A; symbol @) and CANA (Table 1 Panel B; symbol @) treatment in Kimba mice.
| Characteristic | Panel A | Panel B | |||||||||||||||||||
| Kimba + Vehicle | Kimba + EMPA | Kimba + Vehicle | Kimba + CANA | ||||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | 6 | |
| Capillary non-profusion |
++++ | + | ++++ | ++++ | +++ | ++++ | + | + | ++ | ++ | ++ | +++ | ++++ | ++ | ++++ | +++ | +++ | +++ | ++ | ++ | ++ |
| Loss of vessel integrity and leakage |
+++ | - | + |
+ |
+ |
+ |
- | - | - | - | - | - | + |
- | +++ | - | - | - | - | - | - |
| Vessel tortuosity ^ | +++ | ++ | ++ | ++ | ++ | ++ | -/+ | -/+ | -/+ | + | ++ | ++ | +++ | ++ | +++ | ++ | +++ | ++ | + | ++ | ++ |
| Microaneurysms/IRMA/vessel tufts |
+ |
+ | + |
+ |
++ |
+ |
-/+ |
+ | + | + | ++ | + | + |
++ |
+ |
++ | ++ |
+ | + | + | + |
| Monocytes/macrophages |
++++ | - | ++++ | ++++ | ++ | ++++ | - | - | - | - | ++ | ++ | +++ | ++ | +++ | + | ++ | + | - | + | + |
| &: -, Absent; +, in 1 quadrant; ++, in 2 quadrants, +++, in 3 quadrants, ++++,
in 4 quadrants. $: -, Absent; +, mild; ++, moderate; +++, severe. ^: -, Absent; + mild; ++ moderate; +++, severe. #: -, Absent; +, less than 300 microaneurysms/IRMA/vessel tufts; ++, more than 300 microaneurysms/IRMA/ vessel tufts. @: -, Absent; +, in 1 quadrant; ++, in 2 quadrants, +++, in 3 quadrants, ++++, in 4 quadrants. *: Expansive retinopathy/capillary dropout resulted in the retina showing a lower incident of vascular lesions such as microaneurysms/IRMA/vessel tufts. **: Large convoluted IRMA. ***: Showed less than 50 microaneurysms/IRMA/vessel tufts and the overall retinal vasculature was clearly organised. a: Leakage confined to areas of IRMA. aa: Minimal vascular capillaries remaining, these capillaries could be occluded leading to less vascular leakage. EMPA, Empagliflozin; CANA, Canagliflozin; IRMA; Intraretinal microvascular abnormalities. | |||||||||||||||||||||
| Characteristic | Panel A | Panel B | |||||||||||||||||
| Akimba + Vehicle | Akimba + EMPA | Akimba + Vehicle | Akimba + CANA | ||||||||||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | 6 | |
| Capillary non-profusion |
++++ | +++ | ++ | ++++ | + | ++++ | + | + | ++ | ++ | ++ | +++ | + | ++ | ++++ | ++++ | ++++ | ++ | ++++ |
| Loss of vessel integrity and leakage |
++ | - | - | +++ | - | +++ | - | - | + | - | - | - | - | - | - |
+++ | + |
- | - |
| Vessel tortuosity ^ | +++ | +++ | ++ | +++ | - / + | +++ | -/+ | -/+ | +++ | ++ | +++ | ++ | + | ++ | +++ | +++ | +++ | ++ | +++ |
| Microaneurysms/IRMA/vessel tufts |
+ |
++ | ++ | + |
-/+ |
+ |
-/+ |
-/+ |
++ | + | ++ | ++ |
+ | + | + |
+ |
+ |
+ | + |
| Monocytes/macrophages |
++++ | - | - | ++++ | - | ++ | - | - | +++ | - | - | ++ | - | - | ++ | ++ | ++ | - | ++ |
| &: -, Absent; +, in 1 quadrant; ++, in 2 quadrants, +++, in 3 quadrants, ++++,
in 4 quadrants. $: -, Absent; +, mild; ++, moderate; +++, severe. ^: -, Absent; + mild; ++ moderate; +++, severe. #: -, Absent; +, less than 300 microaneurysms/IRMA/vessel tufts; ++, more than 300 microaneurysms/IRMA/ vessel tufts. @: -, Absent; +, in 1 quadrant; ++, in 2 quadrants, +++, in 3 quadrants, ++++, in 4 quadrants. *: Expansive retinopathy/capillary dropout resulted in the retina showing a lower incident of vascular lesions such as microaneurysms/IRMA/vessel tufts. **: Large convoluted IRMA. ***: Showed less than 50 microaneurysms/IRMA/vessel tufts and the overall retinal vasculature was clearly organised. a: Leakage confined to areas of IRMA. aa: Minimal vascular capillaries remaining, these capillaries could be occluded leading to less vascular leakage. EMPA, Empagliflozin; CANA, Canagliflozin; IRMA; Intraretinal microvascular abnormalities. | |||||||||||||||||||
Overall, Akimba mice showed more pronounced vascular disorganization, microaneurysms, IRMA and neovascular tufts when compared to Kimba mice as previously published [32, 35, 37, 39]. With the treatment of EMPA, 75% of the DR Akimba mice showed a marked improvement in all retinal vascular parameters when compared to vehicles (Table 2 Panel A). However, 25% EMPA treated Akimba mice showed extensive damage to the retina which was similar to vehicle treated mice (Supplementary Fig. 1). The treatment with CANA did not show an overall improvement in the retinal vascular features when compared to vehicle treated Akimba mice (Table 2 Panel B). While the number of microaneurysms/IRMA/vessel tufts appeared to be reduced, there were expansive regions without retinal capillaries in Akimba mice treated with CANA (Table 2 Panel B; symbol # and &, respectively).
SGLT2 inhibitors are now an established therapy for the treatment of diabetes due to their anti-diabetic properties and their potential benefits for cardiovascular and renal complications [40, 41, 42, 43, 44]. Many pre-clinical studies on both Type 1 or Type 2 diabetic animals have shown that glycemic control with SGLT2 inhibitors decreased the progression of major microvascular complications associated with diabetes [21, 45, 46, 47]. In this novel study, we examined the therapeutic effect of two SGLT2 inhibitors, EMPA and CANA, on retinopathy and DR, utilizing the well-characterised Kimba and Akimba mice, respectively. We chose Kimba and Akimba mice for the current study, as these models are regarded as two of the most suitable models of retinopathy and DR [15, 25, 32, 35, 37]. The key findings of this study are (i) both SGLT2 inhibitors significantly lowered fasting blood glucose levels and improved weight gain in diabetic Akimba mice; (ii) EMPA exerts protective effects for both retinopathy and DR as shown by the reduction in retinal vascular abnormalities and (iii) CANA showed protective effects against retinopathy in Kimba mice only.
Similar to human trials, EMPA and CANA reduced fasting blood glucose levels in
our type 1 diabetic Akimba model, in agreement with other rodent studies [45, 48, 49, 50, 51]. Interestingly, CANA showed a more profound reduction in fasting blood
glucose levels compared to EMPA (Vehicle 32.46
Glucose variability or fluctuation of glucose levels are a contributing factor to diabetes-related macrovascular complications [55]. It is shown that high glucose variability can lead to endothelial dysfunction [56, 57], which in turn may contribute to the development and progression of DR. SGLT2 inhibitors have been shown to reduce glucose variability in diabetic patients [58] thereby reducing endothelial dysfunction. Therefore, it is plausible to hypothesise that SGLT2 inhibitors may in fact improve DR by efficiently managing glucose variability and thereby reducing endothelial dysfunction. Therefore, future studies should investigate these aspects in relation to DR and SGLT2 inhibitors.
Typically, impaired or stalled growth is a characteristic feature of uncontrolled hyperglycaemia [59]. Type 1 diabetic Akimba mice typically show poor weight gain which is a hallmark of the failure to thrive phenotype in these animals. We have previously shown that EMPA prevented the failure to thrive phenotype in these animals when treatment started at 4 weeks of age [25]. Similarly, in our current study, the SGLT2 inhibitors EMPA and CANA both induced improved weight gain in Akimba mice. However, an opposite effect on body weight was observed in the non-diabetic Kimba mice. Similar findings have been reported in a study by Vallon et al. [45] in diabetic Akita mice and its non-diabetic counterparts.
Consistent with previous reports in SGLT2-deficient non-diabetic mice [60, 61], the increased loss of glucose calories into the urine (glucosuria) of SGLT2 inhibitor-treated (both EMPA and CANA) non-diabetic Kimba mice could be responsible for the increased fluid intake. Furthermore, Masuda et al. [62], reported that in non-diabetic rats with free access to food and water, SGLT2 inhibition increased glucosuria, urine volume and Na+ excretion, which was associated with increased fluid intake. Similarly, in non-diabetic Kimba mice, the mechanism of action of EMPA and CANA potentially caused hyperosmolar urine (due to the presence of Na+ and glucose) leading to polyuria (excessive urination), due to osmotic diuresis. This was likely responsible for polydipsia (excessive thirst resulting in increased fluid intake) noted in these animals. It can be suggested that the increase in fluid intake in response to SGLT2 inhibition may be compensating the primary diuretic, glucosuric, and natriuretic effect of EMPA and CANA to maintain fluid and electrolyte balance in Kimba mice [62].
A very limited number of studies have investigated the effect of SGLT2 inhibition on the retinal vasculature. A substantial decrease in the number of acellular capillaries were observed with the treatment of Dapagliflozin db/db mouse retinas [63]. In early stages of DR, microaneurysm, exudation, and retinal hypoperfusion was reduced in db/db mice with the treatment of EMPA [19]. Eid et al. [21] showed that EMPA reduced retinal apoptosis and thereby showed a trend toward a salutary effect on DR in type I diabetic animals. Similarly, we have recently demonstrated that the features of DR in young Akimba mice (4 weeks of age) were decreased with the treatment of EMPA [25]. When compared to younger Akimba mice, older mice used in this study showed a severe disease phenotype [25, 32, 39]. In this study, we have shown that in older Akimba mice, EMPA attenuated diabetes-associated increases in retinal microvascular abnormalities such as microaneurysms, IRMA, capillary dropouts and vessel tortuosity. Taken together, our investigations in the specific Type 1 Diabetes Akimba mouse model suggests that the SGLT2 inhibitor EMPA could be a promising therapeutic for the treatment of DR development and progression.
Loss of neural cell populations and thinning and changes in retinal layers are well-documented in both Kimba and Akimba mice [33, 34, 35, 37, 39]. We have recently shown that, in Akimba mice, the SGLT2 inhibitor Dapagliflozin preserved the well-structured neural layers, reduced Muller cell gliosis and minimised abnormal blood vessel transgression to the outer retinal neural layers (outer plexiform and nuclear layer) [15]. Similarly, Ipragliflozin has been shown to improve retinal neural layer modifications in spontaneously diabetic Torii fatty rats [64] and reduced modifications to the nerve fiber layer and ganglion cell layer and prevented retinal neural apoptosis in db/db mice [19]. Therefore, future studies should investigate the effect of both EMPA and CANA on the retinal neural structure of Kimba and Akimba mice to gain a better understanding of the role of SGLT2 inhibition in the neurodegeneration of the retina.
The frequency and severity of DR among patients with diabetes mellitus is heterogeneous [65]. Although not entirely explained, known risk factors such as duration of diabetes and glycaemic control, explains some of the observed heterogeneity in DR [66, 67]. For instance, some diabetic patients develop DR and progress through the disease, despite a short duration of diabetes and/or excellent glycaemic control. Interestingly, other patients do not develop DR with long durations of diabetes and/or long-term poorly managed hyperglycaemia [65]. As summarised in Tables 1,2, this heterogenous disease phenotype was evident in the Kimba mice displaying retinopathy and Akimba mice demonstrating DR.
Differential response to the same treatment by different patients with different characteristics of DR has been shown [68]. Similarly, although EMPA decreased the overall progression of retinal vascular lesions in Akimba mice, a small percentage of animals did not show an improvement in retinal vascular abnormalities due to the deferential response to EMPA treatment by Akimba mice with DR. Furthermore, it is evident that upon the complete disruption of the retinal vasculature (Supplementary Fig. 1), treatment may not achieve improvements in the disease pathology [69]. There is cumulative evidence demonstrating the effectiveness of various treatment options earlier in the pathogenesis of DR [70, 71]. Therefore, choosing the appropriate treatment window with an earlier initiation of treatment may potentially result in improved outcomes in DR [19, 25, 71].
Monitoring vascular and non-vascular alterations of retinopathy and DR using in vivo ophthalmic modalities are often used in translational research [72]. However, one limitation of our study is that we were unable to perform longitudinal in vivo imaging and functional investigations to evaluate the efficacy of SGLT2 inhibitor treatment on the retinal vasculature, neural structure and function. To date, a very limited number of studies have utilised in vivo techniques to study the effects of SGLT2 inhibitors on the retina. For instance, Takakura and others showed that the treatment of Iplegliflozin in spontaneously diabetic Torii obese rats improved the oscillating potential when retinal electroretinogram was conducted [64]. It is also shown that, in db/db mice, Dapagliflozin treatment improved the Electroretinogram (ERG) b-wave amplitude [63] and Empagliflozin alleviated retinal edema around the disc observed by optical coherence tomography (OCT) [19]. Therefore, future studies should utilize in vivo techniques such as OCT, fundus fluorescein angiography (FFA), electrophysiological testing (ERG) and fundus photography (FP) to study the structure (vascular and neural) and function of the retina [72]. These studies will reveal clinically relevant findings and provide important insights into the effectiveness of SGLT2 inhibitor treatment.
Prolonged hyperglycaemia is an established, independent, and prominent risk factor for the development and progression of DR and good glycaemic control is strongly related to the progression and improvement of DR [66, 73]. In part, the beneficial retinal vascular outcomes in the treated diabetic Akimba mice are likely due to glycaemic control conferred by SGLT2 inhibitors. However, the fact that (i) Akimba-CANA mice did not demonstrate an improvement in the retinal vasculature and (ii) the improvement in the retinal vascular phenotype in the non-diabetic Kimba mice (with EMPA or CANA) indicates that there are perhaps other mechanisms influenced by the treatment of this drug class. Hence, inflammation, oxidative stress, blood flow autoregulatory mechanisms, increased sorbitol in retinal cells as a consequence of osmotic effects, advanced glycosylation end product formation and the role of growth-factor/alternative angiogenic factor production should be investigated in future studies to determine the mechanisms of action of SGLT2 inhibitors [15, 16, 18, 20, 74, 75, 76, 77].
The relationship between dyslipidemia and DR is controversial and not yet fully understood [78, 79, 80]. Some studies have shown diabetic patients display high levels of triglycerides and low-density lipoprotein (LDL) cholesterol, along with low high-density lipoprotein (HDL) cholesterol and these patients may have an increased risk of development and progression of DR [81, 82, 83, 84]. However, the NO BLIND study showed that high HDL values are associated to DR [85] and as revealed by the United Kingdom Prospective Diabetes Study (UKPDS), higher levels of HDL cholesterol levels correlated with more advanced retinopathy [86]. A recent study reported that the parental strain of the Akimba, the Akita mouse, showed significantly increased LDL cholesterol while HDL cholesterol levels were significantly higher [87]. Therefore, it is likely that the Akimba mouse model with DR may possess a similar lipid profile. However, future studies should firstly investigate the lipid profiles of Akimba mice and secondly, conduct dose dependant HDL therapy studies [88] to determine the effect of HDL in the development and progression of DR in the Akimba model. As recently reviewed by Yaribeygi et al. [89], evidence suggest that SGLT2 inhibitors improved dyslipidaemia by decreasing LDL-levels, triglycerides and increasing HDL-levels, thus providing cardiovascular benefits in diabetic patients. Given improving dyslipidaemia may improve retinal endothelial function, retinal blood flow, inflammation, retinal vascular leakage, and thus delay the development and progression of DR [90], further research is needed to determine the long-term effects of SGLT2 inhibitors on HDL levels and DR.
Tahara and colleagues used diabetic and non-diabetic rodents to demonstrate that each SGLT2 inhibitor has distinct pharmacokinetics, pharmacodynamics, and pharmacologic effects [91, 92, 93]. Although all SGLT2 inhibitors block the function of the SGLT2 protein in the kidneys, there are differences in their onset of action, half-life, renal threshold, selectivity, absorption, distribution, and metabolism. Additionally, their relative affinity for the SGLT2 protein versus the SGLT1 protein is significant in understanding the distinct effects of these medications [94]. While both SGLT2 inhibitors in this study effectively increased urinary glucose excretion and decreased hyperglycemia, the actual differences reflected in the pharmacokinetics, pharmacodynamics, and pharmacologic profiles of each of these SGLT2 inhibitor might be responsible for the distinct SGLT2 inhibitor mediated variations in metabolic parameters, retinopathy and diabetic retinopathy observed in our specific mouse models.
Our findings suggest beneficial effects of SGLT2 inhibition on DR, which now warrants clinical investigations to determine the overall effect of SGLT2 inhibition on the full spectrum of DR. Such clinical studies will be able to address the differences in outcomes between individual SGLT2 inhibitors and also determine if these outcomes are due to differences in SGLT2 selectivity.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conceptualization, LYH, VBM, MPS and EPR; formal analysis, LYH and VBM; funding acquisition, LYH, VBM and MPS; investigation, LYH, JRM, VBM and MPS; writing-original draft, LYH, JRM and VBM; writing-review and editing, LYH, JRM, MPS, EPR and VBM. All authors have read and agreed to the published version of the manuscript.
Animal ethics was approved by both the Royal Perth Hospital Animal Ethics Committee (R537/17-20; approved: 15/08/17) and Harry Perkins Institute for Medical Research Animal Ethics Committee (AE141/2019; approved: 12/02/19).
We thank Dr. Aaron Magno (University of Western Australia, Western Australia) for technical assistance. Authors thank Animal Resources Centre (Murdoch, Western Australia) and the Bioresources, Harry Perkins Institute of Medical Research (Nedlands, Western Australia) for providing animal care services.
This study was funded by the Royal Perth Hospital-Research Foundation WA (VM2018), Diabetes Australia Research Program (Y20G-MATV), Diabetes Research WA (DRWA-LHerat-2020) and Raine Medical Research Foundation, WA (Raine-LHerat-2020).
MS has received research support from Boehringer Ingelheim. The other authors declare no conflicts of interests.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/j.fbl2804083.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.








