1 Department of Neurology, Shanghai East Hospital, Tongji University School of Medicine, 200120 Shanghai, China
2 Department of Neurology, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, 200080 Shanghai, China
3 School of Medicine, Tongji University, 200092 Shanghai, China
4 Department of Joint Surgery & Shanghai Institute of Stem Cell Research and Clinical Translation and Institute for Regenerative Medicine, Shanghai East Hospital, Tongji University School of Medicine, 200120 Shanghai, China
5 Shanghai Clinical Research Center for Aging and Medicine, 200040 Shanghai, China
†These authors contributed equally.
Abstract
Background: Parkinson’s disease (PD) is a common selective and
progressive neurodegenerative disorder of nigrostriatal dopaminergic (DA)
neurons. Quercetin is a bioflavonoid with antioxidant, anti-inflammatory,
anti-aging and anti-cancer properties. However, the exact mechanism by which
quercetin exerts its protective effect on DAergic neurons remains unclear.
Purpose: To investigate the underlying molecular mechanism of
quercetin’s protective effect on DA neurons using 1-methyl-4-phenylpyridinium (MPP
Keywords
- Parkinson's disease
- quercetin
- Nrf2-dependent signaling pathway
- ferroptosis
Parkinson’s disease (PD) is a common, age-related, chronic, disabling
neurodegenerative disease [1]. It is characterized by an abnormal accumulation of
The pathogenesis of PD has been shown to be related to oxidative stress and lipid peroxidation-induced damage and death of DA neurons [4]. Excess reactive oxygen species (ROS) generated by multiple pathways play an important role in the progression of several neurodegenerative diseases, including PD [5, 6]. Ferroptosis is an iron-dependent and caspase-independent form of regulated cell death initiated by intracellular reduced glutathione (GSH) and lipid peroxidation [7]. The process of ferroptosis involves iron-dependent lipid oxidation [8] and GPX (glutathione peroxidase) is the first line of defense against mitochondrial damage and oxidative stress [9], which directly reduces phospholipid hydroperoxides and oxidized lipoproteins in biological membranes [10, 11].
Nuclear factor erythroid 2-related factor 2 (Nrf2), expressed in various brain cells including astrocytes, microglia and DA neurons, is a redox-sensitive regulator of antioxidant enzymes. Previous studies have shown that the Nrf2 signaling pathway plays a role in protecting against neuroinflammation, stroke, and oxidative damage-induced neurodegeneration [12, 13], and helps to defend against oxidative and toxic neurological damage [14]. In the resting state (i.e., non-oxidative stress), Kelch-like ECH-associated protein 1 (Keap1) binds to Nrf2, leaving it in an inactive state in the cytoplasm, where it is degraded by proteasomal ubiquitination [15, 16]. However, under oxidative stress, Keap1 dissociates and translocates Nrf2 from the cytoplasm to the nucleus, where it activates antioxidant response element (ARE)-mediated gene transcription of detoxification and antioxidant enzymes [17] [including glutathione peroxidase (GPX), oxidoreductase (NQO1), and superoxide dismutase (SOD)] to maintain cellular redox homeostasis [18]. In addition to regulating antioxidant enzymes, Nrf2 signaling pathways have anti-inflammatory [19], anti-fibrosis [20], and anti-apoptotic [21] effects. Furthermore, since the development and progression of PD have been associated with ferroptosis [22] targeting Nrf2, an antioxidant transcription factor that inhibits iron degeneration, may be an attractive new option for the treatment of PD [23, 24].
Quercetin is a catecholic flavonol that undergoes enzymatic decomposition during plant metabolism [25] into a dimer that with weak antioxidant and antiferritin activities [26, 27]. Thus, it can coexist in the same plant with its dimer metabolite quercetin Diels-Alder anti-dimer [28]. Quercetin has several pharmacological effects, including antioxidant, anti-inflammatory, anti-cancer, anti-allergic , and anti-aging [29, 30, 31, 32]. For example, studies have shown that quercetin can inhibit ferroptosis, thereby improving the symptoms of kidney damage, type 2 diabetes, and several other diseases [33, 34]. Another study showed that the antioxidant properties of quercetin helped prevent ferroptosis in the liver of mice fed a high-fat diet and reduced fat accumulation, iron overload, lipid peroxidation, and inflammation [35]. Thus, quercetin has a broad therapeutic potential and may be useful for the treatment of many different diseases [36, 37, 38], as well as being a candidate for preventive medicine. However, its potential application in the treatment of PD still remains unclear.
Prior studies have shown that quercetin has a protective effect on DA neurons through anti-lipoperoxidation, mitochondrial function improvement, and cell death inhibition [39, 40, 41]. However, the mechanism underlying its protective effects against cellular lipid peroxidation have not been fully elucidated. Therefore, in the present study, an animal model was used to explore how quercetin achieves anti-lipid peroxidation in PD and we hypothesized that quercetin may protect DA neuronal activity by downregulating ferroptosis.
ML385 (Cat.no.HY-100523), RSL3 (Cat.no.HY-100218a) and Ferrostatin-1 (Cat.no.HY-100579) chemicals were purchased from MCE Corporation (Monmouth Junction, NJ 08852, USA). Quercetin (Cat.no.117-39-5) was obtained from Macklin Biochemical Technology Co., Ltd (Shanghai, China), and the molecular structure of quercetin was shown in Supplementary Fig. 1.
SH-SY5Y cell line was a kind gift from the Department of Neurology, Tongji
Hospital Affiliated with Tongji University. The cells of the human neuroblastoma line (SH-SY5Y) were provided by the Cell Bank/Stem Cell Bank of Type Culture Collection Committee of Chinese Academy of Sciences. The SH-SY5Y cell line was cultured in DMEM-F12 medium supplemented with 10% fetal bovine serum (10100147, Gibco, CA, USA) and 1% Penicillin-Streptomycin (P/S). All cell lines were maintained at 37 °C in a 5% CO
Apoptosis was quantified using the apoptosis detection kit Annexin V FITC/PI
(Cat.no.AP101-100-AVF, Linktech, Hangzhou, China). Briefly, SH-SY5Y/primary
neuronal cells (3
The C11-BODIPY 581/591 (Thermo Fisher Scientific, Waltham, MA, USA) instrument was used to
measure lipid ROS (LPs) levels. Cell medium was added with a final concentration
of 2
A standard curve for MDA was prepared according to the manufacturer’s
instructions (Cat.no.S0131S, Beyotime Biotechnology, Beijing, China) and target
cells were lysed to assess MDA levels. MDA concentration was determined using the
Lipid Peroxidation MDA Assay Kit (Beyotime). MDA levels (nmoL/mL) were calculated
as follows: [sample optical density (OD) value - blank OD value]/(standard OD value - blank OD
value)
The CCK-8 assay kit (Cat.no.RM02823, ABclonal Technology, Woburn,
Massachusetts, USA) was used to quantify cell viability after treatment. The
optical density (OD) of CCK-8 was examined at 450 nm. The cell
survival rate of cells was calculated as follows: OD treatment/OD control
An iron assay kit (Cat.no.BC5415, Solarbio, Beijing, China) was used to measure intracellular iron concentrations.
Cells were fixed with fixative disruptor (eBioscience, Cambridge, UK), stained with 1 mg/mL of GPX4 antibody (Abcam, Cambridge, UK), and then incubated with Alexa Fluor 647-labeled anti-rabbit GPX4 antibody according to the manufacturer’s instructions, and rabbit IgG monoclonal (ab172730; Abcam) was used as primary isotype control. Cell sampls were tested on beckmancytoflex flow cytometer (BDBiosciences). Next, GPX4 expression was measured by flowcytometric analysis using FlowJo 10.4.2 software (Ashland, OR, USA).
Changes in the intracellular MtMP were measured using the JC-1 Mitochondrial Membrane Potential Assay Kit (Cat.no.C2006, Beyotime Biotechnology, China) according to the manufacturer’s instructions, and fluorescence intensity was measured using flow cytometers at wavelengths of 488 nm and 525 nm.
Cell samples were collected and lysed in 1
The preparation of primary cortical cultures was performed as previously described [42]. Briefly, cells were isolated from the cortex of male and female neonatal mice at P0-P1 days postnatally. Primary neuronal cell cultures were prepared as described previously. Microtubule-associated protein (MAP2) was used as a neuronal marker and immunolabelled with rabbit polyclonal MAP2 antibody (Abcam, ab32454, 1:750). MAP2 and 4′,6-diamidino-2-phenylindole (DAPI) were used to stain neurons and nuclei, respectively, see Supplementary Fig. 2.
GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA,
www.graphpad.com) statistical software was used to perform the statistical
analysis. Means were expressed as standard deviations (SD). Statistical analysis
included one-way ANOVAs and the Tukey-Kramer method for multiple comparisons in
all experiments. p
The chemical structure of quercetin is shown in Supplementary Fig. 1.
As previously described, high concentrations of quercetin can induce apoptosis in
certain cells. To determine the optimal concentration of quercetin for treating
neuronal cells, SH-SY5Y/primary neuron cells were coincubated with different
concentrations of quercetin for 24 h, and then cell viability was measured by
CCK-8 assay. Quercetin was found to affect the viability of SH-SY5Y/primary
neuron cells, as shown in Fig. 1A,B. After exposure to lower concentrations of
quercetin (
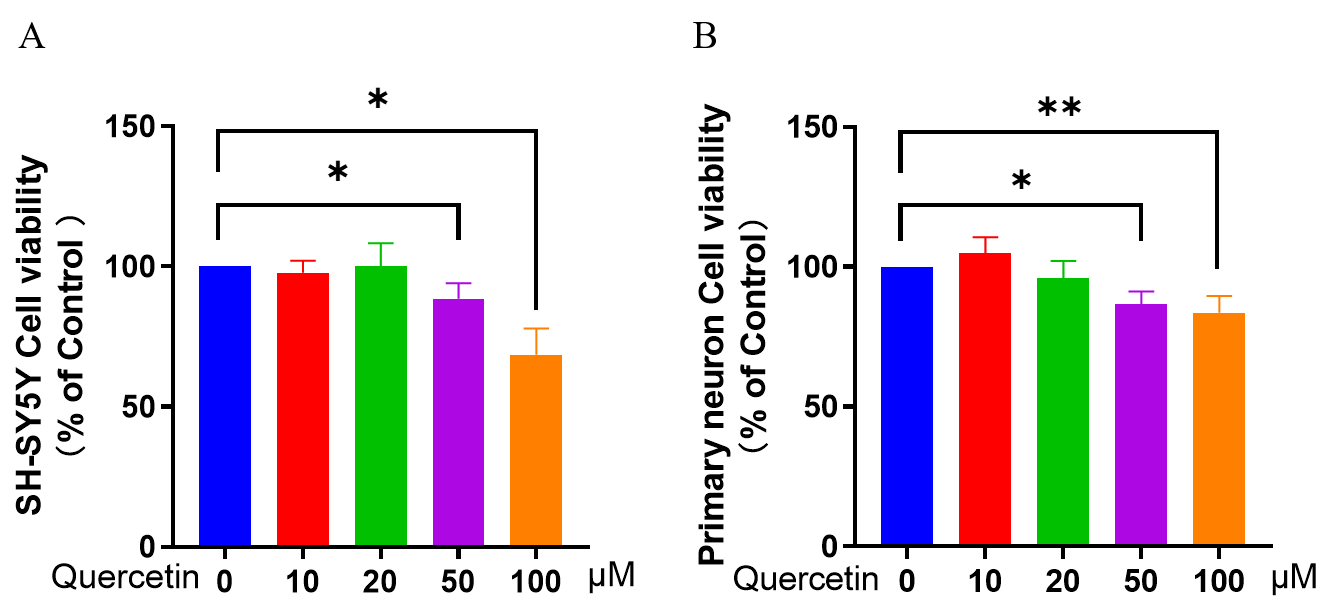 Fig. 1.
Fig. 1.Effect of different concentrations of quercetin on SY-SY5Y/
Primary neuron cell viability. CCK-8 assay was used to determine neuronal
viability. (A) Effect of quercetin on cell viability of SH-SY5Y/Primary neurons.
(B) Effect of quercetin on primary neuron cell viability. Three independent
experiments were used to calculate the mean
Next, the effect of quercetin on neuronal cell apoptosis was examined by flow
cytometry (Fig. 2A,B). Compared to control cells, decreased neuronal cell
viability and increased cell death were observed in the MPP
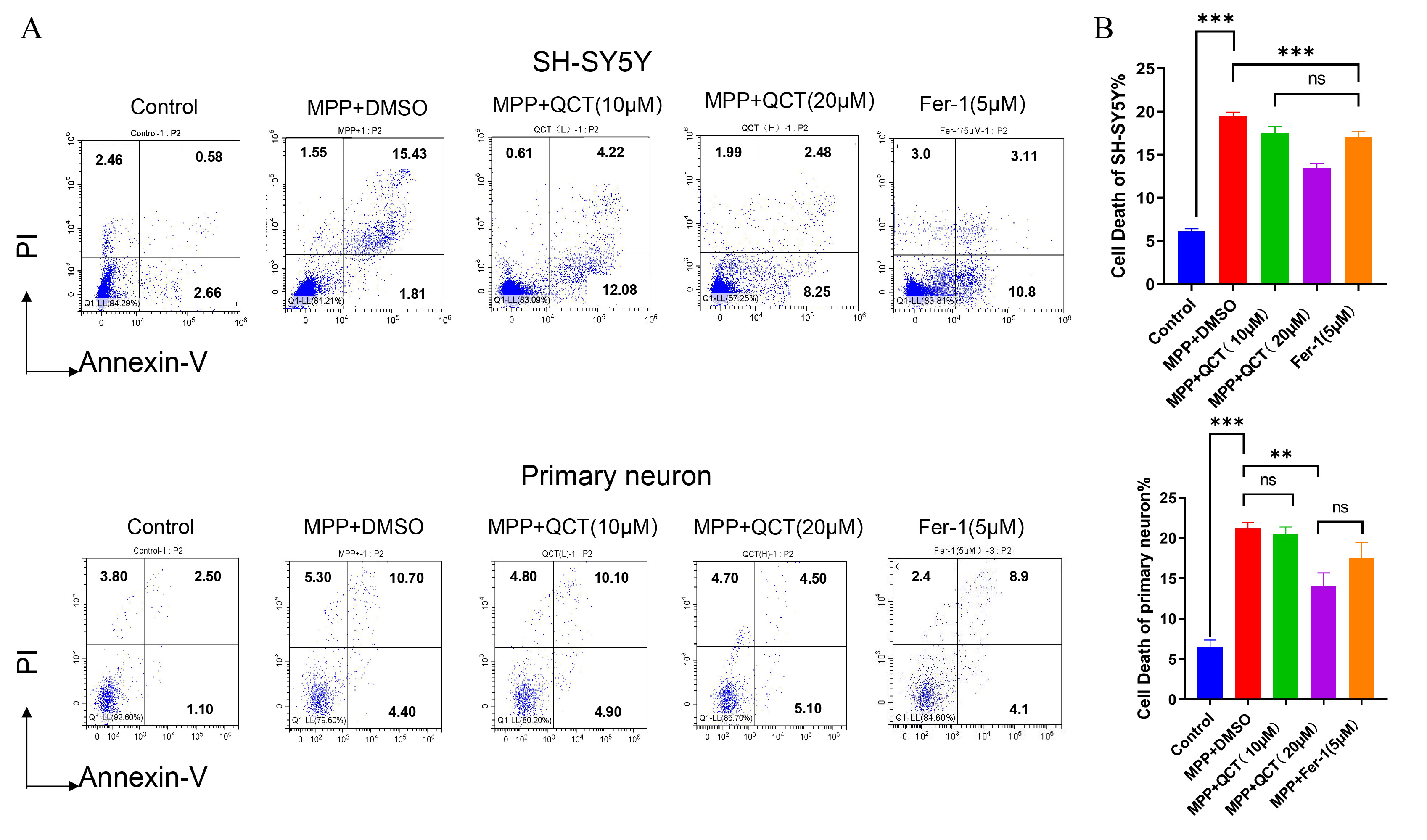 Fig. 2.
Fig. 2.Quercetin reduces MPP
Lipid peroxidation is a key indicator of oxidative stress and plays an important
role in the progression of neurodegenerative diseases, including PD [43]. MDA is
a commonly used marker of lipid peroxidation. In the present study, the MDA level
increased significantly in the MPP
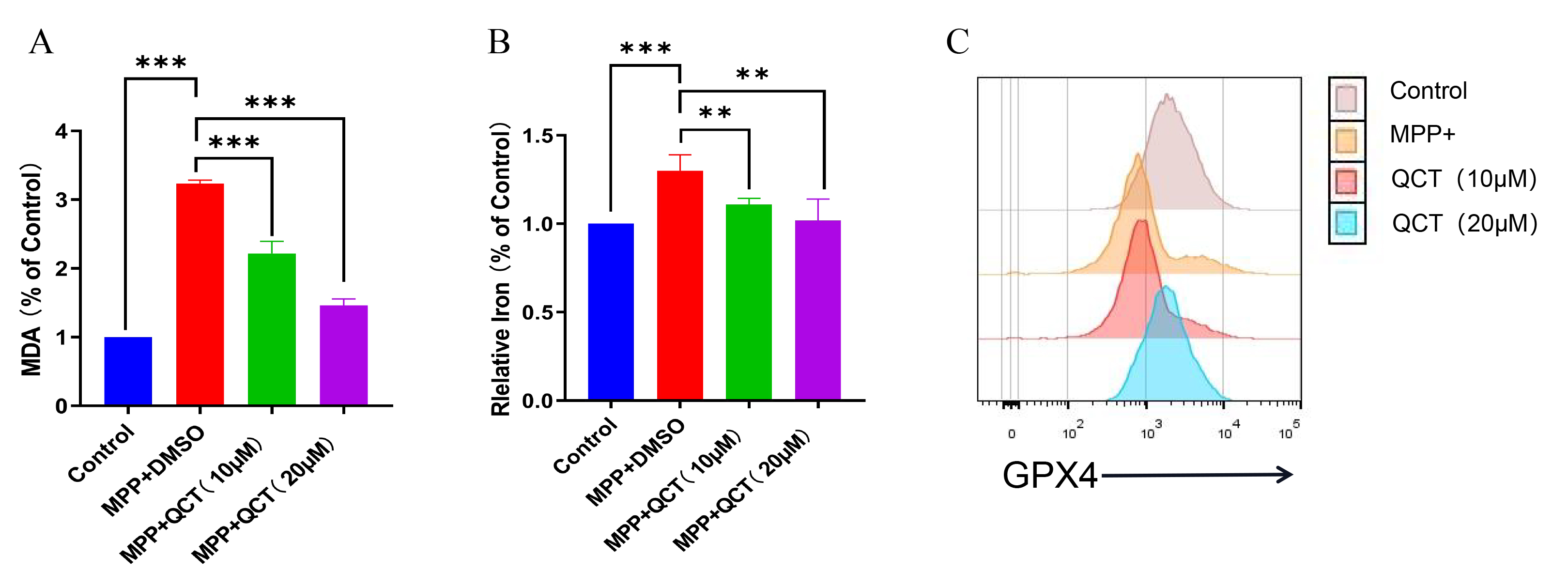 Fig. 3.
Fig. 3.Quercetin reduces the changes of MDA, iron content and GPX4
levels caused by MPP
Iron is a stimulator of oxidative stress and iron overload that promotes
ferroptosis [44]. The iron content of MPP
GPX4 is an important antioxidant enzyme in the ferroptosis pathway. In the
MPP
The previous findings suggest that quercetin inhibits ferroptosis through
upregulation of GPX4, thereby reducing DA neuron death. To further investigate
the underlying mechanism of this regulatory effect, we examined the effect of
quercetin on DA neurons after blocking GPX4 expression with RSL3. Compared with
the control group, the viability of PD cells was significantly reduced in the
RSL3 group, while PD cell death was increased (Fig. 4A). This confirmed the
significant iron toxicity observed after RSL3 induction. GPX4 levels were
significantly increased in the quercetin and RSL3-treated group compared with the
RSL3-treated group. Therefore, a 5
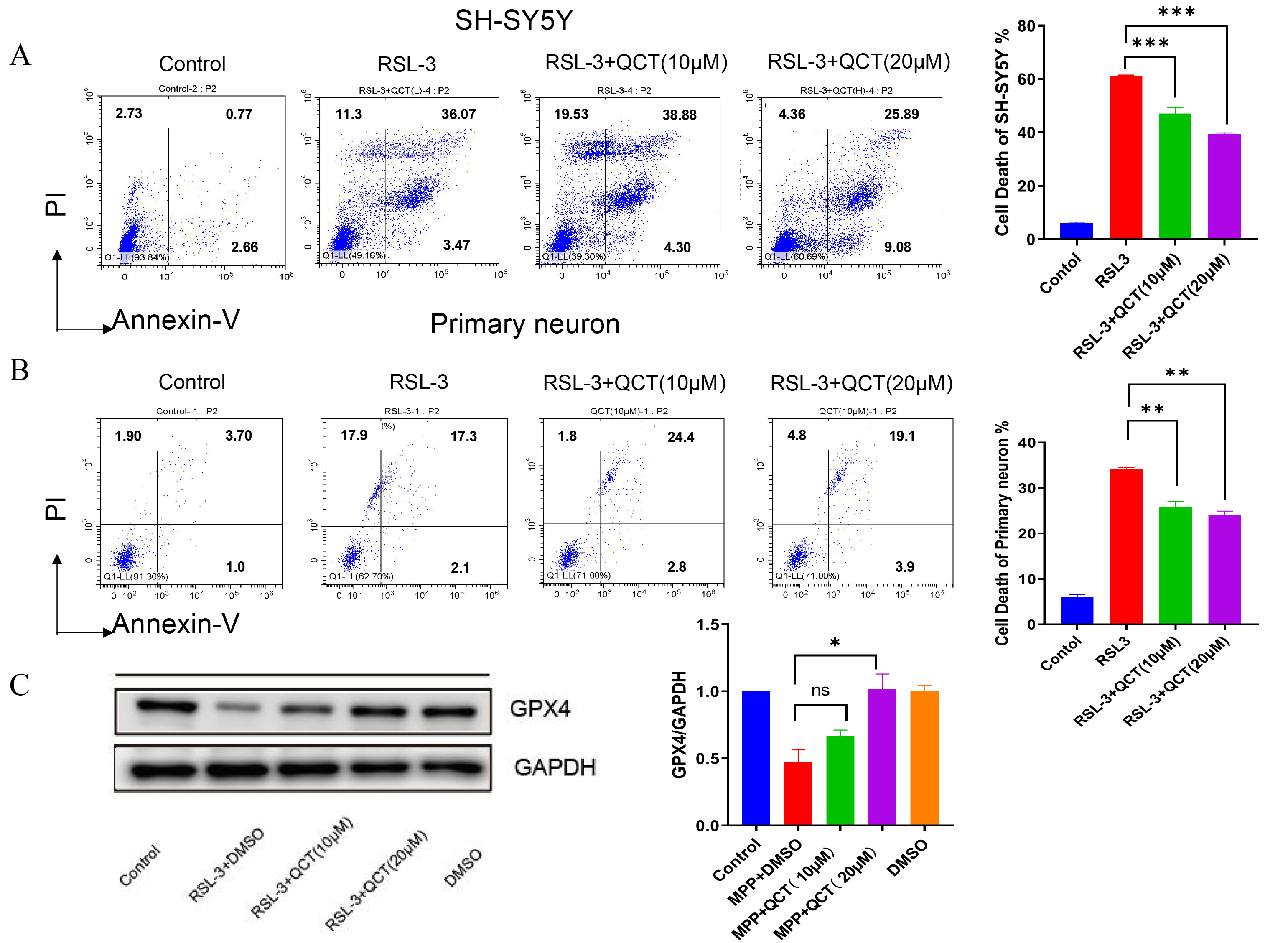 Fig. 4.
Fig. 4.Quercetin promotes cell viability and GPX4 expression which
inhibited by RSL3 induction. (A) Cell viability was assessed by
Annexin-PI assay used to assess apoptosis in SH-SY5Y neurons treated with RSL3
followed by treatment with quercetin (10/20
Previous studies showed that NCOA4 acts as a mediator of ferritin phagocytosis,
causing iron accumulation and ferroptosis. In the current study, NCOA4 levels
were significantly upregulated in the MPP
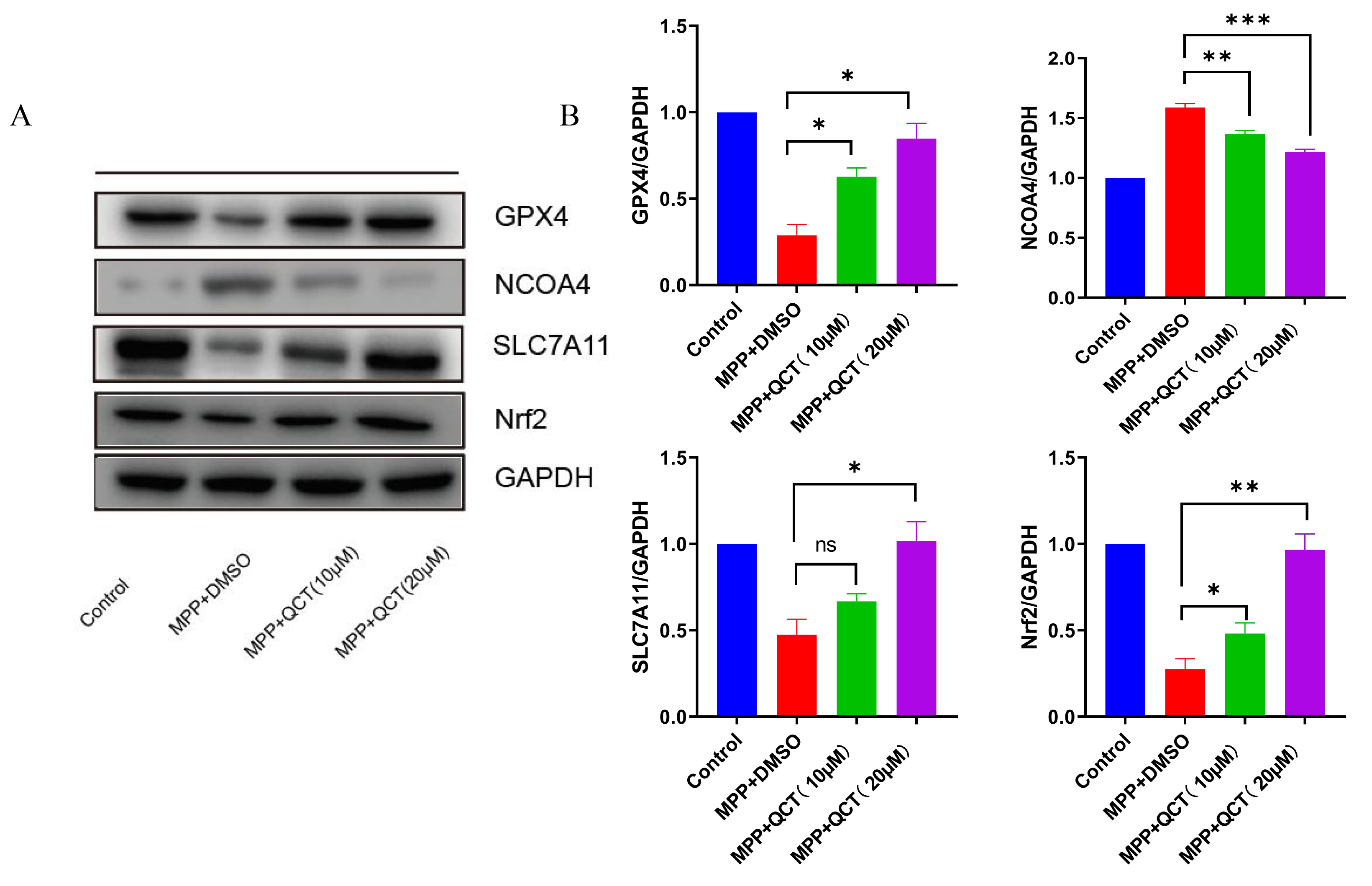 Fig. 5.
Fig. 5.The effect of quercetin on ferroptosis-related protein levels.
(A) Representative WB images are shown on the left. (B) The corresponding
relative grayscale statistics of protein levels are shown on the right. The
results of five independent experiments are expressed as mean
The Nrf2/GPX4 axis is a key factor in the inhibition of ferroptosis, and
Nrf2/GPX4 expression is significantly reduced in MPP
The results of the aforementioned study showed that Nrf2 protein expression in
the PD cell model increased with increasing quercetin concentration. Next, the
ability of quercetin was evaluated to induce cytoprotective/antioxidant protein
expression in Nrf2-driven SH-SY5Y cells. Cells were pretreated with quercetin and
the specific Nrf2 inhibitor ML385 for 2 hours, and then treated with MPP
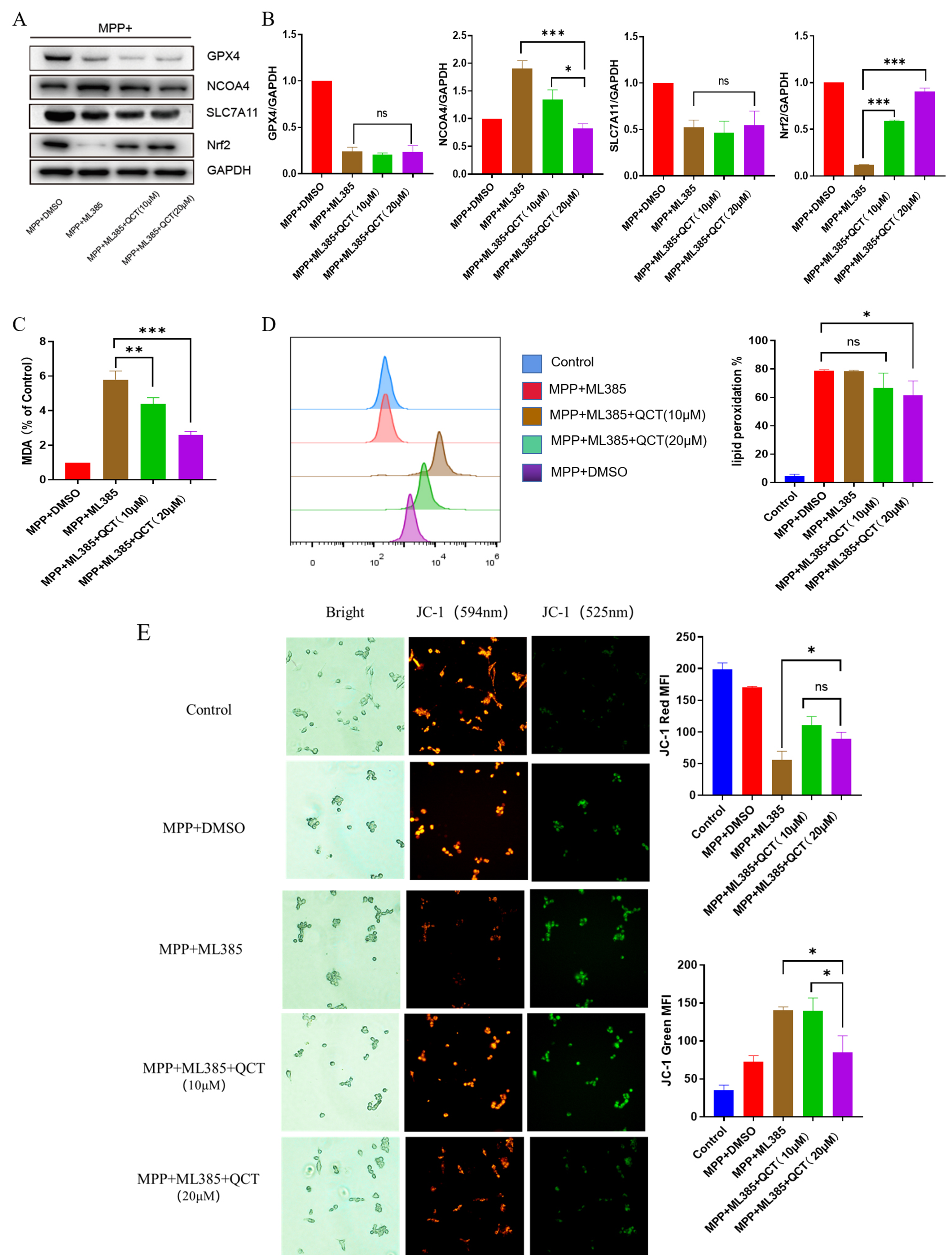 Fig. 6.
Fig. 6.Nrf2 is protective in the effect of quercetin against
MPP
Subsequently, JC-1 staining revealed a similar mitochondrial membrane potential signature, which is consistent with the trend of ferroptosis, suggesting that quercetin protects against ferroptosis through the Nrf2-mediated pathway and that blocking the Nrf2 pathway inhibits the protective effect of quercetin.
Quercetin, a phenolic compound widely distributed in the plant kingdom, is an
antioxidant that helps to maintain oxidative homeostasis [45]. Tumor cells can
undergo apoptosis in the presence of high concentrations of quercetin [46]. In
this study, we investigated the effect of different concentrations of quercetin
on the anti-apoptotic capacity of neuronal cells, and the graphic abstract was
shown in Fig. 7. SH-SY5Y cells were used as a cell model for in vitro
study of PD in this study because they have some properties of DA neurons. Our
study showed that SH-SY5Y neuronal cells and primary cultured neurons exhibited
similar sensitivity to quercetin. In this study, we found that quercetin inhibits
MPP
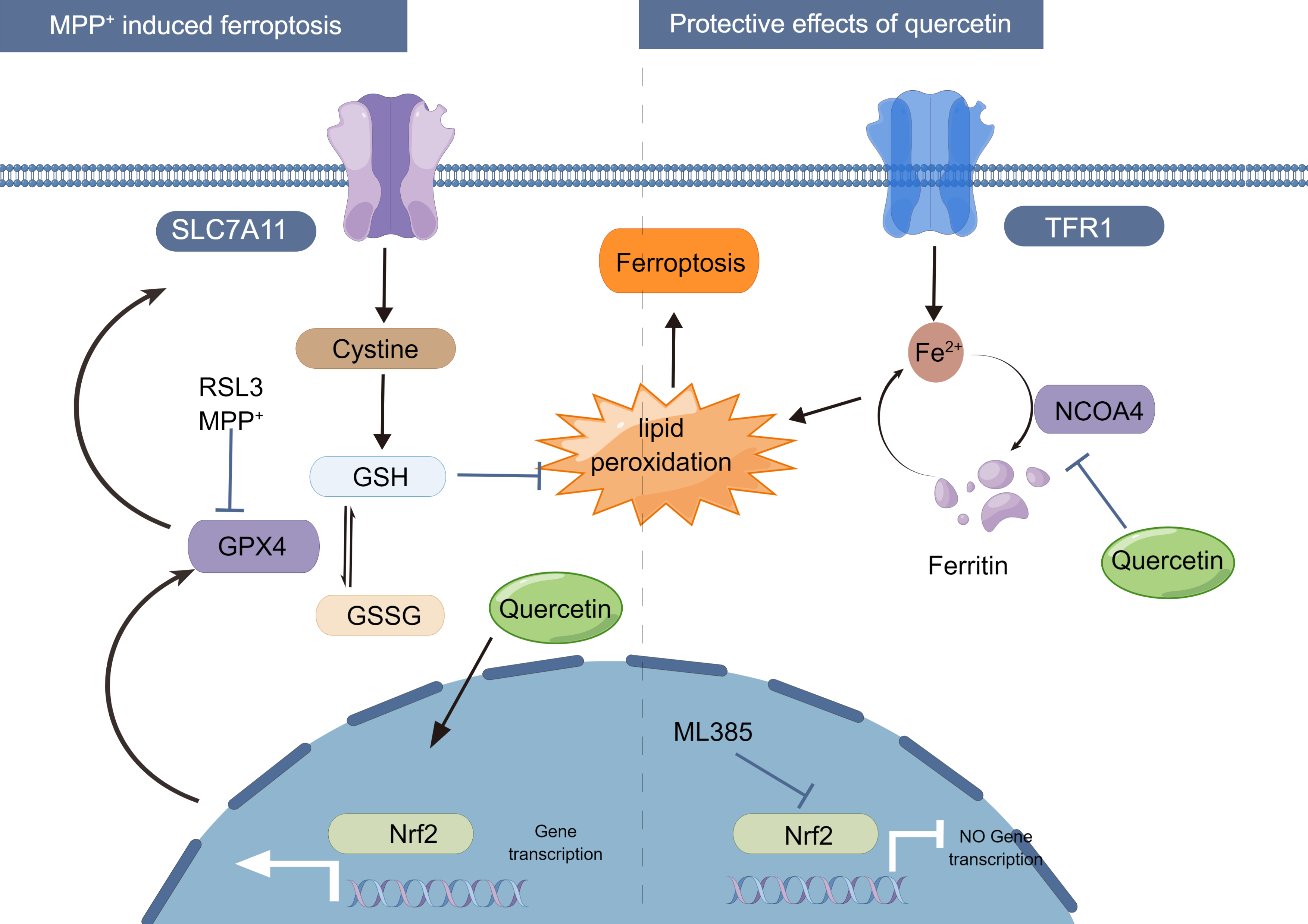 Fig. 7.
Fig. 7.
Proposed mechanism for the protective effect of quercetin on
MPP
ROS-induced lipid peroxidation plays a key role in cell death, including
apoptosis and ferroptosis. Unlike other cell death modes such as autophagy and
apoptosis, ferroptosis is a non-apoptotic cell death characterized by lipid
peroxidation and iron overload [47]. MPP
Previous studies have shown that MDA and Fe
To further evaluate the effect of quercetin against ferroptosis, the iron atrophy agonist ML385 was used to treat SH-SY5Y cells to induce a classical ferroptosis phenotype. Again, it was found that quercetin could counteract this process and exert a neuroprotective effect on cell viability. Therefore, we suggest that quercetin may protect neuron cells by modulating the ferroptosis phenotype. Many studies have also shown that the flavonoid quercetin exerts antioxidant, anti-inflammatory, and anti-proliferative properties [51]. These may be exerted through several mechanisms, including direct effects on signal transduction pathways [52]. Previous studies have shown that quercetin can enhance the antioxidant capacity of the inherent antioxidant gene Nrf2 and its downstream GPx, reduce the excessive production of MDA, and maintain cellular redox properties [53]. Studies have also shown that quercetin can reduce inflammation and oxidative stress by activating the Nrf2 signaling pathway to promote cell proliferation, upregulate SLC7A11, and reduce ferroptosis [54, 55]. Therefore, SLC7A11 might be a potential target for a single pathway via Nrf2 [56, 57, 58].
In this study, MPP
In conclusion, the Nrf2/GPX4 pathway was found here to regulate lipid ROS
levels, leading to ferroptosis. Quercetin was able to reduce the effect of
MPP
The present study has a few limitations. First, no in vivo studies were conducted. In addition, since the brain is protected by the blood-brain barrier (BBB), which acts as a vital boundary between neural tissue and the circulating blood, new drugs proposed for the treatment of PD need to penetrate the blood-brain barrier and the blood–spinal cord barrier. Our in vitro experience also suggests that different concentrations of drugs are needed to mimic any potential in vivo effects.
The current study has shown that quercetin treatment reduces oxidative stress
and inhibits ferroptosis in an MPP
PD, Parkinson’s disease; DA neuron, dopaminergic neuron;
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
DH is the recipient of fundings and supervise the present project, as well as reviewed and edited the manuscript; FY administrated the project, wrote and reviewed the manuscript; WY performed formal analysis, data curation and supervision; HX made the investigation and visualization; YJ performed the methodology, data resources collection, data validation, and writing the original draft; GX performed the data validation and resources collection; softwares application was in charge of AA. All authors have read and approved the final manuscript.
Not applicable.
We would like to thank all participants and co-workers who participated in this study. SH-SY5Y cells were a kind gift from the Department of Neurology, Tongji Hospital Affiliated with Tongji University. Fig. 7 was created by Figdraw.
This research was supported by the National Natural Science Foundation of China (grant No. 81771258) and the Ministry of Science and Technology of China (grant No. 2020YFC2002800).
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/j.fbl2803042.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.







