1 Department of Applied Chemistry, Dong-Eui University, 614-714 Busan, Republic of Korea
Abstract
Background and Aims: Normal cells become tumorigenic owing to mutations in
oncogenes and tumor suppressor genes modulating cell division. Cancer cells break
down extracellular matrix to metastasize other tissues. Therefore, the
development of natural and synthetic substances that suppress metastatic enzymes
such as matrix metalloproteinase (MMP)-2 and MMP-9 is useful to inhibit metastasis. Silibinin is the main
ingredient of silymarin extracted from the seeds of milk thistle plants having
lung cancer-suppressing effects and liver protection. The purpose
of this study was to investigate the inhibitory effect of silibinin on the
invasion of human fibrosarcoma cells. Methods: The effect of silibinin
on cell viability was measured in HT1080 cells using an MTT assay. The MMP-9 and
MMP-2 activities were analyzed using a zymography assay. The expression of
proteins in cytoplasm related to metastasis was examined by western blot analysis
and immunofluorescence assay. Results: In this study, silibinin above 20
Keywords
- silibinin
- invasion
- IL-1β
- p-p38
- MMPs
Cancer cells that develop as a result of chronic inflammation move to peripheral tissues via blood vessels through angiogenesis. To metastasize to surrounding tissues, cancer cells need to degrade the extracellular matrix. In particular, the gelatinases such as matrix metalloproteinase (MMP)-2 and MMP-9 among MMPs degrade collagen 4, the main component of the basement membrane [1], involved in angiogenesis and metastasis. Therefore, it is crucial to regulate the expression of MMPs to limit the metastatic ability of cancer cells. Therefore, treatment of tumor cells with a substance that inhibits the expression of those inflammatory cytokines involved in MMPs regulation is also expected to limit metastasis formation.
While screening medicinal plants for anti-metastasis research, it was found that
the inhibitory effect of milk thistle (Cirsium japonicum) extract was
excellent. The silibinin employed in this study corresponds to 50–70% of the
three isomers of silymarin which makes up roughly 2% of milk thistle’s active
component [2]. It has been known to have a role in the anti-tumor drug
cisplatin’s hepatoprotection, antioxidation, anti-angiogenesis, inhibition of
inflammation, and nephrotoxicity [3]. The action mechanism of silibinin on
metastasis remains unclear. The great efficacy of silibinin to target cancer
cells’ migratory and invasive features as well as their capacity to metastasize
to distant organs has also been demonstrated in recent pre-clinical trials.
According to thorough mechanistic investigations, silibinin targets signaling
molecules that control the epithelial-to-mesenchymal transition (EMT), activation
of proteases, adhesion, motility, and invasiveness as well as the components of
the supporting tumor microenvironment, preventing metastasis [4]. Therefore, we
tried to investigate whether silibinin could inhibit cell invasion and MMPs in
the model of human fibrosarcoma cells (HT1080 cell line) widely used for the
study of metastasis. Moreover, the expression of proteins such as MAPKs and
IL-1
Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS),
Trypsin-EDTA, and antibiotics such as penicillin (10,000 U/mL)/streptomycin
(10,000
HT1080 cell line (ATCC No.CCL-12, Homo sapiens, fibroblast, lung) and IMR90 cell
line (ATCC No.CCL-186, Homo sapiens, fibroblast) purchased from ATCC (American
Type Culture Collection) were cultured using DMEM containing 10% of FBS and
subcultured with trypsin-EDTA. Antibiotics such as
penicillin/amphotericin/streptomycin were used to prevent cell culture from
bacterial contamination. Mycoplasma testing was performed to authenticate the
cell lines used in this study. This was accomplished using the
MycoAlert™ Mycoplasma Detection Kit (Lonza, Bend, OR, USA), which detects
enzymatic activity associated with viable mycoplasma in cell cultures. Briefly,
cells were harvested and lysed, and the resulting lysate was incubated with the
MycoAlert™ substrate for 10 minutes at 37 °C. The
fluorescence of each sample was then measured to check mycoplasma
contamination. The silibinin was freshly dissolved in dimethyl sulfoxide (DMSO) as
a solvent before use. Silibinin at 2.5, 5, 10, 15, 20, and 25
Silibinin (500
Different doses of silibinin (6
The growth inhibitory effect of silibinin on HT1080 cells was evaluated using
3-(4,5-Dimethyl-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) [5]. The cells at a
density of 5
The activities of MMP-2 and MMP-9 were examined using gelatin zymography
according to a previous study [6]. HT1080 cells were cultured in the presence of
silibinin at 2.5, 5, 10, 15, 20, and 25
Western blot analysis was carried out according to standard procedures. HT1080
cells were exposed to silibinin at 2.5, 5, 10, 15, 20, and 25
HT1080 cells were cultured in a slide chamber at 37 °C for 24 hours. After treatment with each concentration of silibinin, for 1 h, PMA at 1 ng/mL was added and incubated for 24 h. After fixing with 10% formalin for 15 min, the cells were permeabilized with phosphate-buffered saline (PBS) containing 0.5% of Tween 20 (PBS T-20) for 30 min and washed 3 times with 0.1% PBS T-20. After blocking with 5% of donkey normal serum, primary antibodies (MMP-2) were added for 2 h. Then, after washing 3 times for 5 min each with 0.1% PBS T-20, secondary antibodies (donkey anti-goat conjugated CY3, donkey anti-mouse conjugated CY3, donkey anti-rabbit conjugated FITC) were added for 1 h. Then, after washing them, the slides were exposed to DAPI reagent for nuclei staining and observed with the iRiS Digital Cell Imaging System (Logos Biosystems, Gyeonggi-do, Korea). All reagents were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
The invasion of HT1080 cells was carried out in accordance with the
Chemicon® methodology. The invasion chamber from the Cell
Invasion Assay Kit (ECM550) consists of a 24-well tissue culture plate and 12
cell culture inserts containing polycarbonate membrane (8.0
Data were analyzed using ANOVA and post hoc (Duncan) test as means of values
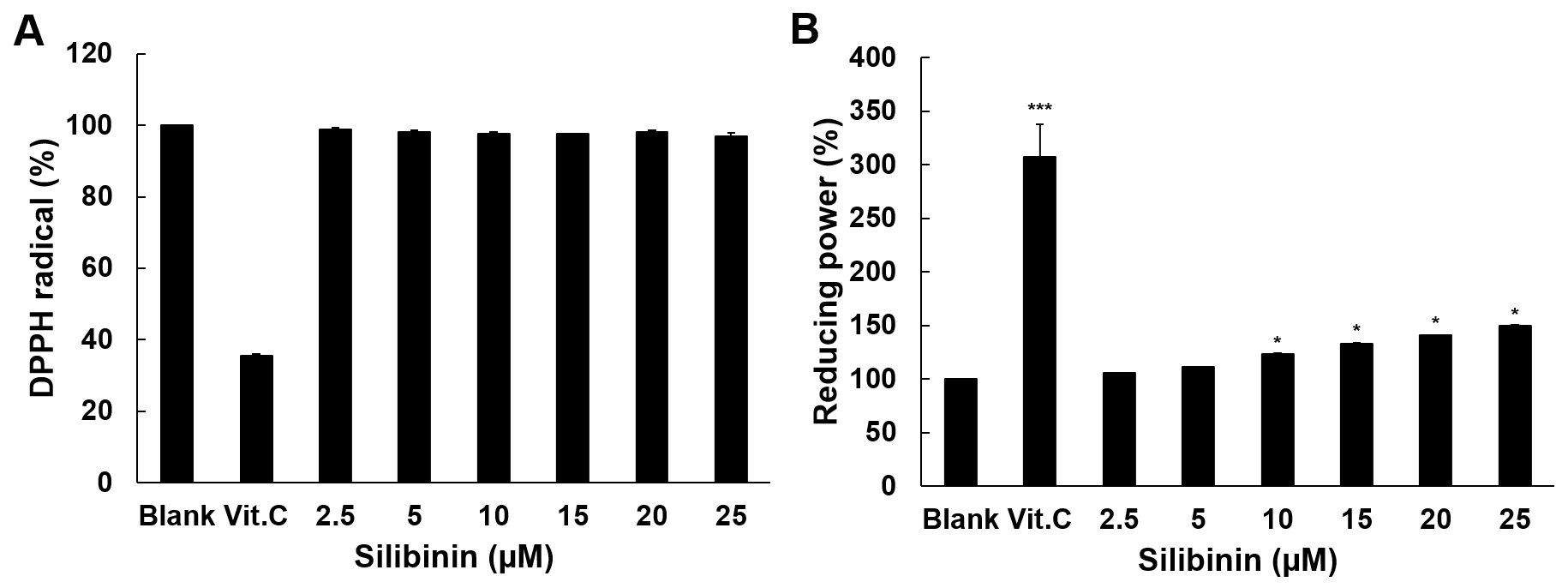
The antioxidant activity of silibinin was examined using the DPPH radical
scavenging assay and the reducing power assay. Vitamin C (Vit. C) at 100
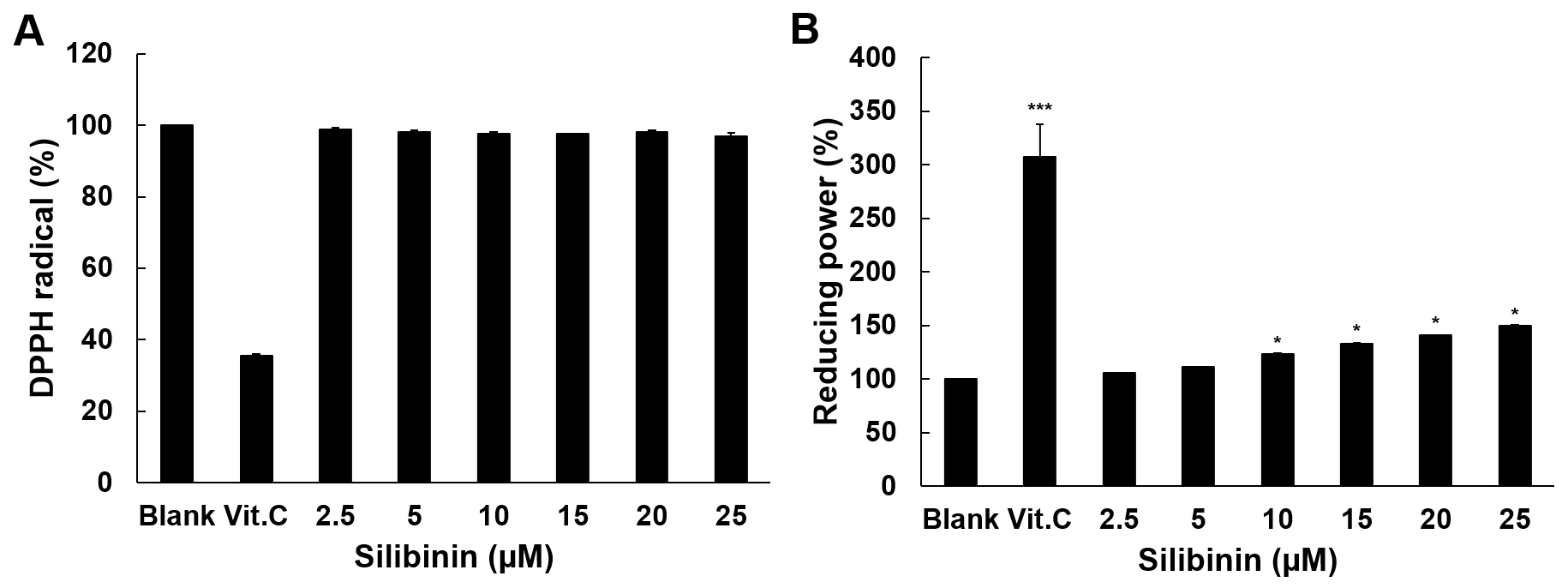 Fig. 1.
Fig. 1.Antioxidant activity of silibinin. (A) The scavenging effect of
silibinin on DPPH radicals is shown in this experiment. Vitamin C was used as
positive control at 100
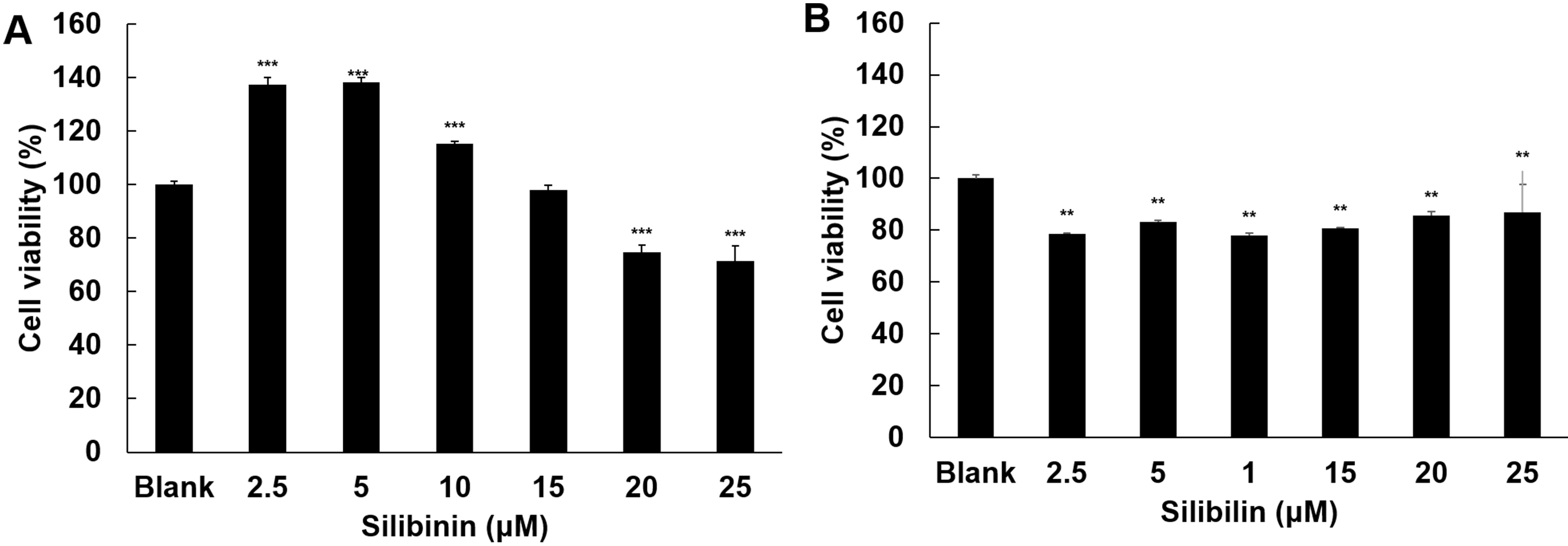
The effect of silibinin on cell viability was examined in HT1080 and IMR-90
cells. In HT1080, silibinin at low dose (2.5
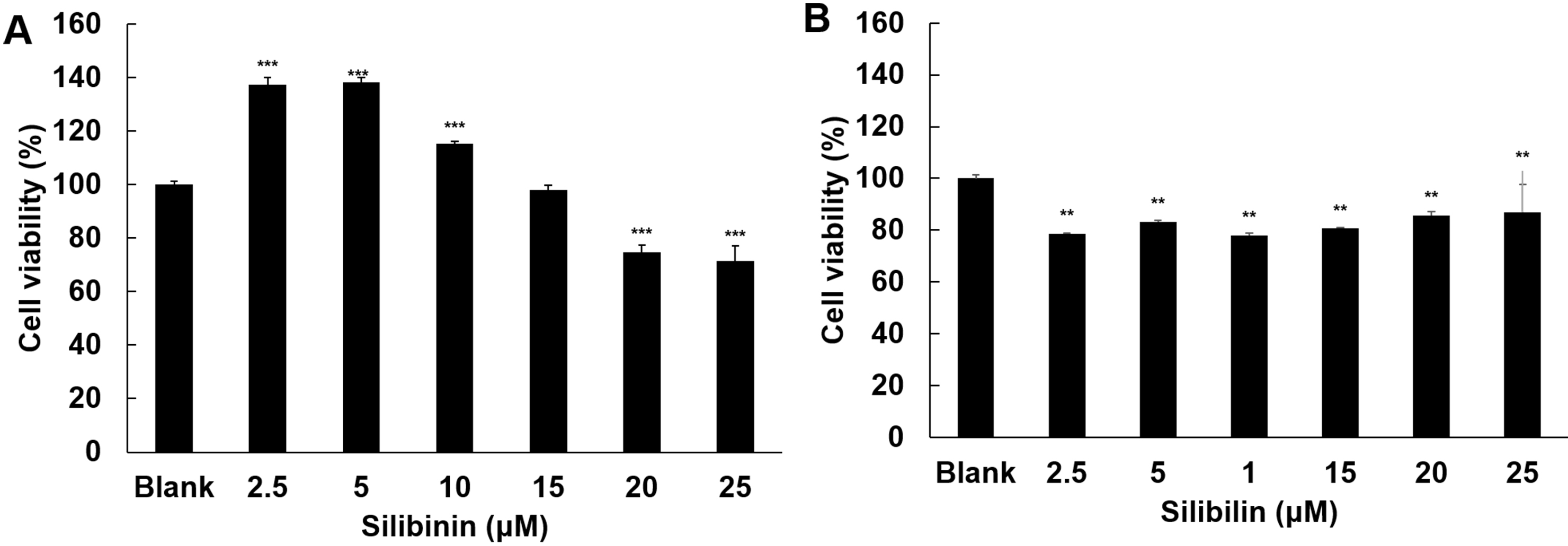 Fig. 2.
Fig. 2.Effect of silibinin on cell viability. The effects of silibinin
on cell viability were examined in HT1080 cells (A) and IMR-90 cells (B),
respectively. The cells were treated with silibinin at 2.5, 5, 10, 15,
20, and 25
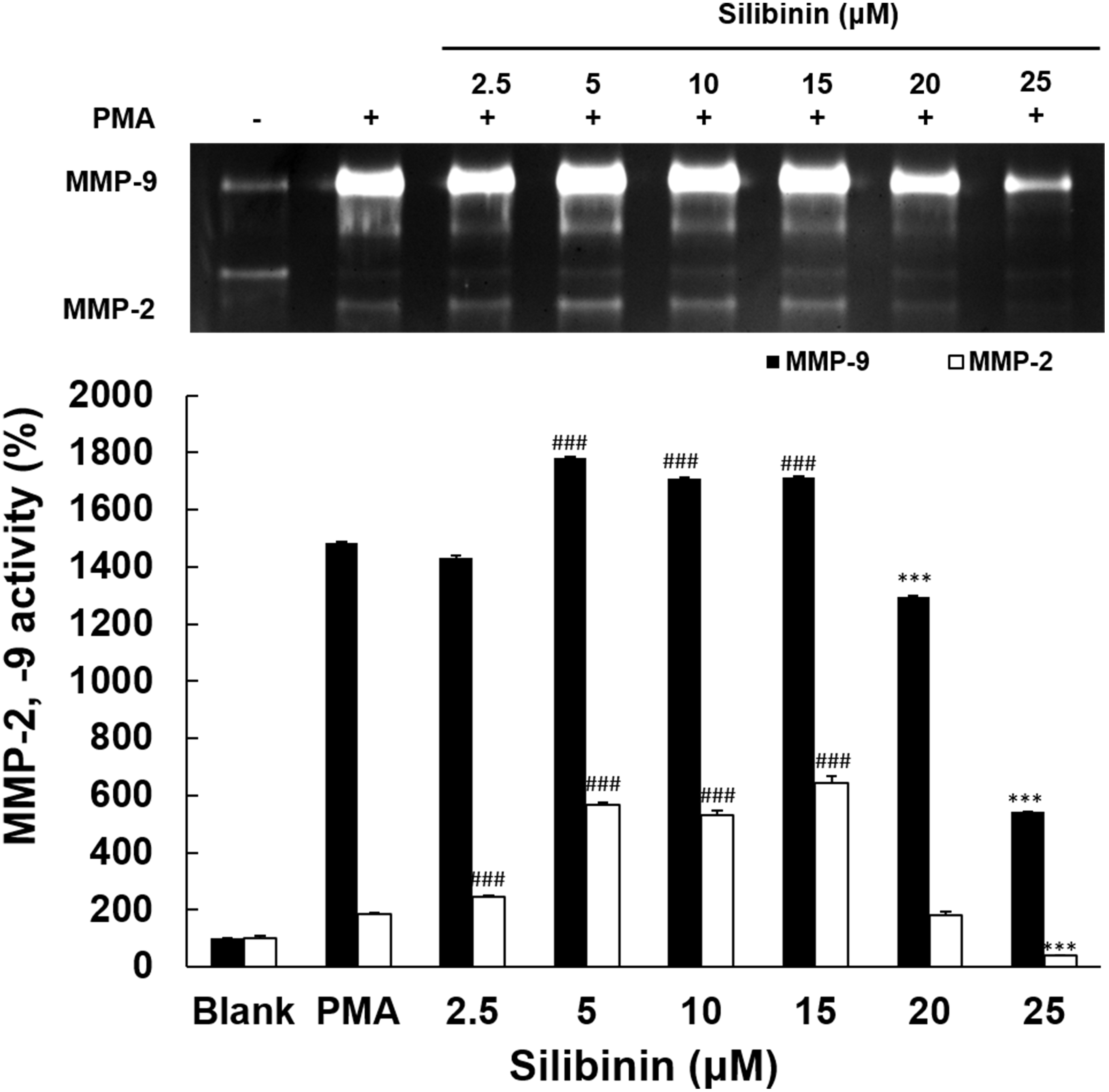
The effect of silibinin on MMPs activity was examined in HT1080 cells. Silibinin
at low doses had no inhibitory effect, and only at the two higher doses of 20
 Fig. 3.
Fig. 3.Effects of silibinin on MMPs activation. The inhibitory effects
of silibinin on the inhibitory activities of MMP-9 and MMP-2 was evaluated in
PMA-stimulated HT1080 cells to induce MMPs expression. The silibinin at 2.5, 5,
10, 15, 20, and 25
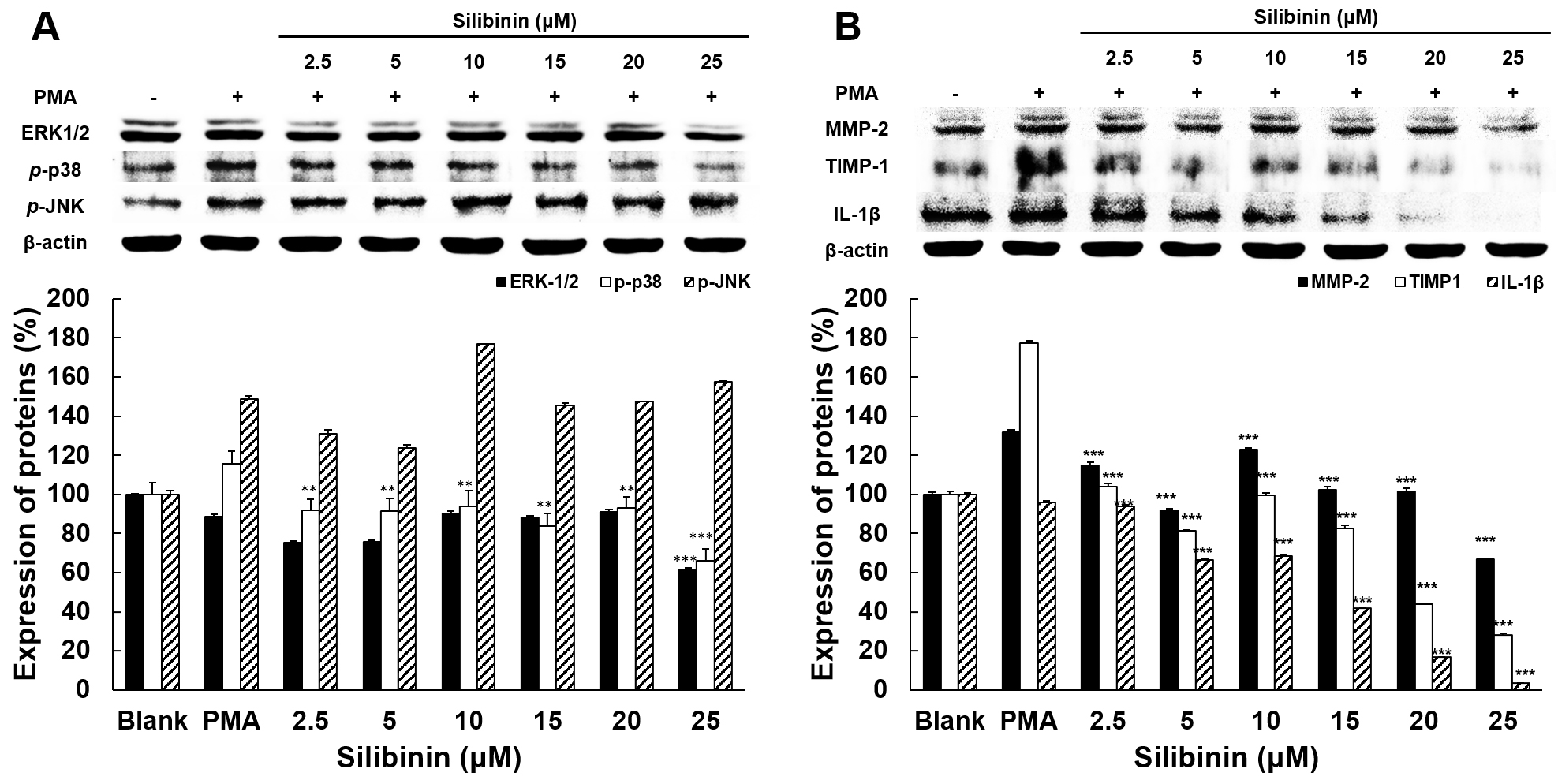
The expressions of MAPK and other mediators regulating MMPs were examined to
elucidate how silibinin influences the regulation of MMP-2 and MMP-9 expression
and activity. The expression of ERK-1/2, p-p38, p-JNK (Fig. 4A), MMP-2, TIMP-1, and IL-1
 Fig. 4.
Fig. 4.The effect of silibinin on the expression of proteins associated
with invasion and metastasis in HT1080 cells. (A) Effects of silibinin on the
expressions of ERK-1/2, p-p38, and p-JNK. (B) Effects of
silibinin on the expressions of MMP-2, TIMP-1, and IL-1
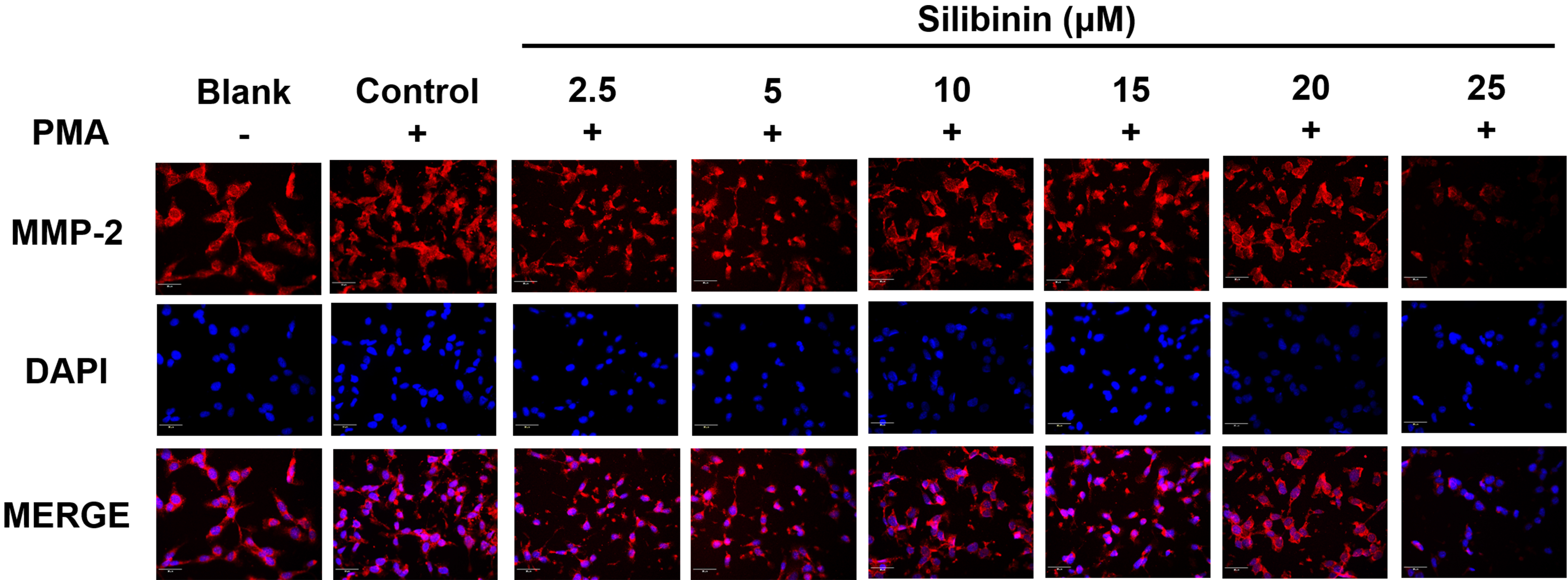
To investigate the effect of silibinin on the expression of metastasis-related
proteins, immunofluorescence staining of MMP-2 was performed in PMA-stimulated
HT1080 cells, with or without silibinin treatment. The cell’s nuclei were
observed in blue color after being stained with DAPI. MMP-2 were stained with CY3
and displayed in red color. Silibinin treatment at 25
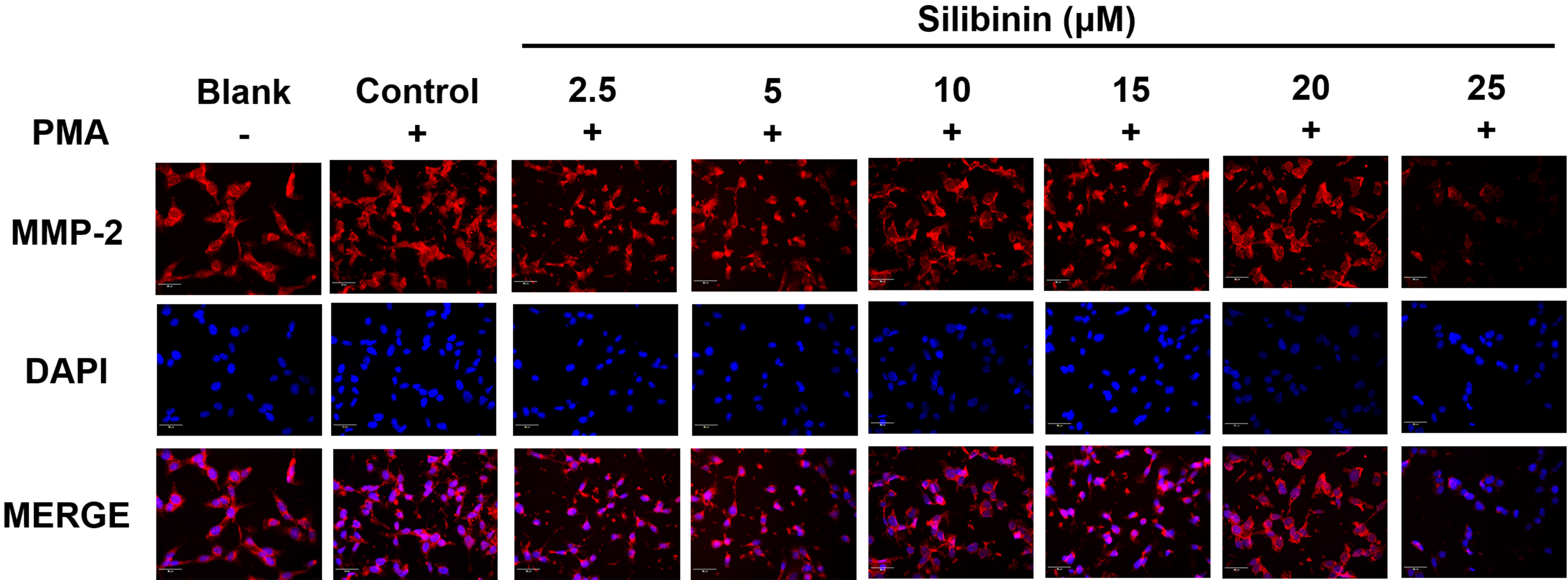 Fig. 5.
Fig. 5. The immunofluorescence staining analysis of MMP-2. HT1080 cells
were treated with silibinin at 2.5, 5, 10, 15, 20, and 25
Tumor cells degrade collagen in the extracellular matrix to obtain more
nutrients and make space to move into other tissues through blood vessels.
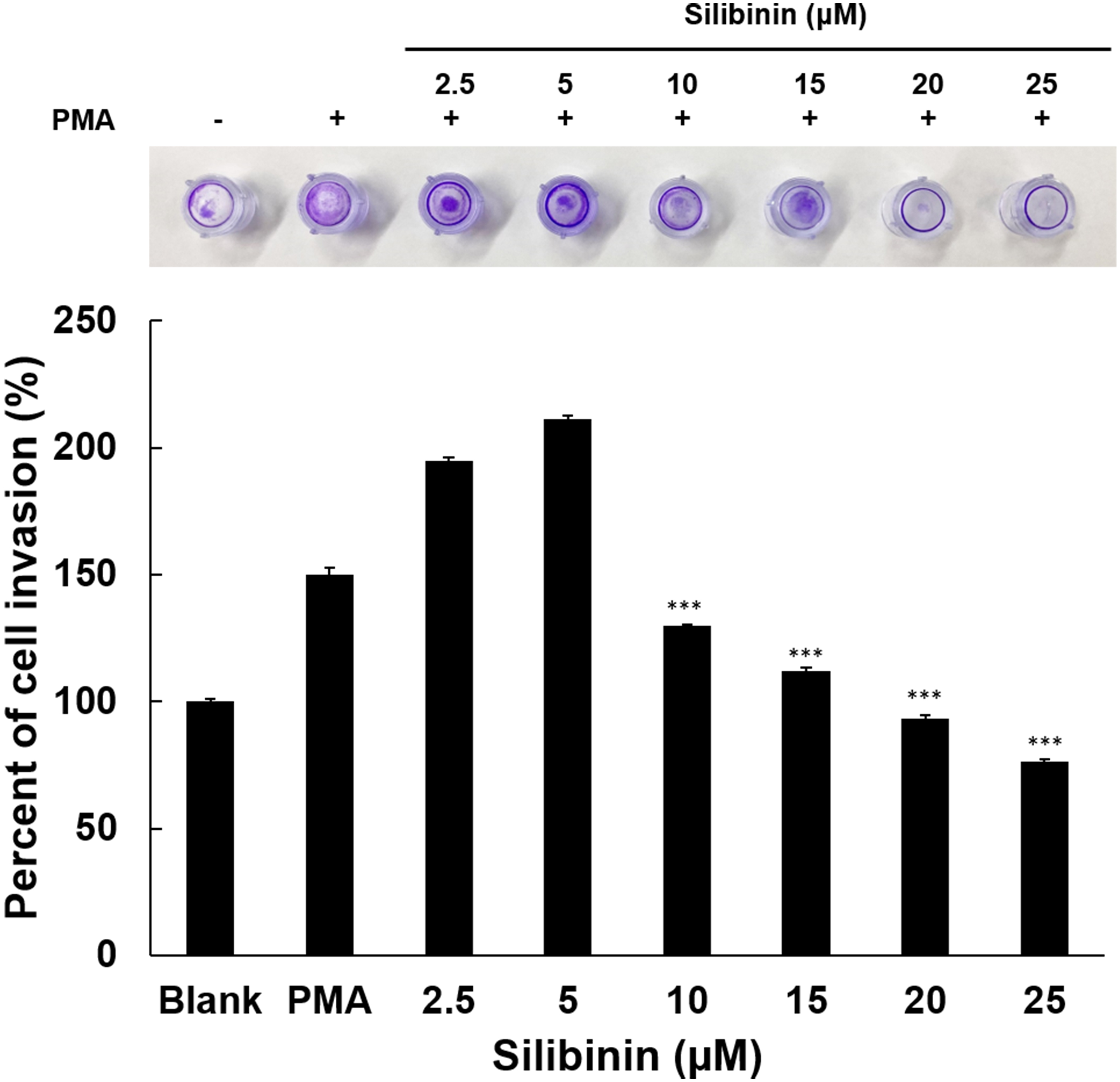
Therefore, in this study, an invasion assay was performed using HT1080 cells
stimulated with PMA, in order to evaluate the efficacy of silibinin in the
inhibition of cell invasion. Paradoxically, though in line with data on cell
growth and effects on MMP2, silibinin treatment at low doses promoted invasion,
while at concentrations above 10
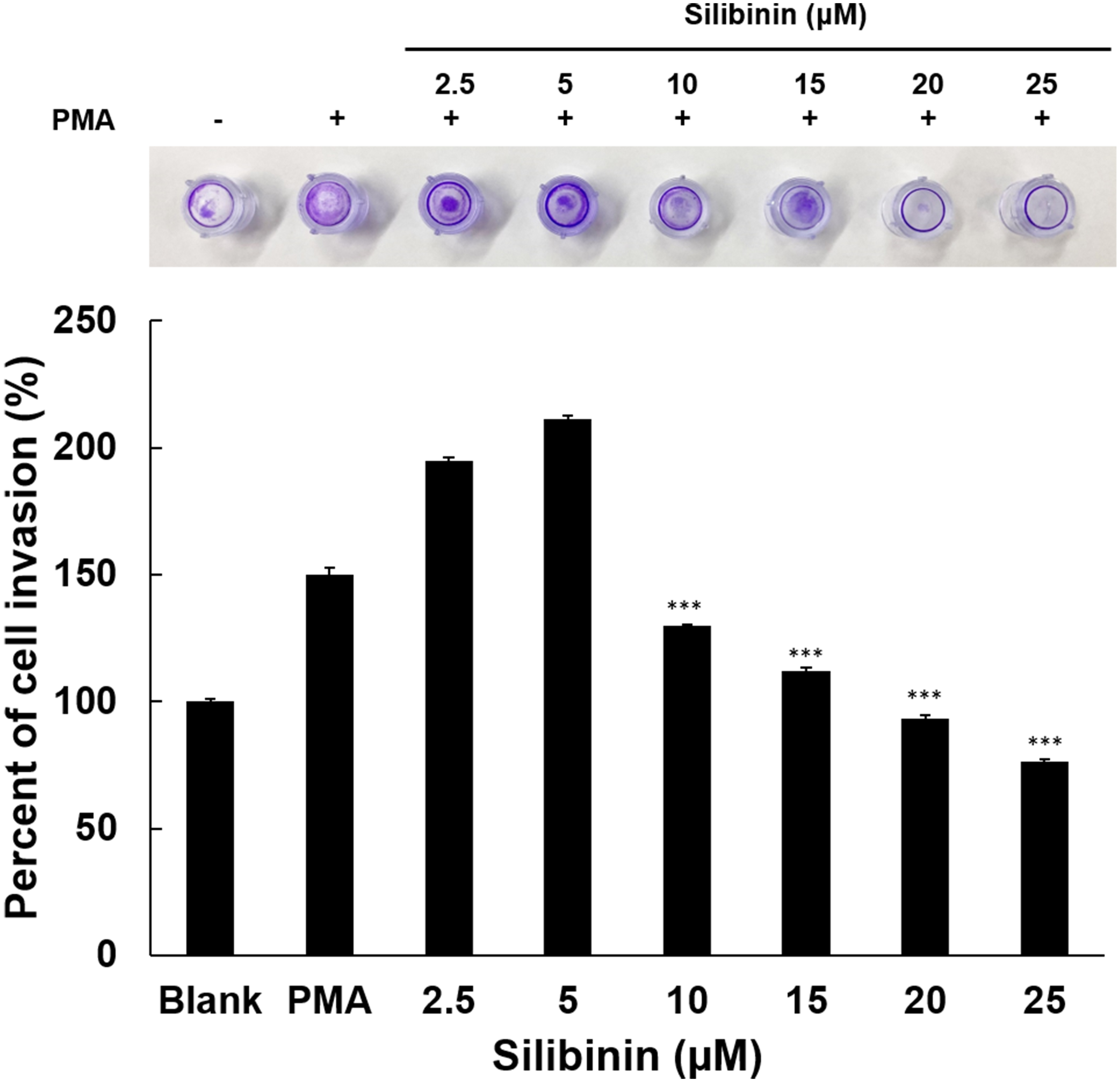 Fig. 6.
Fig. 6.Effects of silibinin on HT1080 cell invasion. Cell penetration
into the ECM layer through the polycarbonate membrane was examined in the
presence of silibinin. Using a 24-well tissue culture plate with an insert and a
polycarbonate membrane with an 8
Chronic inflammation and the continuous release of inflammatory cytokines may
contribute to cancer development and its evolution into a malignancy that demands
oxygen and nutrition over time. As a result, matrix metalloproteinases (MMPs) can
be induced in growing cancers, when the surrounding nutrients are low. MMP-2 and
MMP-9 in particular digest collagen IV, a critical component of the extracellular
matrix. Cancer cells then may reach and travel through the bloodstream, finally
infiltrating metastatic cells into other permissive tissues. Therefore, blocking
cell invasion may play a key role in cancer therapy and the prevention of cancer
metastasis. In this study, we have shown that silibinin at the highest doses
tested may suppress MMP-9 and MMP-2 activity and expression, as well as
IL-1
However, the doses of silibinin here used could inhibit the activity of gelatinases such as MMP-2 and MMP-9 in HT1080 cells previously stimulated with PMA. This effect is consistent with previously published results showing that silibinin inhibited the activity of MMP-2 and MMP-9 in osteoblasts [13].
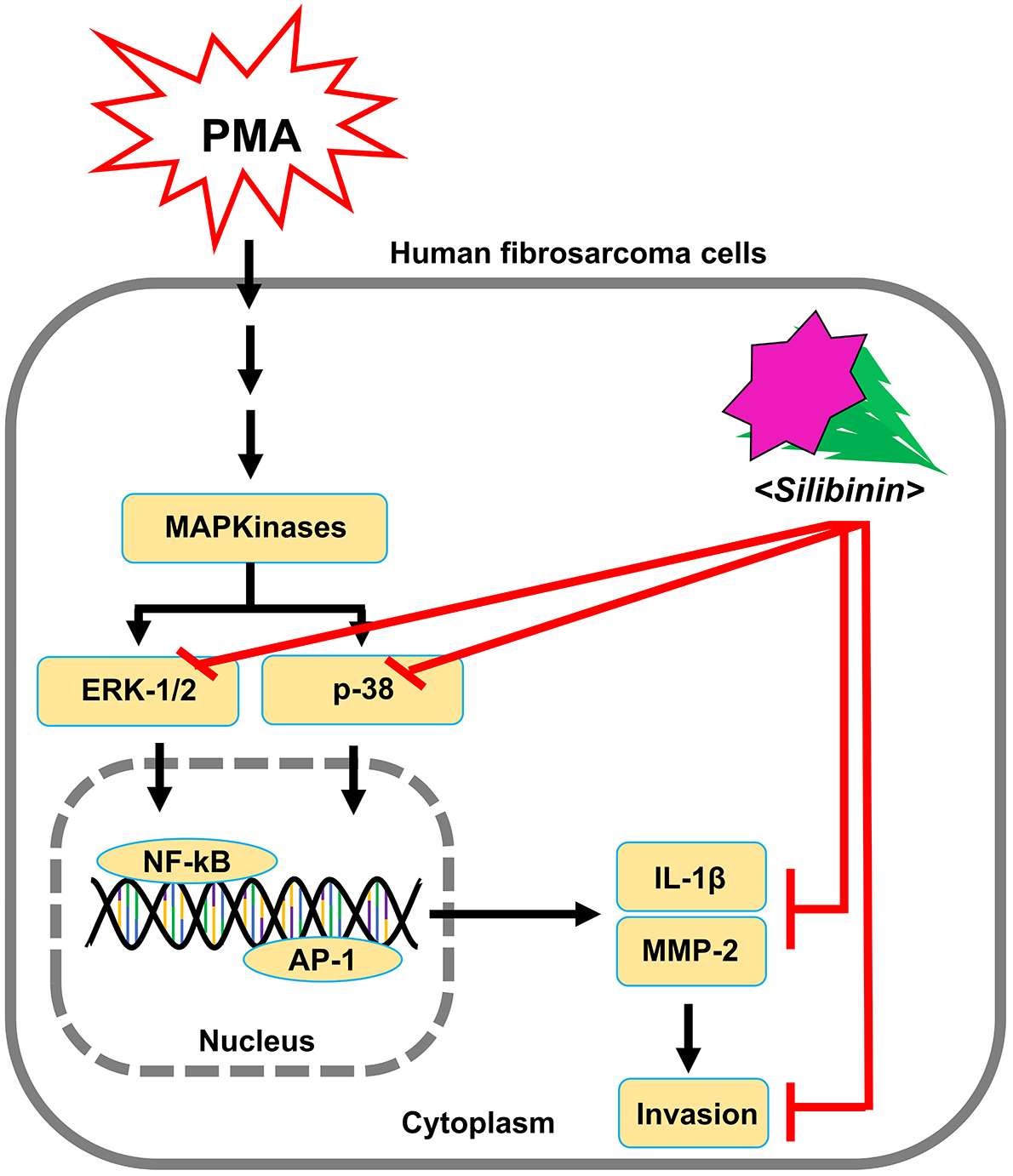
During metastasis, AP-1 and NF-kB are the main mediators that promote the
transcription of tumor necrosis factor (TNF)
In addition, our results indicate that silibinin could modulate the MAPK
signaling pathway of ERK and p38 via IL-1
 Fig. 7.
Fig. 7.Schematic diagram for the effect of silibinin on invasion and metastasis of HT1080 cells stimulated by PMA.
In the end, this study shows a paradoxical effect of silibinin on HT1080 cells. At low doses, it appears to promote growth and MMPs expression, thus favoring invasion. Only at the higher doses it shows inhibitory effects on these parameters, and therefore it might be supposed to inhibit the metastatic ability of cancer cells. In vivo, it is not predictable which dose will reach tumor cells within the tumor mass, and so it remains also unpredictable which effect is expected, whether promotion or inhibition of invasion and metastasis. More studies in vivo, at different doses, will be necessary to elucidate the pharmacokinetics and the pharmacodynamics of silibinin, in order to identify a dose which will exert the desired effect of preventing cell invasion and metastasis.
ATCC, American Type Culture Collection; DMEM, Dulbecco’s Modified Eagle’s
Medium; DMSO, dimethyl sulfoxide; DPPH, 2,2-diphenyl-1-picrylhydrazyl; ERK,
extracellular signal-controlled kinases; FBS, fetal bovine serum; HT1080, human
fibrosarcoma cells; IL-1
The data used to support the findings of this study are available from the corresponding author upon request.
AIJ performed the experiments, analyzed the data, and assisted in writing the manuscript. Prof MMK proposed the concept, designed the experiment, analyzed the data, and revised the manuscript. Both authors read and approved the final manuscript and agree to be accountable for all aspects of the research.
Not applicable.
We wish to thank Sojeong Jeon for her kind advice and help with this study.
This research received no external funding.
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/j.fbl2804064.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.