1 Institute of Pathology, Laboratory and Forensic Medicine (I-PPerForM), Universiti Teknologi MARA, 47000 Sungai Buloh, Selangor, Malaysia
2 Department of Pathology, Faculty of Medicine, Universiti Teknologi MARA, 47000 Sungai Buloh, Selangor, Malaysia
3 Department of Biochemistry & Molecular Medicine, Faculty of Medicine, Universiti Teknologi MARA, 47000 Sungai Buloh, Selangor, Malaysia
Abstract
Background: Various methods were used to induce atherosclerosis in
rabbits. One of the most common methods used is high-cholesterol diet (HCD)
feeding. However, the exact amount and duration of HCD feeding to induce early
and established atherosclerosis in New Zealand white rabbits (NZWR) continue to
be debated among researchers. Therefore, this study aims to evaluate the
effectiveness of 1% HCD feeding in inducing early and established
atherosclerosis lesions in NZWR. Methods: A total of 50 g/kg/day of 1%
HCD was fed to three to four months old male rabbits weighing 1.8 to 2.0 kg for
four and eight weeks to induce early and established atherosclerosis
respectively. The body weight and lipid profile were measured at baseline and
post-HCD intervention. Following euthanasia, the aorta was excised and prepared
for histology and immunohistochemical analysis to confirm the stages of
atherosclerosis. Results: The mean body weight of the rabbits in early
and established atherosclerosis groups increased significantly up to 17.5%
(p = 0.026) and 19.75% (p = 0.019) respectively compared to
baseline. The total cholesterol level dramatically elevated up to 13-fold
(p = 0.005) and 38-fold (p = 0.013) compared to baseline, after
four and eight weeks of 1% HCD feeding respectively. The low-density lipoprotein
level significantly increased up to 42-fold (p = 0.006) and 128-fold
(p = 0.011) compared to baseline, after four and eight weeks of 1% HCD
feeding respectively. Rabbits fed with four and eight weeks 1% HCD significantly
developed 5.79% (p = 0.008) and 21.52% (p = 0.008) aortic
lesion areas compared to the control group. Histological evaluation in the aorta
showed accumulation of foam cells in early atherosclerosis group and formation of
fibrous plaque and lipid core in the established atherosclerosis group. Rabbits
fed with eight weeks HCD showed higher tissue expressions of ICAM-1, VCAM-1,
e-selectin, IL-6, IL-8, NF-
Keywords
- cholesterol
- rabbits
- lipid profile
- immunohistochemistry
- atherosclerosis
It is estimated that 17.9 million people die each year from cardiovascular disease (CVD), accounting for 31% of all deaths worldwide [1]. Atherosclerosis is one of the major causes of CVD [2]. It is an arterial disease associated with dyslipidemia, abnormal arterial calcification, and oxidative stress. It has been shown that the chronic inflammatory state of the arterial wall contributes to the development of atherosclerosis [3]. The risk factors of atherosclerosis include hyperlipidemia, hypertension, smoking, insulin resistance, diabetes, obesity, inflammation, stress, and others [4]. There are several development stages of atherosclerosis. In early atherosclerosis, foam cells could be detected microscopically with the absence of plaque formation while established atherosclerosis could be differentiated from early atherosclerosis through the macroscopic presence of atherosclerotic plaque [5].
The use of animal models, especially rabbits, is essential to provide a better comprehension of the underlying pathological mechanism of atherosclerosis so that improved strategies and interventions can be formulated for the treatment of atherosclerosis. Rabbits were the first experimental animal model used specifically for atherosclerosis research. Besides, the stages of atherosclerotic lesions in rabbits also closely resemble those of humans [6]. Both rabbits and humans show a high expression of very-low-density lipoprotein (VLDL) receptors, which promote the foam cells to form, as compared to mice that do not [7]. Moreover, rabbits exhibit abundant cholesteryl ester transfer protein (CETP) in the plasma which regulates cholesterol metabolism, as opposed to mice and rats [8].
Due to these resemblances to humans, rabbits were the first animal model used to test many hypolipidemic drugs, including statins [9, 10] fibrates [11, 12] and probucol [13, 14, 15]. The anti-atherogenic effects of the infusion of high-density lipoproteins were also first established in cholesterol-fed rabbits [16, 17]. Besides, a systematic review concluded that HCD fed rabbits represented the best model to study the cholesterol-lowering activity of statins [18].
Earlier studies have shown that the animal model of choice for atherosclerosis
induction was the Watanabe heritable hyperlipidemic (WHHL) rabbits that were of
genetically atherogenesis-prone strain. They were widely used in the screening of
drugs against atherosclerosis during the development stage. However, WHHL rabbits
are costly and difficult to manage. Another model that can be used to induce
atherosclerosis in rabbits is by using transgenic or genetically modified rabbits
such as apolipoprotein E knockout (
Several reviews on the utilization of rabbits in atherosclerosis research have been published recently [6, 21, 22]. Each review contributed valuable components to this study. Fan et al. [6] provided an overview of recent progress on how rabbits can be used as a practical alternative to humans for the study of atherosclerosis. Lee et al. [21] focused on describing numerous species used in the atherosclerosis models, including rabbits, where they discussed the practicality of their use in experimentation. Finally, a review by Baumgartner et al. [22] described the investigations of therapeutic interventions in rabbits and the measurements of relevant parameters in vivo and ex vivo.
Despite decades of research, the exact amount and duration of high-cholesterol diet feeding to induce early and established atherosclerosis in NZWR continues to be debated among researchers. Therefore, this study would be beneficial for researchers who are new to the concept of working with rabbits. In short, we aimed to evaluate the effectiveness of a 1% high-cholesterol diet for four and eight weeks in the induction of early and established atherosclerosis in NZWR.
The standard normal diet (ND) was purchased from A Sapphire Enterprise
(001303794-M) whereas the high-cholesterol diet (HCD) 1% was purchased from
Specialty Feeds, Western Australia (SF00-221). Low-density lipoprotein
cholesterol Gen.3 (LDLC3) for Cobas c 501 was obtained from Roche Diagnostic
(Basel, Switzerland). The monoclonal antibodies for Interleukin 6, Interleukin 8
and Intercellular Adhesion Molecule 1 were purchased from MyBioSource (San Diego,
CA, USA) while NF-
The HCD is composed of 18% protein, 4% total fat, 13% crude fiber, 13% A
delta fiber whereas the digestible energy is 11 MJ/kg. The HCD is made up of
barley, lupins, oaten hay, oat hulls, lucerne, soya meal, canola meal,
DL-methionine (also known as racemethionine, is a mixture of two enantiomers
D-methionine and L-methionine), magnesium oxide, dicalcium phosphate, salt, mixed
vegetable oils, United States Pharmacopoeia (USP) cholesterol and a vitamin and
mineral premix. The mineral contents of the HCD composed of 1.10% calcium,
0.70% phosphorus, 0.30% magnesium, 0.20% sodium, 1.10% potassium, 0.20%
sulphur, 356 mg/kg iron, 23 mg/kg copper, 1.8 mg/kg iodine, 126 mg/kg manganese,
0.7 mg/kg cobalt, 95 mg/kg zinc, 1.0 mg/kg molybdenum, 0.3 mg/kg selenium, 0.008
mg/kg cadmium and 3.4 mg/kg boron. The HCD contains 48,640 IU/kg of vitamin A, 60
mg/kg of vitamin E, 3.3 mg/kg of vitamin K, 5.6 mg/kg of vitamin B1, 6.6 mg/kg of
vitamin B2, 56 mg/kg of niacin, 5.7 mg/kg of vitamin B6, 19 mg/kg of pantothenic
acid, 141
A total of 15 male NZWR aged 3–4 months old with body weights of 1.8 to 2.0 kg were used. For the induction of early and established atherosclerosis, the NZWR were given HCD for 4 weeks and 8 weeks to promote atherogenesis for the development of early and established atherosclerotic lesions.
The experimental protocol is depicted below (Fig. 1). A total of 15 rabbits were randomized into the control group, group A (n = 5) and HCD group (n = 10). Rabbits in the control group were given a normal diet i.e., the chow pellet for two weeks while the HCD group was further divided into two groups which were group B (n = 5) and group C (n = 5). Group B was fed with 50 grams/kg/day of HCD for 4 weeks to induce early atherosclerosis while group C was fed with the same amount for 8 weeks to induce established atherosclerosis. The key readout parameters include the assessment of body weight, serum biochemical marker analysis, morphological examination, histopathological analysis and immunohistochemical analysis.
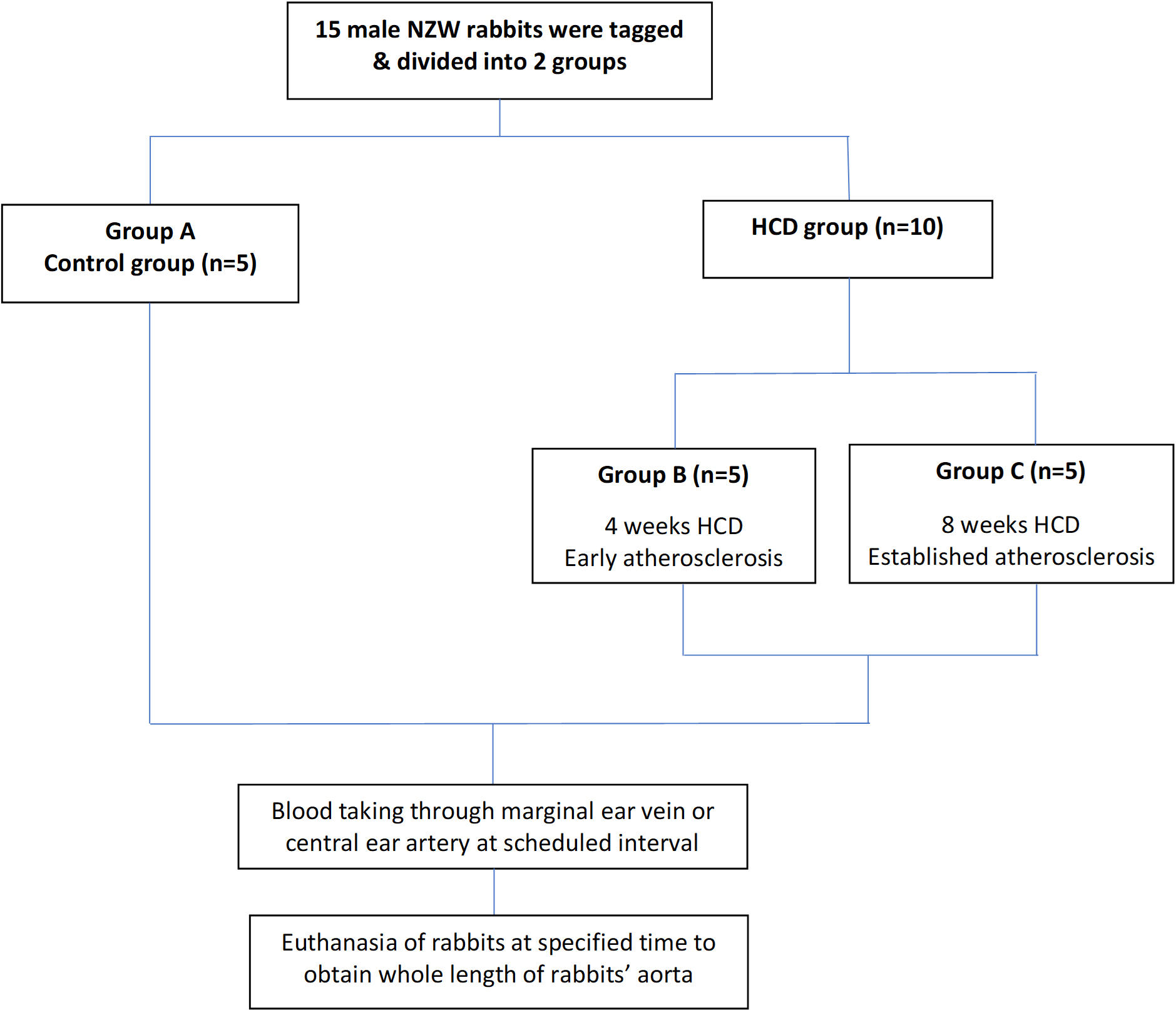 Fig. 1.
Fig. 1.Experimental protocol to induce early and established atherosclerosis in rabbits.
Rabbit’s blood was collected at baseline and after completing the 4 and 8 weeks of HCD feeding for Group B (Fig. 2) and Group C (Fig. 3) respectively. Rabbits were fasted for ten hours from 10 pm to 8 am the next morning. A total of 8 ml of blood was taken from either the marginal ear vein or the central ear artery of the rabbits.
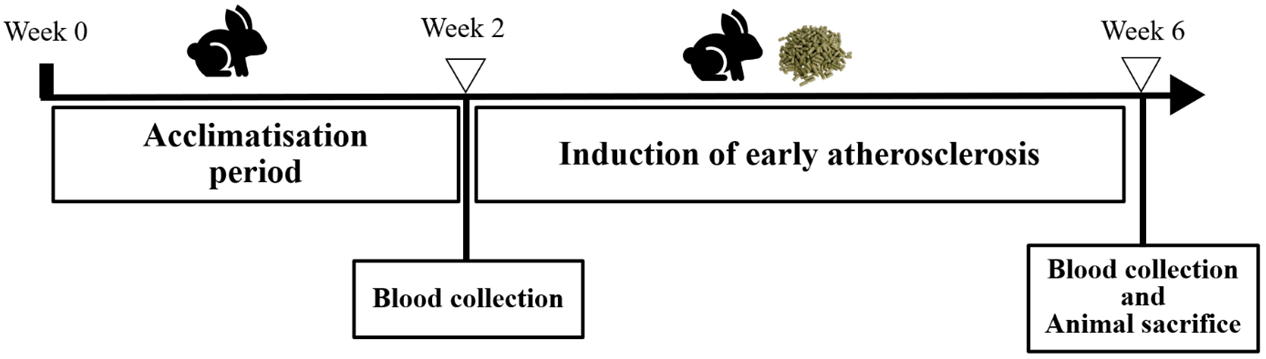 Fig. 2.
Fig. 2.Rabbit study timeline for group B (early atherosclerosis).
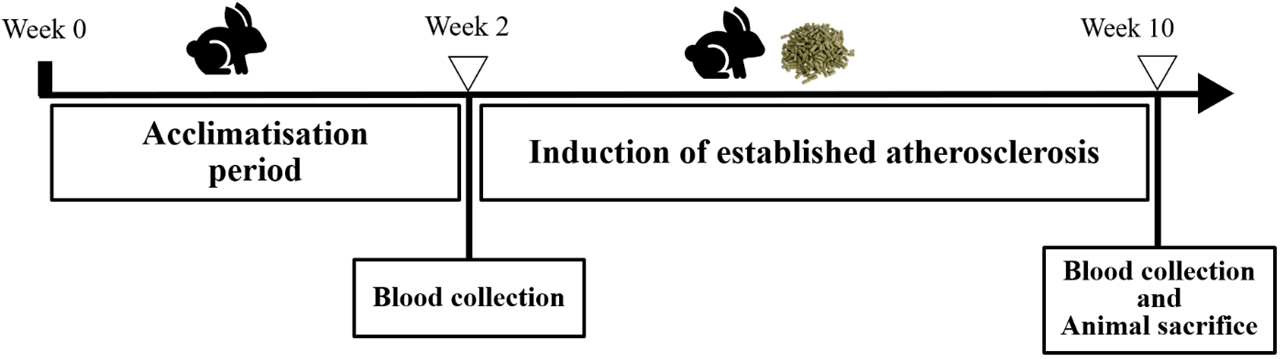 Fig. 3.
Fig. 3.Rabbit study timeline for group C (established atherosclerosis).
Following that, the blood samples were centrifuged at 4000 rpm for 10 minutes to obtain the serum. Aliquoted serum was stored at –80 °C until analysis. The samples were then analyzed for lipid profile which include direct low-density lipoprotein cholesterol (LDL), total cholesterol (TC), triglycerides (TG), and high-density lipoprotein (HDL). All the tests were analyzed on automated analyzer, Roche Cobas c 501 (Indianapolis, IN, USA). Direct LDL was used because it is more precise than calculated LDL and it is not influenced by other lipid profile components [23]. All the tests were measured based on the standard calibration procedure and quality control check as per guidelines in the laboratory.
At the end of the experiment, the rabbits were euthanized with sodium pentobarbital (100 mg/kg) intravenously. The whole length of aorta was obtained after dissection to evaluate the atherosclerotic lesions. The aorta must be isolated to facilitate the macroscopic evaluation of the aortic lesions in terms of size and quality. The dissection started from the ascending aorta down to the bifurcation of the iliac arteries. The inner surface of the aorta was stained with Sudan IV according to the method in previous study [24].
First, the aortas were gently rinsed with normal saline before being cut open
longitudinally to expose the lumen. The opened aortas were then pinned flat on a
cardboard in a stainless-steel tray (8
For histological evaluation, three different areas of aortic tissue were
sampled, i.e., 10 mm away from the aortic arch, 10 mm away from upper thoracic,
and 10 mm above the iliac bifurcation. Each section measured 15 mm in length
(Fig. 4). All the aortic specimens of the rabbits were fixed in 10% formalin
solution before grossing was performed and the tissues were placed in tissue
cassettes. The tissues were then processed using an automated tissue processor
MICROM STP120, Thermo Scientific™ (Waltham, MA, USA) for 21 hours
(Table 1). The processed tissues were embedded in paraffin using
Tissue-Tek® TEC™ (Torrance, CA, USA) to form
paraffin-embedded tissue blocks and sectioned to 4–6
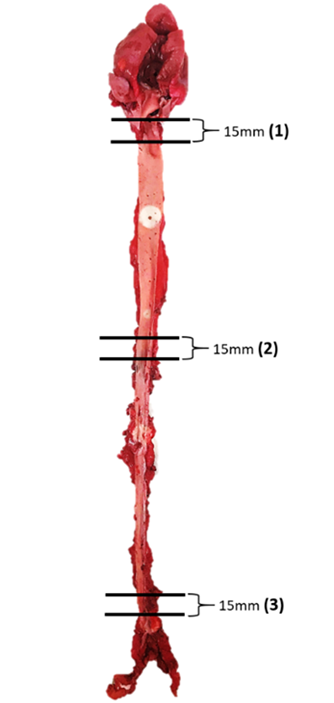 Fig. 4.
Fig. 4.Schematic diagram of the aortic tissue of rabbits and selected lesion sites for section analyses.
| No. | Chemical/Reagent | Quantity | Duration (Hour) |
|---|---|---|---|
| 1 | 10% Formalin | 1.8 liters | 2:00 |
| 2 | 50% Alcohol | 1:00 | |
| 3 | 70% Alcohol | 1:00 | |
| 4 | 80% Alcohol | 1:00 | |
| 5 | 95% Alcohol | 1:00 | |
| 6 | Absolute Alcohol | 2:00 | |
| 7 | Absolute Alcohol | 2:00 | |
| 8 | Mixture of absolute alcohol and xylene (1:1) | 1:00 | |
| 9 | Xylene | 2:00 | |
| 10 | Xylene | 2:00 | |
| 11 | Paraffin wax | 3:00 | |
| 12 | Paraffin wax | 3:00 |
Three different sites of aortic tissue were sampled with each section measuring 15 mm in length. The number in parentheses indicates the representative areas of each part of the artery selected for tissue section and the measurement of intimal lesion areas. (1) At the origin of the aortic arch; (2) at the origin of the abdominal aorta; (3) at the end of abdominal aorta before the bifurcation of iliac artery.
Immunohistochemical (IHC) staining for all antibodies; endothelial activation
biomarkers (intracellular adhesion molecule-1 (ICAM-1), vascular cell adhesion
mole-cule-1 (VCAM-1), and e-selectin), inflammatory biomarkers (interleukin-6
(IL-6), interleukin-8 (IL-8), and nuclear factor kappa B (NF-
The IHC was commenced by incubation of slides in peroxidase block for 10 minutes to reduce non-specific background staining. Slides were washed 3 times using wash buffer at 5 minutes interval and followed by application of primary antibodies at 1:150 dilutions. The antibodies were incubated overnight at 4 °C. Slides were washed thoroughly and the tissue was subjected to biotinylated primary antibody for 1 hour and followed by streptavidin-peroxidase application for another hour. Slides were then washed and subjected to application of DAB and substrate-chromogen for 5 minutes. The sections were then immersed in distilled water for 5 minutes, stained with hematoxylin and subsequently dehydrated through 50, 70, 80, 95, and 100% ethanol for 3 min each, and then cleared in xylene. Finally, the sections were mounted with dibutylphthalate polystyrene xylene (DPX) and glass coverslips, and allowed to dry. The specificity of the immunostaining was confirmed using negative control experiment in which primary antibodies were substituted with antibody diluent.
The Statistical Analysis Package for Social Sciences (SPSS) for Windows, Version
27.0 (Chicago, IL, USA) was used for all analyses. All the data were tested for
normality using the Shapiro-Wilk test. The results were expressed as mean
The mean body weight of group B and group C increased significantly up to 17.5% (p = 0.026) and 19.75% (p = 0.019) respectively compared to before treatment (B0) (Fig. 5).
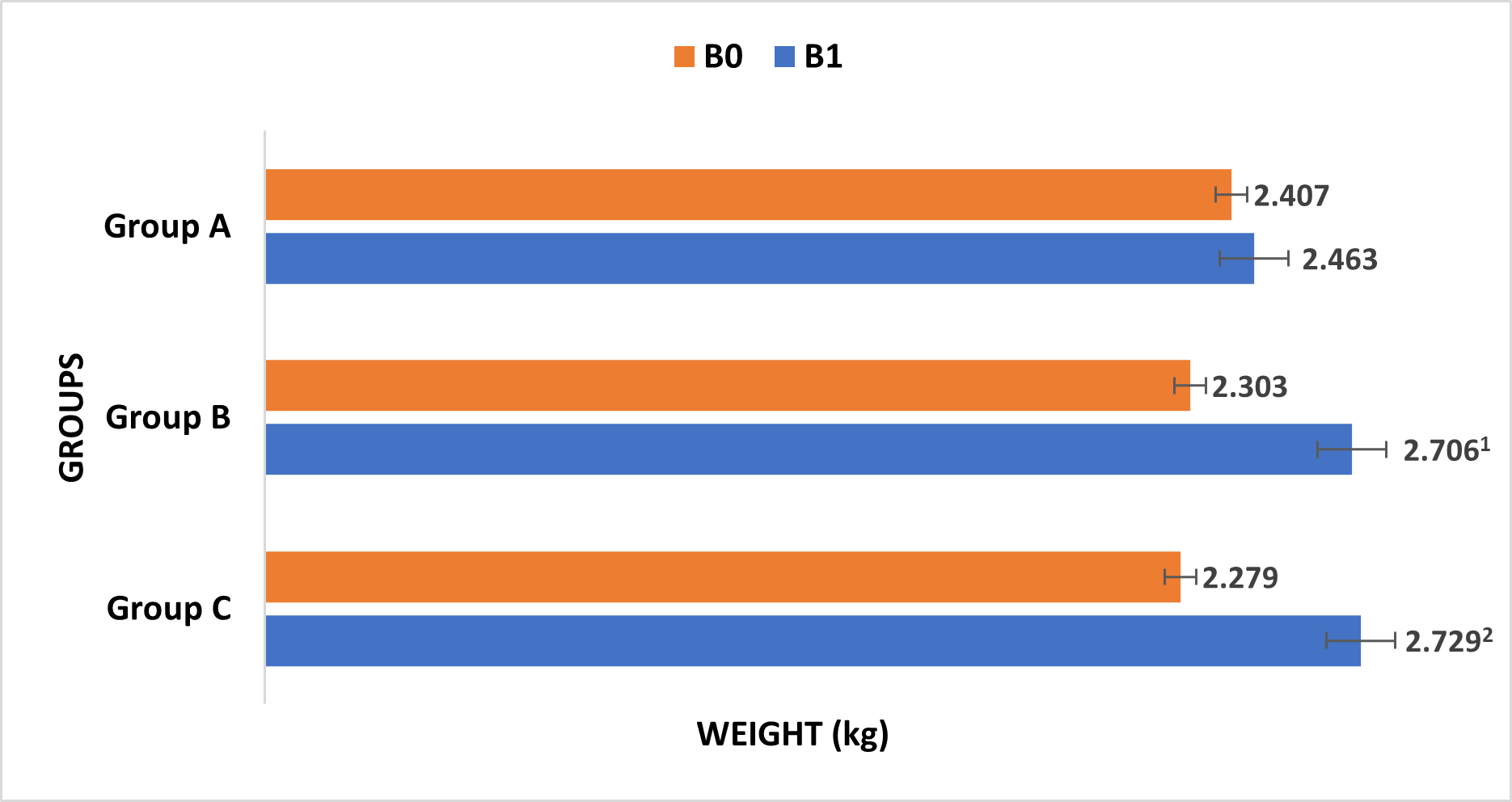 Fig. 5.
Fig. 5.Changes in body weight of rabbits after fed with normal diet for 2 weeks (Group A), high-cholesterol diet for 4 weeks (Group B) and 8 weeks (Group C). B0: before consumption of ND and HCD; B1: after consumption of ND and HCD. 1 vs. before treatment (B0), p = 0.026; 2 vs. before treatment (B0), p = 0.019.
After 4 weeks feeding of 1% HCD, serum TC dramatically elevated up to 13-fold which is from 0.83 mmol/L to 10.68 mmol/L (p = 0.005). Accordingly, 8 weeks feeding of 1% HCD causes an increase of TC up to 38-fold, which is from 1.01 mmol/L to 38.63 mmol/L (p = 0.013). The LDL level significantly increased up to 42-fold, from 0.26 mmol/L to 10.83 mmol/L (p = 0.006) in group B whereas in group C, the LDL elevated up to 128-fold which is from 0.32 mmol/L to 40.93 mmol/L (p = 0.011). The TG level decreased from 0.67 mmol/L to 0.26 mmol/L (p = 0.025) in group B, while the HDL level significantly increased up to 3-fold in group B and C. The HDL in group B increased from 0.49 mmol/L to 1.24 mmol/L (p = 0.014) while in group C, the HDL increased from 0.45 mmol/L to 1.3 mmol/L (p = 0.008) (Table 2).
| Parameters (mmol/L) | 4 weeks HCD | 8 weeks HCD |
|---|---|---|
| Before treatment (B0) | ||
| TC | 0.83 |
1.01 |
| LDL | 0.26 |
0.32 |
| TG | 0.67 |
1.24 |
| HDL | 0.49 |
0.45 |
| After treatment (B1) | ||
| TC | 10.68 |
38.63 |
| LDL | 10.83 |
40.93 |
| TG | 0.26 |
1.5 |
| HDL | 1.24 |
1.3 |
Values are expressed as the mean
The control group, group A showed extremely little or relatively no atherosclerotic lesion (0.037%) while group B showed higher percentage of atherosclerotic lesions which is 5.79% (p = 0.008). Almost half of the aortic area was significantly covered with atherosclerotic lesions in group C (21.52%) (p = 0.008) due to pro-longed exposure of the aortic walls to cholesterol (Table 3 and Fig. 6).
| Group | Relative Area (%) |
|---|---|
| A | 0.04 |
| B | 5.79 |
| C | 21.52 |
Values are expressed as the mean
1 vs. control group (group A), p = 0.008; 2 vs. control group (group A), p = 0.008.
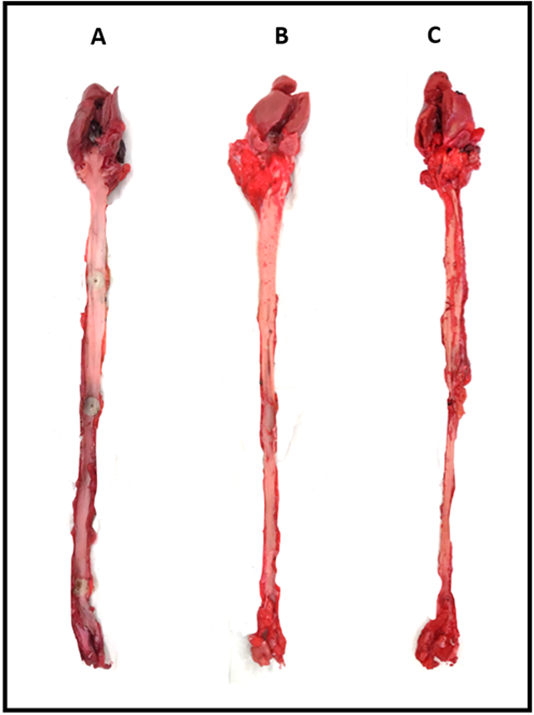 Fig. 6.
Fig. 6.Quantification of the atherosclerotic lesions of rabbits’ aorta using ImageJ (NIH, USA). (A) Rabbits fed with 2 weeks normal diet. (B) Rabbits fed with 4 weeks high-cholesterol diet. (C) Rabbits fed with 8 weeks high-cholesterol diet.
The histological examination of aorta in group A, the control group showed intact tunica intima with thin wavy corrugated endothelium, a normal subendothelial layer, and wavy internal elastic fibers. The tunica media is comprised of elastic fibers and smooth muscle. The outermost layer is the tunica adventitia and is made up of loose connective tissue (Figs. 7,8). In group B, the tunica intima showed the presence of focal thickening, filled with vacuolated cells or foam cells, resulting in the widening of the subendothelial layer (Fig. 9). The tunica intima of the aorta in group C showed intimal thickening with subendothelial collection of foam cells as well as formation of a lipid core and fibrous cap (Fig. 10).
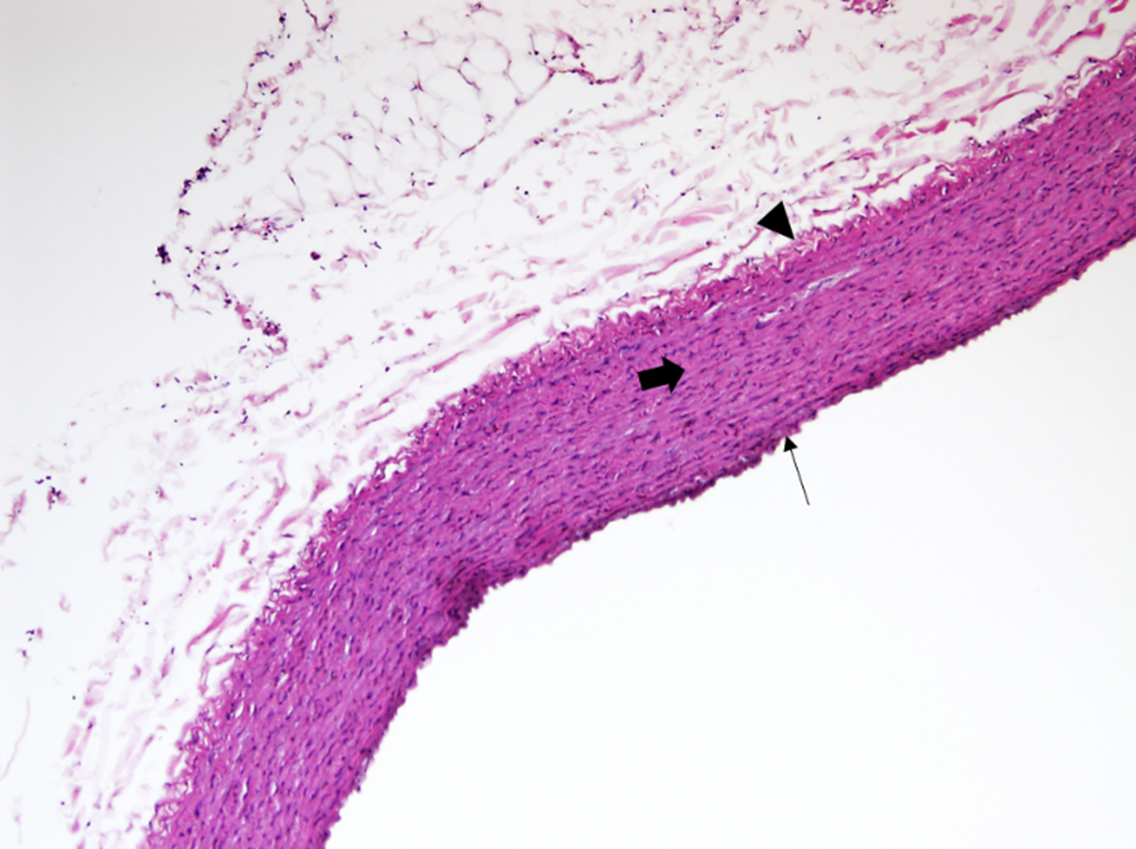 Fig. 7.
Fig. 7.A photomicrograph of a section of the aorta in group A stained
by hematoxylin and eosin (10
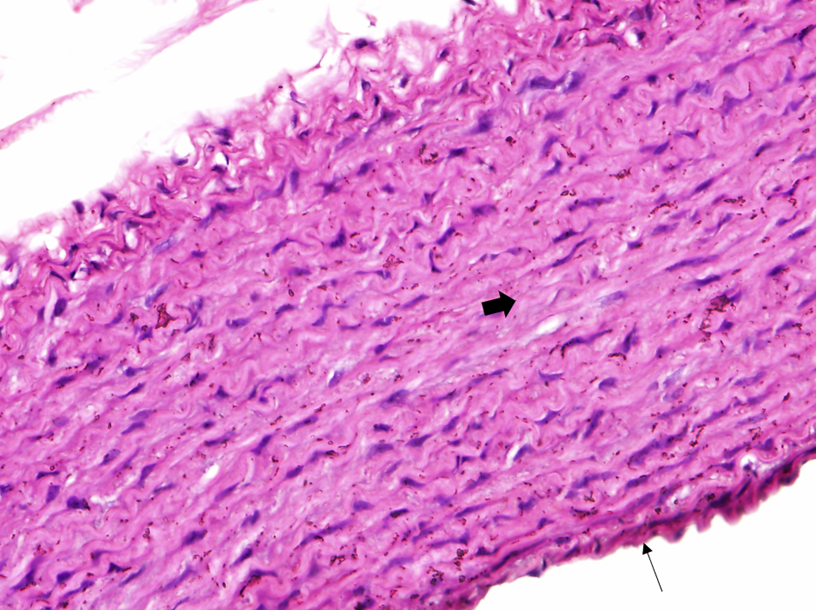 Fig. 8.
Fig. 8.A photomicrograph in a section of aorta in group A stained by
hematoxylin and eosin (40
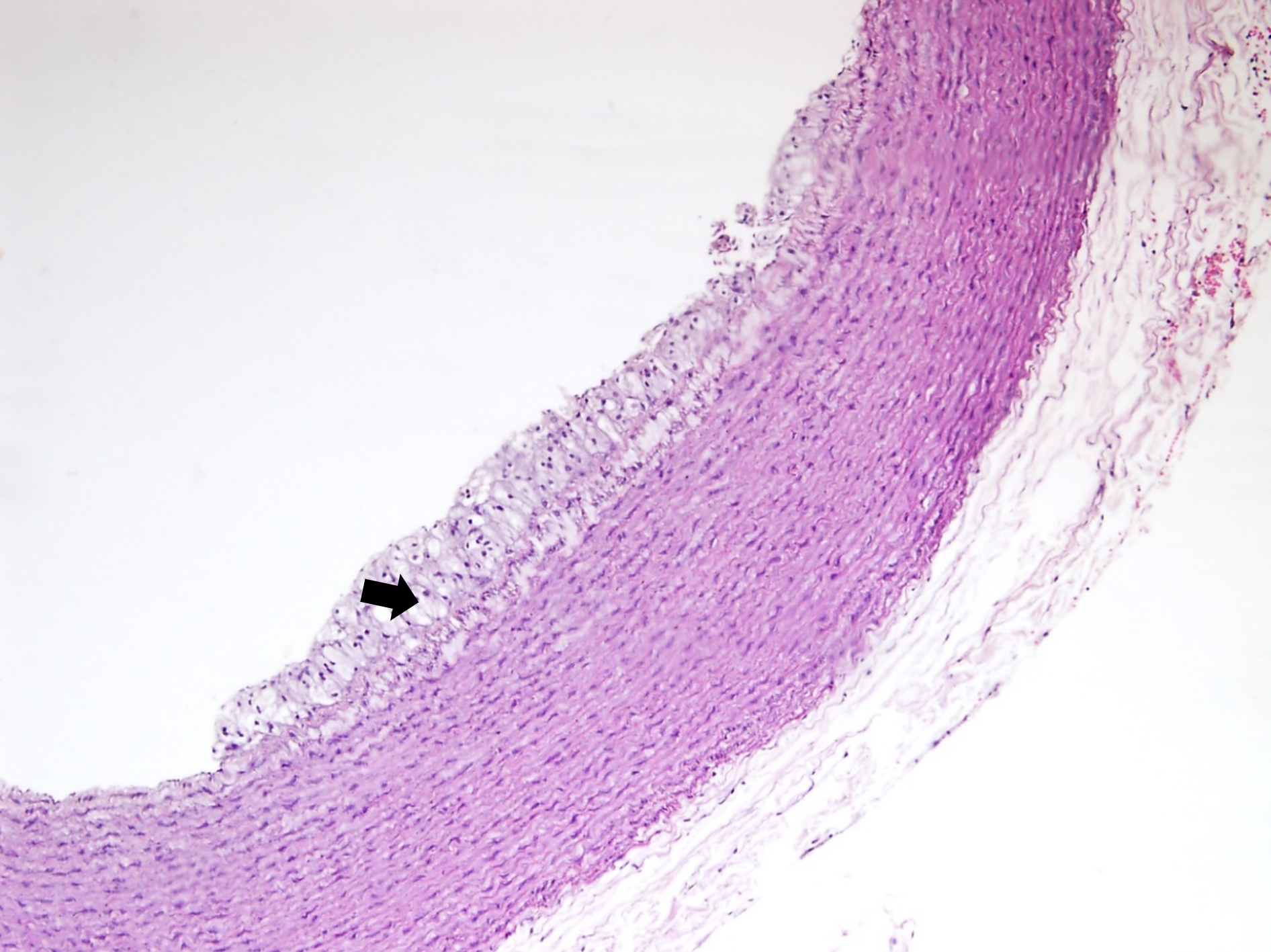 Fig. 9.
Fig. 9.A photomicrograph of a section of aorta in group B stained by
hematoxylin and eosin (10
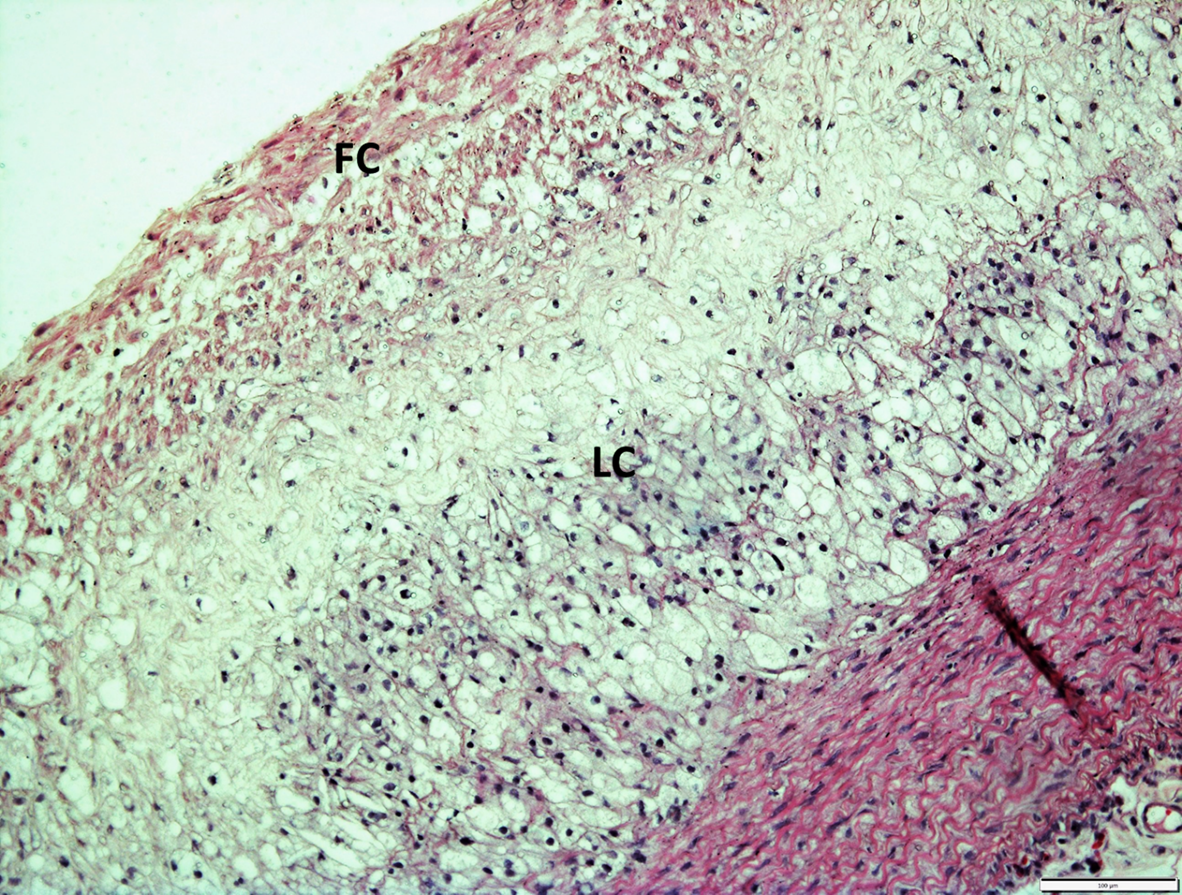 Fig. 10.
Fig. 10.A photomicrograph of a section of aorta in group C stained by
hematoxylin and eosin (10
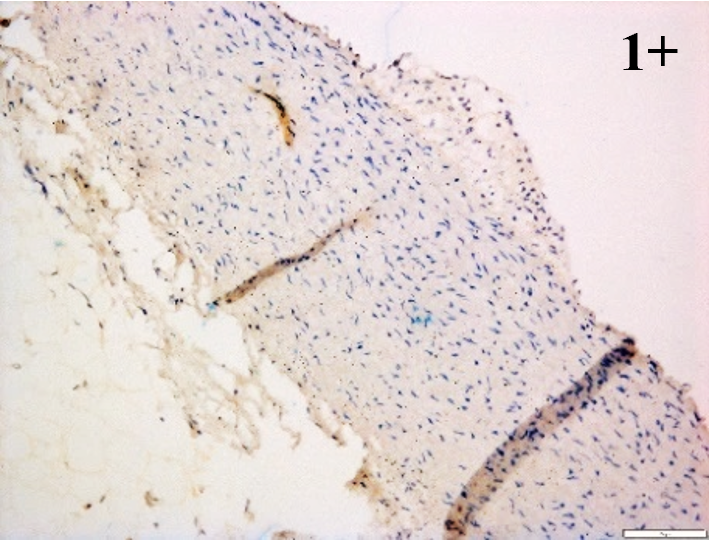
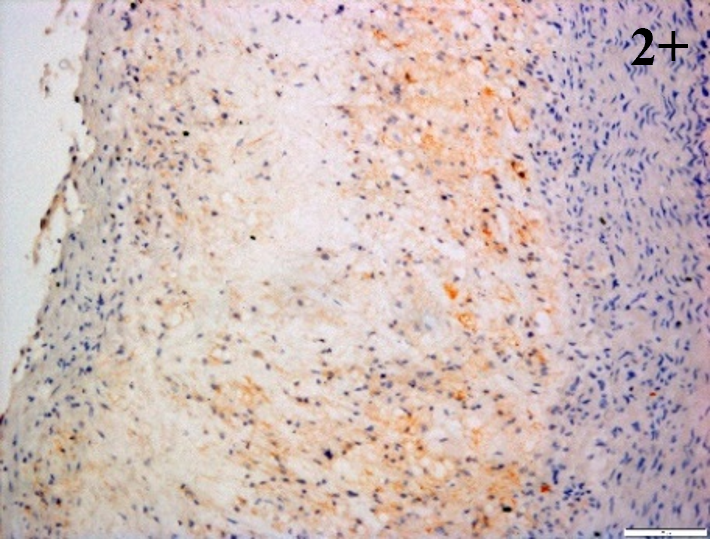
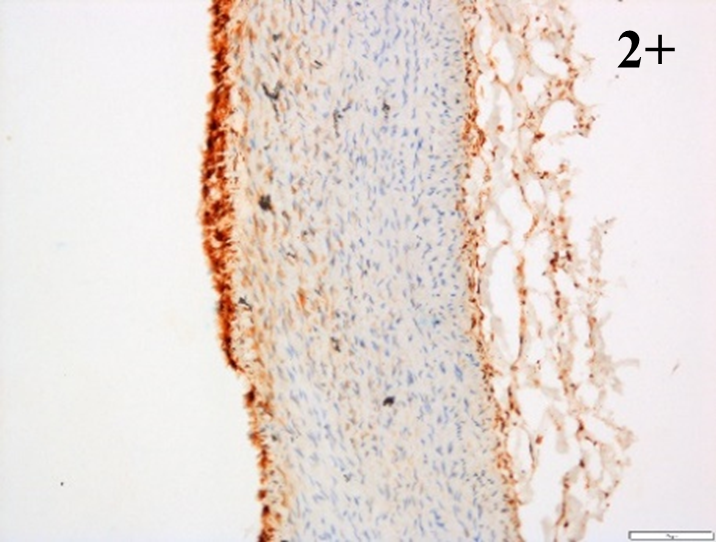
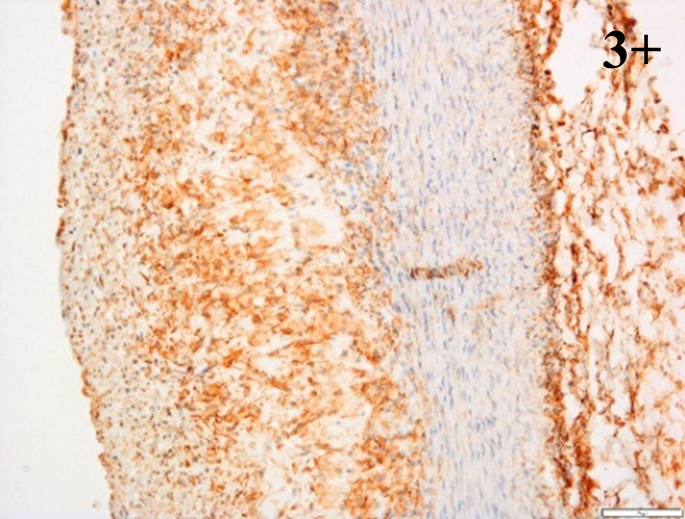
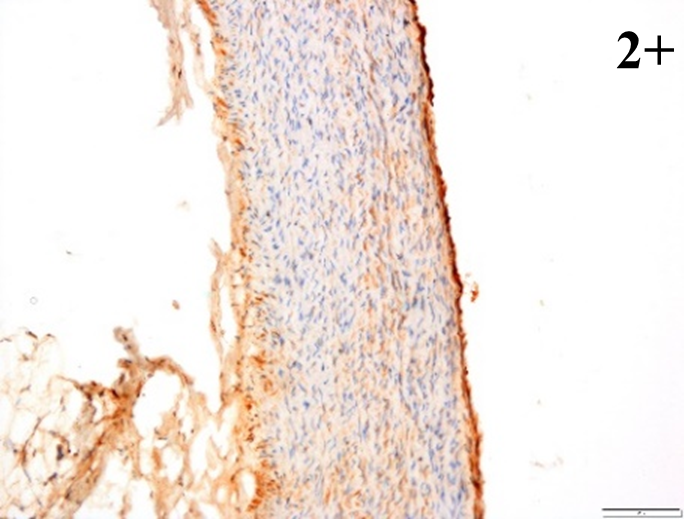
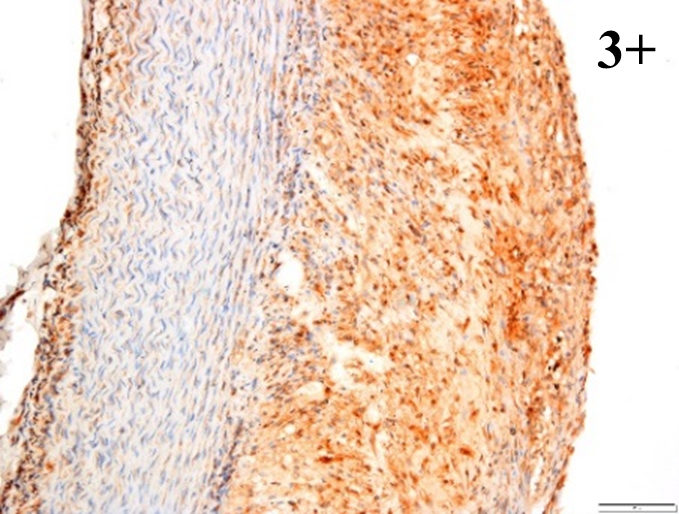
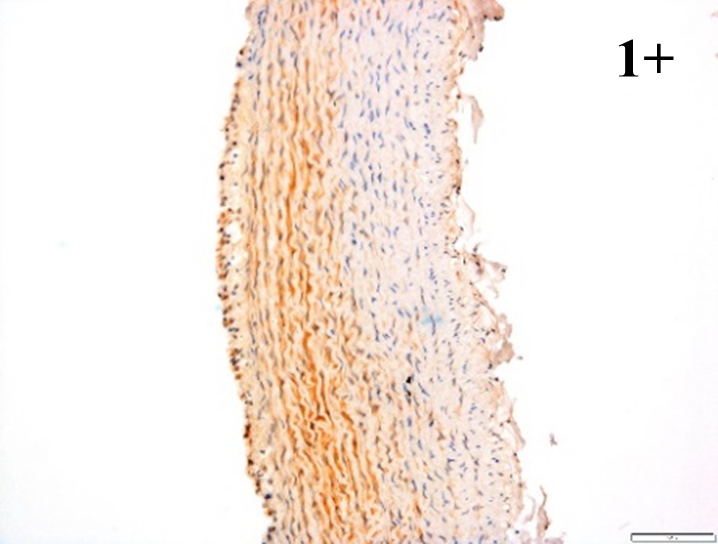
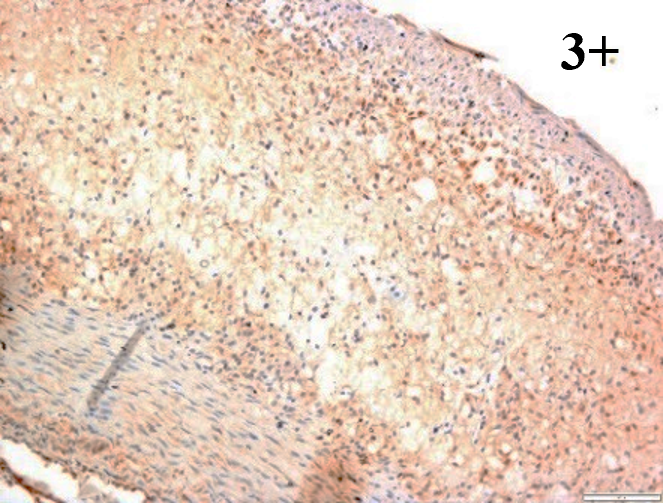
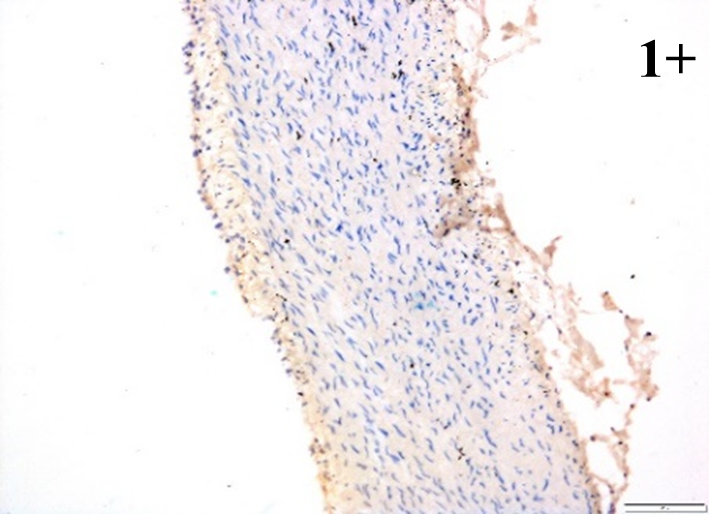
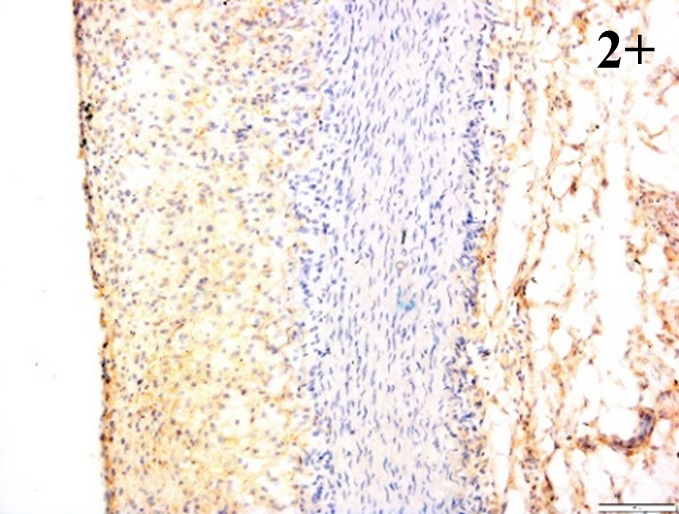
Immunoreactions of ICAM-1, VCAM-1, e-selectin, IL-6, IL-8, NF-
| Control | 4 weeks HCD | 8 weeks HCD | |
|---|---|---|---|
| E-selectin | 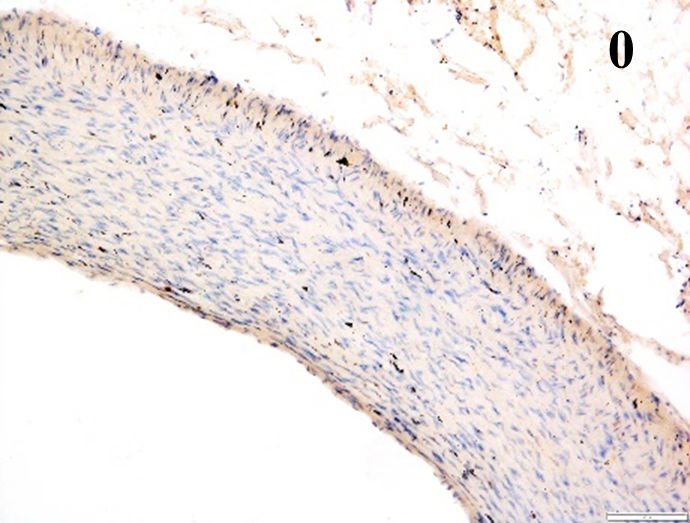 |
 |
 |
| VCAM-1 | 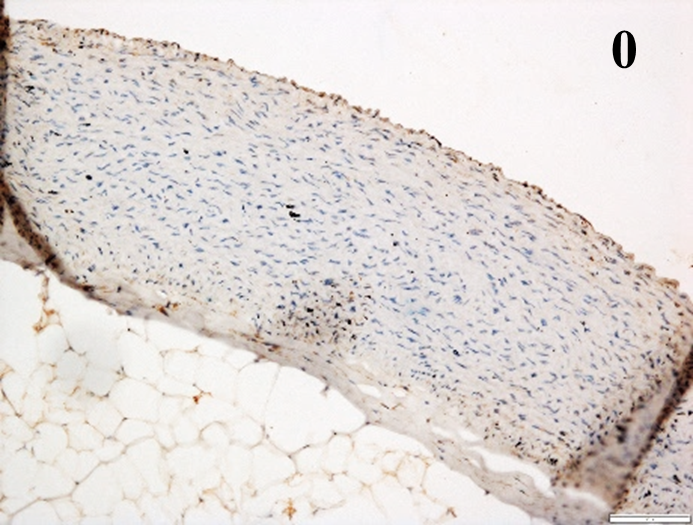 |
 |
 |
| ICAM-1 | 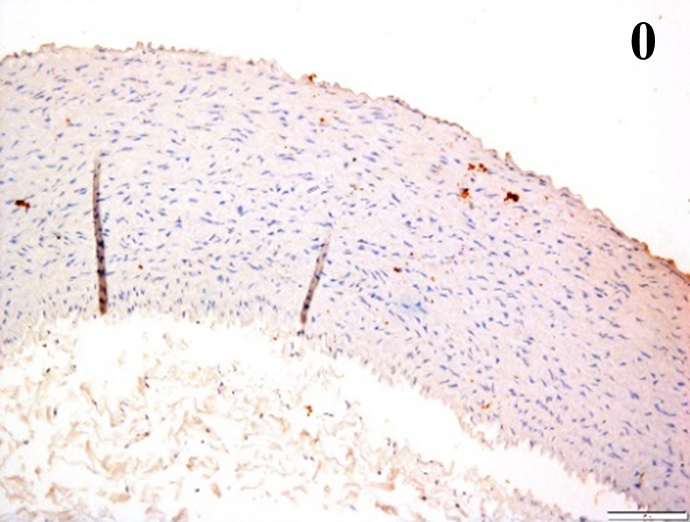 |
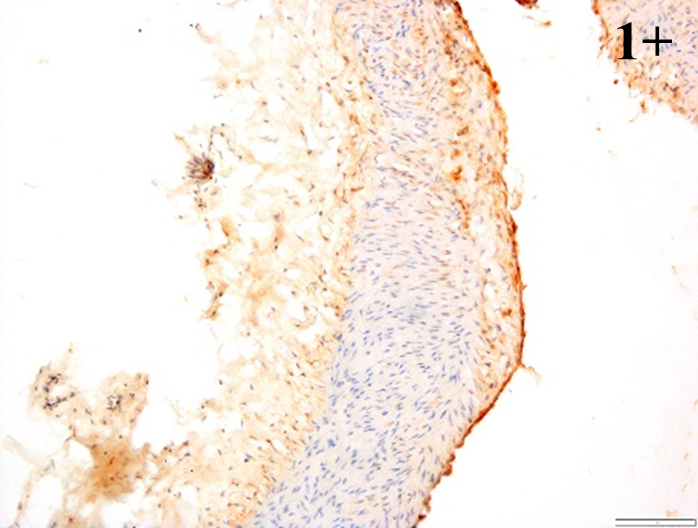 |
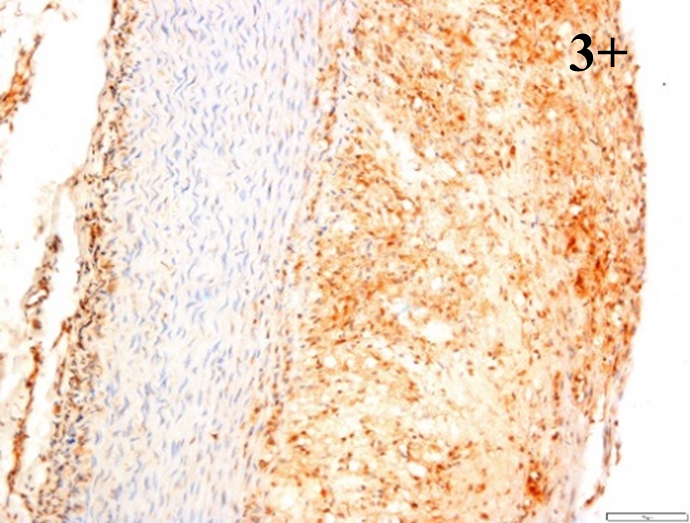 |
| IL-6 | 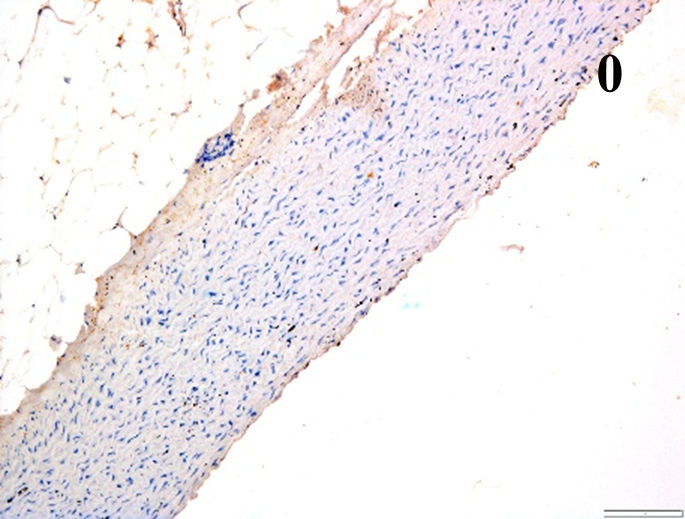 |
 |
 |
| IL-8 | 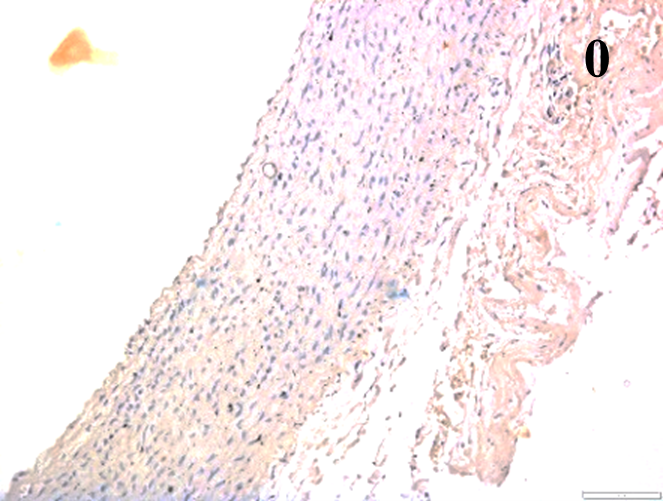 |
 |
 |
| NF-κB p65 | 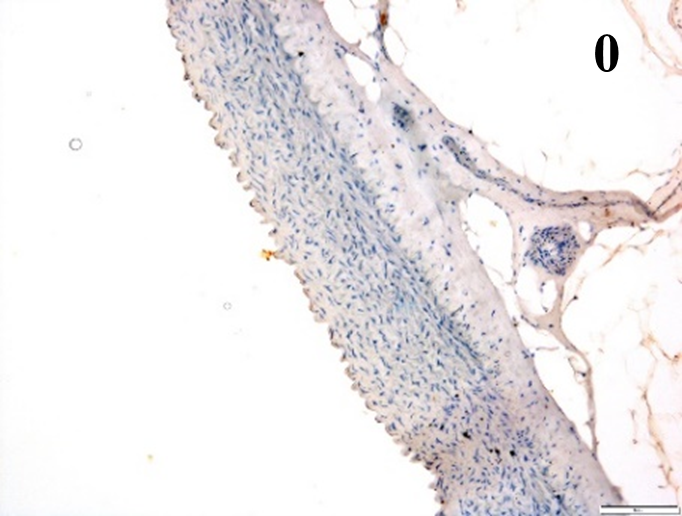 |
 |
 |
| MMP-12 | 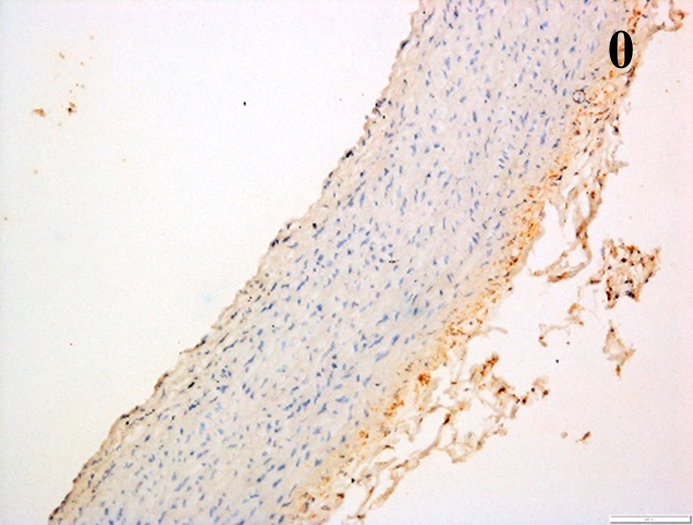 |
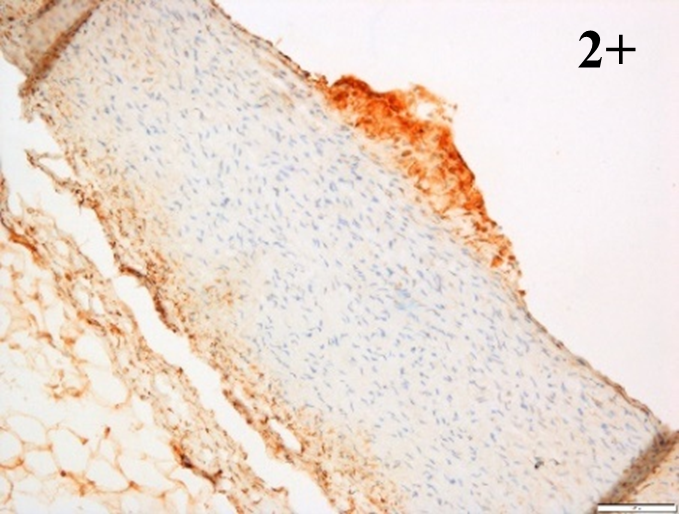 |
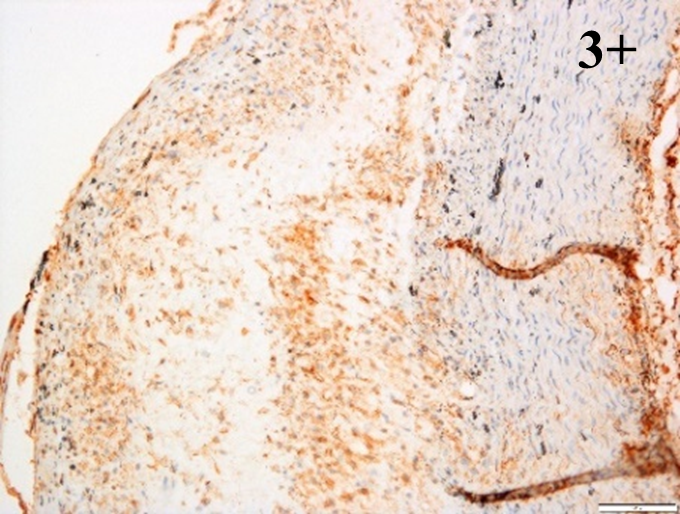 |
According to the percentage endothelial staining of cells, semi-quantitative
scores were applied. Score: 0 (negative); 1+ (1–10% positive cells); 2+
(11–25% positive cells) and 3+ (
There are various methods available to induce atherosclerosis in rabbits which include oral feeding with HCD, inducing injury to the aorta of the rabbits and by combination of high fat diet, intravenous injection of bovine albumin and arterial injury. Balloon injury of the aortas or wire injury of the iliac arteries in rabbits can help in developing atherosclerotic lesions. This method may also be conducted in rabbits fed with cholesterol to accelerate the lesion formation. However, atherosclerotic lesions induced by injuries are primarily composed of “neo-intima” that are formed by proliferating smooth muscle cells as compared to fatty streaks induced by cholesterol diet feeding [6]. A study by Tian et al. [25] found a novel method to induce atherosclerosis in rabbits by using ferric chloride to stimulate injury in the right common carotid artery of rabbits. However, spontaneous intraplaque hemorrhage, plaque rupture, and thrombus were not observed by using this method. Another method is by combining several methods which are high-fat diet feeding, intravenous injection of bovine albumin and induction of arterial injury [26]. Though this method is time saving and cost effective, there are several restrictions that need to be followed to accomplish the same atherosclerotic model. Researchers need to be very precise in calculating the dosage of albumin and anesthesia to prevent the loss of rabbits. Moreover, only trained and expert researchers could proceed with this method as there are lots of technical details that need to be considered. For instance, excessive force during arterial injury could damage the arterial wall, rupture the blood vessels and cause rabbits to die [26].
One of the safe and established methods to induce atherosclerosis and hyperlipidemia in rabbits is by feeding HCD. However, the percentage of cholesterol and the duration of feeding varies between studies. For instance, Lorenz et al. [27] fed a high-cholesterol diet containing 0.5% cholesterol to rabbits for 4 weeks. Another study by Ibrahim et al. [28] fed the rabbits with a 120-gram 1% cholesterol diet daily for 90 days while Ram et al. [29] fed the NZWR with a 200-gram high-fat diet per day (500 mg cholesterol mixed with 5 mL coconut oil/kg orally) for 15 days to induce atherosclerosis. Qiao et al. [26] induced atherosclerosis in Japanese big-ear rabbits by feeding 50-gram high-fat diet (2% cholesterol, 10% lard, 10% egg yolk powder, 0.2% propylthiouracil, and 67.8% regular diet) daily for seven weeks. Majeed et al. [30] kept the rabbits on a cholesterol-rich diet (2% cholesterol) for 12 weeks whereas Wang et al. [31] induced atherosclerosis in the Male Japanese White rabbits by giving a 0.3% cholesterol diet (100 g/day) for 16 weeks. Even though the duration of feeding and the percentage of cholesterol differed between the abovementioned studies, all the methods led to an increased lipid profile and the development of some kind of atherosclerotic lesions in the rabbits.
Rabbits have more tissue due to their bigger sizes compared to rodents. Thus, a single rabbit is adequate to perform multiple analyses. As a result, the use of rabbits should be considered in translational research involving the development of new lipid-lowering and anti-atherosclerotic therapeutic drugs. Furthermore, rabbits were the first experimental animal model used specifically for atherosclerosis research. They are particularly suitable for this purpose due to the distinctive features of their lipid metabolism [32].
Similar to humans, the major plasma lipoproteins in rabbits are LDL while mice have HDL as their major plasma lipoproteins. In addition, mice have no CETP while rabbits and humans have abundant CETP. Humans and rabbits exhibit a similar type of apoB-48, which is made up of chylomicrons. The apoB-100 in humans and rabbits are also similar, where they can be bound to apo (a), while apo (a) in mice do not. HDL is heterogeneous in rabbits and humans, while homogeneous in mice. Like humans, rabbits exhibit VLDL receptors in their macrophages as opposed to mice. Furthermore, the cholesterol pool in rabbits and humans are mainly from hepatic synthesis, whereas, in mice, the pool is mainly from dietary origin. The ability to excrete bile acid derived from cholesterol is low in humans and rabbits, whereas, high in mice. Furthermore, rabbits and humans are very sensitive to cholesterol diets compared to mice [32].
The present study further characterized the effects of consumption of 1% HCD for 4 weeks and 8 weeks on the body weight, lipid profile, morphology and histology of aorta in the rabbits. In this study, it was observed that the body weight of rabbits fed with 8 weeks HCD was higher than the body weight of rabbits fed with 4 weeks HCD. This is consistent with earlier studies [33, 34] which showed that dietary treatment of rabbits with high-cholesterol diets causes significant weight gain.
The significant increase of TC and LDL post HCD feeding might be correlated with an increased risk of atherosclerosis [35]. This was confirmed by positive and significant correlations between total plasma cholesterol and atherosclerosis degree in our experiment in which higher increase in the percentage of TC and LDL showed higher percentage of atherosclerotic lesions.
In fact, elevated levels of plasma TC and LDL concentrations after HCD consumption have been recorded in earlier studies and shown to contribute to the development of atherosclerotic plaques [36]. On the other hand, it is suggested that HCD accelerate atherogenesis through increasing blood viscosity and disturbing the mechanical fragility of atherosclerotic plaques hence making them vulnerable to rupture and thrombosis [37].
Hypercholesterolemia in rabbits is due to the accumulation of exogenous cholesterol. Rabbits easily developed atherosclerosis by feeding HCD since they are unable to increase sterol excretion [32]. Therefore, high amount of cholesteryl ester-rich lipoproteins was exported into the circulation by the liver causing hypercholesterolemia to occur.
The inconsistent results of the TG levels may be due to variations in responses to HCD and plasma lipid levels in each rabbit. Such variations in lipids will certainly affect the extent of atherosclerosis. Therefore, it is vital to adjust the cholesterol intake and carefully screen the animals before performing the experiment so that rabbits with similar responsiveness to cholesterol will be selected for the experiment [38].
An increase in the HDL levels may be due to the composition of HCD that act as cholesteryl ester transfer protein (CETP) inhibitors. According to Javandoost et al. [39], HDL is elevated by the inhibition of CETP. CETP inhibitors prevent the transfer of cholesteryl esters from HDL towards apo B containing lipoproteins in exchange for triglycerides and therefore increases plasma HDL level [40]. Besides, CETP only exists in humans and a few laboratory animals, such as rabbits, guinea pigs, and hamsters, whereas mice and rats do not [41]. Another possibility of high HDL level is due to deficiency of CETP in the rabbits. A study by Zhang et al. [42] demonstrated that genetic ablation of the CETP gene in rabbits led to increase in plasma HDL.
Besides, the HCD composition could also contribute to the rise of HDL level. The HCD (SF00-221) contains several vitamins such as vitamin A, vitamin K, vitamin E, vitamin B1, vitamin B2, niacin (Nicotinic acid), vitamin B6, calcium pantothenate and vitamin B12. Some of these vitamins, for example, niacin, are evident to raise HDL level by decreasing the hepatic CETP expression and plasma levels of CETP [43]. Furthermore, niacin has been shown to selectively increases the plasma levels of apolipo-protein-A1 in patients with low HDL [44].
The histological results of this study agree that the severity of the atherosclerotic lesions in the aorta is associated with hypercholesterolemia, which is one of the most important risk factors of endothelial dysfunction in human arteries [45, 46]. In this study, the histology analysis confirmed that HCD can cause atherosclerotic lesions formation in a time-dependent manner. These histopathological results are parallel to the biochemical and morphological findings in this study. This is in agreement with other literatures that showed HCD feeding is responsible for the thickening of tunica intima and the formation of foam cells [28, 46].
Medium to high expressions of adhesion molecules, such as ICAM-1, VCAM-1 and
e-selectin were observed in the aortas of rabbits fed with HCD. This represents
the initiation of the lesion stage, where the endothelial cells have been
stimulated by the ox-LDL to express the adhesion molecules, which could lead to
the recruitment of monocytes and transmigrate into the intima by diapedesis as
well as differentiate into pro-atherogenic macrophages [47]. An increased
expression of pro-inflammatory biomarkers, such as IL-6, IL-8 and NF-
As scientifically proven in our study, there are several inflammatory biomarkers that participate in all stages of atherosclerosis. The inflammatory processes incite the induction and development of atheroma, and at the same time contribute significantly to hastening the acute thrombotic complications of atheroma [49]. Interestingly, a previous study found that treatment with canakinumab reduced inflammatory biomarkers without reducing LDL levels, resulting in a significantly lower incidence of recurrent cardiovascular events [50].
Therefore, the comprehensive method in this study can be useful for the researchers, as most of the current studies not only focused on LDL lowering agents but also on the suppression of inflammatory processes [51], including inflammasomes. Recently, emerging evidence showed that inflammasomes play a significant role in the inflammatory processes related to atherosclerosis and other cardiovascular diseases. Accumulating evidence supports the notion that the activation of inflammasomes such as nucleotide-binding domain leucine-rich repeat pyrin domain containing 1 (NLRP1), nucleotide-binding domain leucine-rich repeat pyrin domain containing 3 (NLRP3), nucleotide-binding domain leucine-rich repeat family caspase activation and recruitment domains (CARD) containing 4 (NLRC4), and absent in melanoma 2 (AIM2) triggered by cardiovascular disease risk factors plays a pivotal role in the progression of atherosclerosis [52].
In recent years, the role of gasdermins in atherosclerosis has received much
attention. Gasdermins are a family of pore-forming effector proteins that cause
membrane permeabilization and pyroptosis, a lytic form of cell death [53]. One of
these proteins, gasdermin D (GSDMD), has been identified as the final executor of
the inflammasome activity. A recent study has shown that GSDMD is involved in
multiple pro-inflammatory pathways that promote atherosclerosis progression [54].
Besides, interesting results from a previous study found that a deficiency in
GSDMD reduced the formation of inflammatory plaques and delayed the progression
of atherosclerotic plaque in
However, further investigations and more research are needed to explore these areas so that promising therapeutic approaches can be developed for the treatment and prevention of atherosclerosis. Future studies aimed at identifying and describing new triggers and mechanisms regulating atherosclerosis development are encouraged to use the method described in this study.
This study confirms that 50 g/kg of 1% HCD feeding for 4- and 8-weeks duration is effective in inducing early and established atherosclerosis in NZWR. This is evident by the significant increase in the body weight, LDL and TC levels in the intervention groups which are in parallel with the histological and immunohistochemical results. Compared to other methods, HCD feeding is a non-time consuming, easy to maintain and affordable method that can be used in atherosclerosis studies. However, researchers need to be extra careful in planning the experiment that relates to HDL. Nevertheless, this study could provide new insights into the understanding of atherosclerosis and expand the power of the rabbit model for translational research in atherosclerosis besides facilitating future research experiments in discovering potential preventions and treatments to reduce atherosclerosis.
All data generated or analyzed during this study are included in this published article.
NAMK, EO and HN designed the research study. INAR performed the research. NAMK, EO, SAM and HN provided help and advice on conception, acquisition of data and supervision. INAR, NAMK and EO analyzed the data. INAR wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
All experiments were conducted in an appropriate animal experimentation facility. The animal experiments in this study were approved by and conducted in conformity with the rules and regulations of the Universiti Teknologi MARA Committee on Animal Research & Ethics (UiTM CARE). The ethical approval number was UiTM CARE: 326/2020.
The authors would like to acknowledge the Institute of Medical Molecular Biotechnology (IMMB) and Laboratory Animal Care Unit (LACU) of Faculty of Medicine, Universiti Teknologi MARA for providing the facilities to complete this study.
This research was funded by the Ministry of Higher Education Malaysia under Fundamental Research Grant Scheme, grant number (FRGS) 600-IRMI/FRGS 5/3 (366/2019).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.










