1 Department of Medical Imaging, Medical School of Yangtze University, 434023 Jingzhou, Hubei, China
2 Medical Imaging Department, Wuhan Hospital of Traditional Chinese and Western Medicine, 430022 Wuhan, Hubei, China
3 Department of General Practice, Wuhan Fourth Hospital, 430033 Wuhan, Hubei, China
4 Radiation Physiology Lab, Singapore Nuclear Research and Safety Initiative, National University of Singapore, 138602 Singapore, Singapore
†These authors contributed equally.
Abstract
Ischemic stroke and cranial radiotherapy may induce brain inflammatory response,
oxidative stress, apoptosis and neuronal loss, and impairment of neurogenesis.
Lycium barbarum has anti-oxidation, anti-inflammatory, anti-tumor and anti-aging
properties, may produce both neuroprotective and radioprotective effects. In this
narrative review paper, we described the neuroprotective effect of Lycium
barbarum in different animal models of experimental ischemic stroke and limited
studies in irradiated animal models. Relevant molecular mechanisms are also
summarized. It has been shown that in experimental ischemic stroke models, Lycium
barbarum produces neuroprotective effects by modulating neuroinflammatory factors
such as cytokines and chemokines, reactive oxygen species, and neurotransmitter
and receptor systems. In irradiation animal models, Lycium barbarum prevents
radiation-induced loss of hippocampal interneurons. Given its minimal
side-effects, these preclinical studies suggest that Lycium barbarum may be a
promising radio-neuro-protective drug that can be used as an adjunct treatment to
radiotherapy for brain tumor and in the treatment of ischemic stroke. At
molecular levels, Lycium barbarum may regulate PI3K/Akt/GSK-3
Graphical Abstract
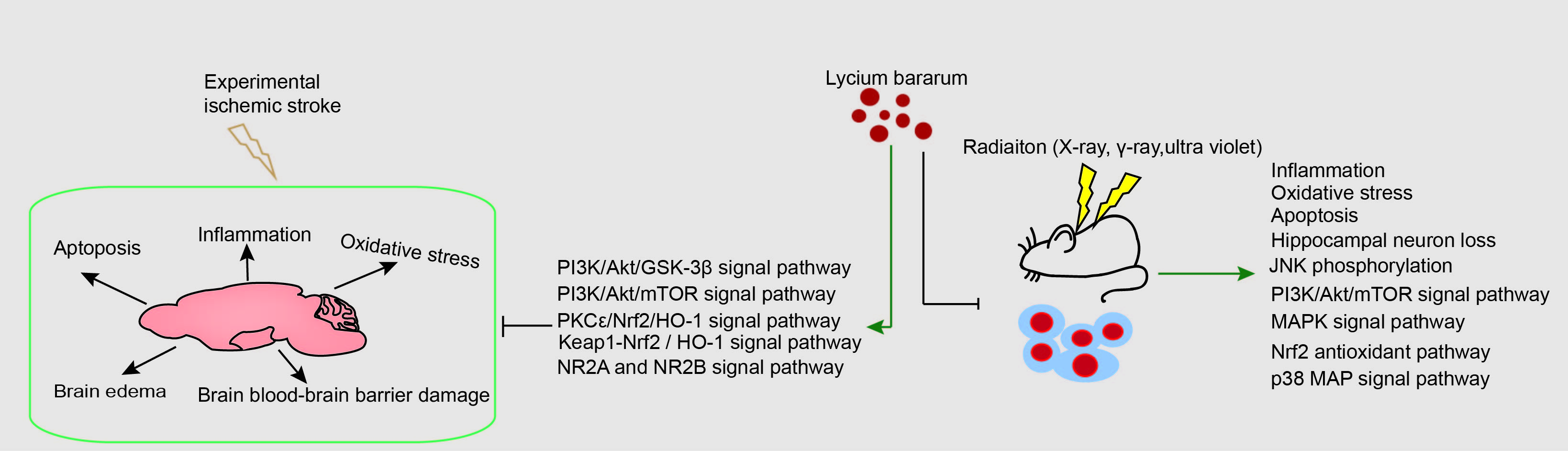
Keywords
- ischemic stroke
- radiation
- radiotherapy
- apoptosis
- inflammation
- oxidative stress
- Lycium barbarum
- neuroprotective
- molecular mechanisms
- Lycium barbarum polysaccharides
Stroke, the leading cause of neurologic disease, is the most common serious manifestation of cerebrovascular disease [1], resulting in a global annual economic burden. It has been reported that ischemic stroke accounts for 70–80% of total stroke events [2]. China is facing the most serious threat of ischemic stroke in the world [3], which is now the second major cause of death worldwide [4], and affects both middle-aged and elderly populations [5, 6]. Ischemic stroke refers to brain tissue and neuronal damage caused by insufficient blood supply [1], and is caused by an interruption in cerebral blood flow induced by thrombosis or embolism [7], leading to neuronal loss, brain atrophy and cognitive decline, which may also occur in patients with Alzheimer’s disease [8], motor functional deficits [9], and multiple cognitive functional deficits [10]. Early and rapid restoration of blood supply within a strict time window is still considered to be the first choice for the treatment of acute ischemic stroke [11, 12]. However, if the recovery of cerebral blood flow exceeds a certain time window, it can result in further neurological damage, resulting in an ischemia-reperfusion injury [13]. Tissue plasminogen activator has been used for the treatment of cerebral infarction for over 20 years, but its side effects limits the prognosis of ischemic stroke [14]. Therefore, there is an urgent need to find new therapies for treating ischemic stroke. It has been shown that drugs that possess anti-apoptosis, anti-inflammatory, and anti-oxidative stress properties, and promote neurogenesis are effective in the treatment of cerebral ischemia [15, 16, 17, 18]. Studies suggest that some Traditional Chinese Medicine (TCM) and natural compounds have neuroprotective properties that can be used for treating ischemic stroke [19, 20].
With the increasing use of radiation for medical diagnosis and therapeutic approaches such as computed tomography (CT), positron emission tomography (PET), radiotherapy and radiopharmaceutical therapy (RPT) of cancers, the risk of exposure to radiation is increasing. Radiotherapy has become one of the most common therapeutic methods for cancer treatment, especially in patients with head and neck tumors, and brain metastases, these patients are exposed to the risk of radiation-induced brain damage [21]. The brain is very sensitive to radiation exposure. Radiation can affect the central nervous system, leading to mental retardation, behavioral changes, cognitive impairment, and neoplastic diseases [22, 23]. Although it is well known that high dose rate radiation causes damage to the brain, skin and eye [24, 25, 26, 27], the effect of low dose radiation on the human brain is still unknown. The anti-radiation drug amifostine is limited in clinical practice due to its side effects [28]. Therefore, it is vital to find drugs with low toxicity, and effective neuroprotection to limit brain damage.
Traditional Chinese Medicines (TCMs) have a long history of application in the treatment of human diseases [29]. Because of their multi-component synergistic effects, multi-targeted therapeutic benefits, low toxicity and side effects, and low price, TCMs may have an advantage when compared to western medicine. Lycium barbarum, a TCM and food supplement [30], has been used for centuries in many countries. Lycium barbarum is also known as Wolfberry or Goji [31]. The fruit, root bark and leaves of Lycium barbarum are widely used as food and pharmaceutical additives [32], and contains rich chemical components [33]. A large number of studies have shown that it has a variety of favorable biological effects in patients with diabetes [32, 34], impaired reproductive systems [35, 36], eye diseases [37, 38], cardiovascular diseases [39, 40] and cancers [41, 42]. Lycium barbarum polysaccharide (LBP), the main active component of Lycium barbarum, has been shown to possess anti-oxidation, anti-inflammatory, anti-tumor and anti-aging properties [43, 44, 45, 46, 47]. Numerous studies have suggested the potential use of LBP or Lycium barbarum in protecting against damage induced by experimental ischemic stroke and radiation exposure [48, 49]. In this study, we review the neuroprotective effects and molecular mechanisms of Lycium barbarum in experimental ischemic strokes and radiation exposure.
Mitochondria, the “energy factory” of cells, play an important role in cellular homeostasis [50]. The brain is an energy dependent organ and performs its functions via aerobic metabolism [51]. In the mitochondrial apoptotic pathway, cerebral ischemia leads to altered mitochondrial membrane potential and increased permeability [52], which then causes the release of cytochrome C (Cyt-C) and apoptosis inducing factor (AIF) and the formation of apoptotic bodies [53], promoting the activation of pro caspase-9. Activated caspase-9 can induce the caspase cascade, resulting in the cleavage and activation of caspase-3 [54]. As a key link in the mitochondrial apoptotic pathway, caspase-3 specifically cleaves substrate proteins, such as poly (ADP-ribose) polymerase (PARP), which in turn leads to PARP hyper-activation, DNA damage and apoptosis [55, 56]. In a mouse model of middle cerebral artery occlusion (MCAO), prophylactic gavage with LBP for 7 days significantly reduced neurological deficit scores, the area of cerebral infarction in the ischemic side, and the apoptosis of neurons. In addition, LBP pretreatment reversed the increase of caspase-3 protein activity, the decrease of B-cell lymphoma-2 (Bcl-2) protein expression, and the increase of Bcl-2-associated X (Bax) protein expression [57, 58]. Furthermore, LBP treatment reduced the expression of Cyt-C, caspase-9 and cleaved PARP-1 [59]. Caspase-12 precursors are located in the endoplasmic reticulum [60]. Endoplasmic reticulum stress specifically activates caspase-12, which then cleaves downstream proteins such as caspase-3, causing apoptosis [61]. Cerebral ischemia-reperfusion can induce an increase of caspase-12 protein and mRNA expression, suggesting that the endoplasmic reticulum pathway is involved in the regulation of neuronal apoptosis [62]. In a rat model, gavage of LBP twice a day for 3 days improved neurological function and reduced the water content of brain tissue, and reduced the expression of caspase-12 protein and mRNA [63]. This demonstrated that LBP reduced neuronal apoptosis by inhibiting caspase-12 in the rat model. Similarly, a recent study indicated that LBP played a neuroprotective role by increasing the expression of cyt-C, cleaved caspase-3, and Bcl-2-associated death promoter, through the NR2B signal pathway in experimental ischemic stroke [64] (Fig. 1, Table 1 (Ref. [57, 58, 59, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74])).
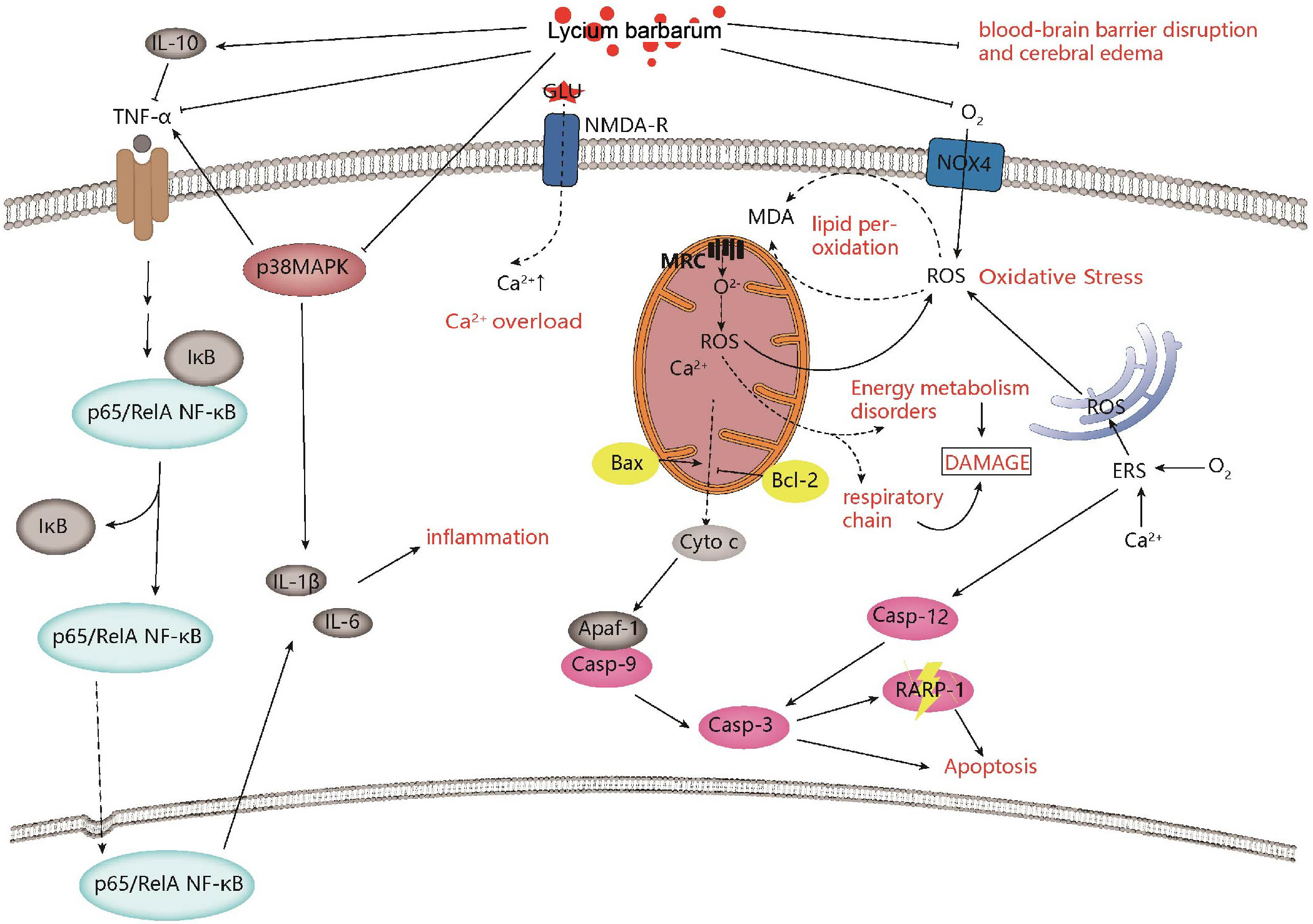 Fig. 1.
Fig. 1.Neuroprotective effects and mechanisms of Lycium barbarum on ischemic stroke. Lycium barbarum shows neuroprotective effects against ischemic stroke by improving inflammation, regulating calcium levels, improving mitochondrial function, inhibiting apoptosis, and improving oxidative stress.
| Compounds | Models | Strain, sex, age, and weight | Dosage and time | Administration and End point | Behavioral change | Brain effect at cellular level | Molecular mechanism | References |
| LBP | MCAO model | Institute of Cancer Research (ICR) mice, male, 27–32 g | 10, 20, 40 mg/kg, before insults | Once daily, intragastric administration for 7 days | Neurological deficit scores↓, Time spent on the rotating-stick↑, tail flick latency↓, number of autonomous activities↑ | p-Akt↑, p-GSK3 |
PI3K/AKT-GSK3 |
[72] |
| LBP | MCAO model | ICR mice, male, adult, 20–25 g | 10, 20, 40 mg/kg, before insults | Once daily, intragastric administration for 7 days | Neurological deficit scores↓ | caspase-3↓, Bax↓, Bcl-2↑ | Inhibit apoptosis | [57] |
| LBP | MCAO model | ICR mice, male, 5–6 weeks, 23–28 g | 10, 20, 40 mg/kg, before insults | Once daily, intragastric administration for 7 days | Neurological deficit scores↓ | Bax↓, Bcl-2↑ | Inhibit apoptosis | [58] |
| LBP | MCAO model | ICR mice, male, 6 weeks, 20–25 g | 20, 50, 100 mg/kg, before insults | Once daily, intragastric administration for 7 days | Neurological deficit scores↓ | p65 NF-κB↓, TNF- |
Inhibit the activation of P65NF-κB, anti-inflammatory | [65] |
| LBP | MCAO model | ICR mice, male, adult, 20–25 g | 10, 20, 40 mg/kg, before insults | Once daily, intragastric administration for 7 days | Behavioral test performance↑ | p65 NF-κB↓, p38 MAPK↓, TNF- |
Anti-inflammatory, partly through inhibiting the activation of p65 NF-κB and p38 MAPK | [66] |
| LBP | MCAO model | SD rats, half male and half female, 220–240 g | 60, 30, 15 mg/kg, 2 days before insults | Twice daily, intragastric administration for 3 days | Neurological deficit scores↓ | caspase-12 protein↓, caspase-12 mRNA↓ | Inhibit apoptosis | [63] |
| LBP | Four-vessel occlusion model | Wister rats, male, 220–300 g | 20 mg/kg, 7 days before or/and after modeling | Once daily, intragastic administration for 7days | Memory deficits↑ | Neuronal survival↑ | Inhibit apoptosis | [64] |
| Oxygen-glucose deprivation (OGD) model | Primary cortial neuron from Wister male rats | 100mg/L, after oxygen-glucose deprivation | Incubate, 24 h | Viability of cortical neurons↑ | ROS↓, Bcl-2-associated death promoter protein ↓, CytC protein ↓, NR2A protein ↑, pAkt protein ↑, pCREB protein ↑ | Activation of NR2A and inhibition of NR2B signialing pathway | ||
| LBP | MCAO model | Kunming mice, male, 25–30 g | 10, 20, 40 mg/kg, before insults | Once daily, intragastric administration for 7 days | - | MDA↓, SOD↑, GSH-Px↑, CAT↑, LDH leakage↓, ATP↑ | Antioxidant, Improve energy metabolism | [69] |
| LBP | MCAO model | Kunming mice, male, 25–30 g | 10, 20, 40 mg/kg, before insults | Once daily, intragastric administration for 5 days | - | MDA↓, SOD↑, GSH-Px↑, CAT↑, LDH leakage↓, ATP↑ | Antioxidant, Improve energy metabolism | [68] |
| LBP | MCAO model | ICR mice, male, 6 weeks, 20–25 g | 20, 50, 100 mg/kg, before insults | Once daily, intragastric administration for 7 days | Neurological deficit scores↓ | Nox4↓, ROS↓, MDA↓, SOD↑, GSH-Px↑ | Antioxidant | [67] |
| LBP | OGD/RP model | primary hippocampal neurons | 15, 30, 60 |
Incubate | - | ROS↓, MDA↓, cleaved-caspase3/caspase3↓, Bcl-2/Bax↑, Beclin 1↓, LC3II/LC3I↓, p62↑, p-Akt↑, p-mTOR↑ | Antioxidant, Inhibit autophagy, Inhibit apoptosis, PI3K/Akt/mTOR signal pathway | [58] |
| LBP | MCAO model | C57BL/6N mice, male, adult, 10–20 weeks | 1, 10 mg/kg, before insults | Once daily, intragastric administration for 7 days | Neurological deficit scores↑, | AQP-4↑, glial fibrillary acidic protein↑, EB extravasation↓, IgG-leaky↓, occludin↑ | Improve injury of cerebral BBB | [70] |
| LBP | MCAO model | SD rats, male, 200–220 g, 8 weeks old | 25 mg/kg, before MCAO | Once daily, injected intraperitoneally for 4 weeks | Neurological deficits scores↓ | Brain edema ↓, apoptosis↓, IgG leakage↓, occluding protein↑, claudin-5↑, ZO-1↑ | Ameliorate ischemia injury via protecting BBB | [71] |
| LBP | MCAO model | ICR mice, male, 20–25 g | 10, 20, 40 mg/kg, before insults | Once daily, intragastric administration for 7 days | Neurological deficit scores↓ | Bcl-2↑, Bax↓, Cyt-C↓, caspase-3↓, caspase -9↓, cleaved PARP-1↓ | Attenuate the mitochondrial apoptosis pathway | [59] |
| Lyciumamide A (LyA) | MCAO model | SPF grade, SD rats, male, 280 |
20, 40, 80 mg/kg, immediately after the surgery | Peritoneal injection | Neurological deficit scores↓ | SOD↑, GPx↑, MDA↓, nuclear Nrf2↑, cytoplasmic HO-1↑, LDH leakage↓, Bax↓, Bcl-2↑, cleaved caspase-3↓, p-PKCε↑, Nrf2↑, HO-1↑ | Antioxidant via PKCε/Nrf2/HO-1 pathway | [73] |
| OGD/RP model | SH-SY5Y cells | 0, 5, 10, 20, 40, 80 |
Incubate for 8 h before OGD | |||||
| LBP | MCAO model | ICR mice, male, adult, 28–30 g | 40 mg/kg, 7 days before insults | Once daily, intragastric administration for 7 days | Neurological deficit scores↓ | Nrf2 mRNA↑, HO-1 mRNA↑, LC3 mRNA↓, Ca2+↓, MMP level↑, ROS↓, Nrf2↑, HO-1↑, Keap-1↑, LC3-II↓, Beclin-1↓ | Antioxidant via Keap1-Nrf2/HO-1 pathway | [74] |
| OGD/RP model | PC12 cells | 40 |
Incubate for 24 h before reoxygenation | |||||
| LBP | Transient global ischemia injury rats model | Adult male Wister rats, 220–300 g | 20 mg/kg | Once daily, intragastric administration for 7 days | Memory deficits↓ | LDH leakage↓, NR2B↓, nNOS↓, Bad↓, Cyt-C↓, cleaved caspase-3↓, ROS↓, Ca2+↓, NR2A↑, p-Akt↑, p-CREB↑ | Regulate NR2A and NR2B signal pathway containing NMDA receptors | [64] |
| OGD/RP model | Embryos of female Wistar rats at E18 gestation | 100 mg/L | Incubate for 24 h before reoxygenation |
The inflammatory response is an important target following an acute ischemic
stroke. In the classical nuclear factor-
Experiments in mice suggested that LBP significantly improved the neurological
symptoms of the brain and reduces the water content and infarct size of brain
tissue [65]. LBP reversed the elevated expression of NF-
| Compounds | Radiation source and dose | Strain, sex, age, and weight | Dosage and Time | Administration and end point | Behavioral change | Brain effect at cellular level | Molecular mechanism | References |
|---|---|---|---|---|---|---|---|---|
| The fruits extract of L. barbarum (LBE) | C57BL/6 mice, male, 6–8-week-old | 1.0, 3.0, 6.0, and 9.0 g/kg, from 7 days before irradiation to 21 days post irradiation | Once daily, oral administration, 28 days | - | TNF- |
Immunomodulation and the synergistically modulating effect on the gut microbiota and related metabolites | [81] | |
| X-ray (whole body), 5.5 Gy | BALB/c mice | 3.0 g/kg, 7 days before irradiation to 21 days post irradiation | Once daily, oral administration, 28 days | - | ||||
| Rat small intestinal epithelial cell line 6 (IEC-6) | 100, 250, 500, 2000 |
Incubate, 24 h | - | |||||
| polysaccharide fraction (LBPF) | Ultraviolet (the dorsal region skin) | HRS/J mice, female, approximately 8 weeks, - | 5% LBPF gel, after irradiation | Three times per week, apply, 4 weeks | - | MMP-1↑, MMP-2↑, MMP-9↑ | - | [85] |
| LBP | Ultraviolet | Immortalized human keratinocytes (HaCaT cells) | 300 |
Incubate, 24 h | - | Phosphorylated p38 protein↓, p38 protein↑, cleaved caspase-3↓, caspase-3↑, MMP-9↓ | Nrf2/ARE pathway, p38 MAP pathway | [86] |
| LBP | X-rays, 4.0 Gy | Kunming mice, male and female, 18–22 g | 50, 100, 200 mg/kg, 2 h after irradiation | Intraperitoneal injection, 14 days | - | SOD activity↑, MDA Content↓, CD44↓, CD49d↓ | Inhibit apoptosis, antioxidant, and alter the expression of adhesion molecule | [83] |
| LBP | Ultraviolet | Human skin fibroblast cell line (HSF) | 300 |
Incubate, 0.5, 1, 2, 3 and 4 h | - | Nuclear p-Nrf2↑, ROS↓, lipid peroxide (LPO)↓, SOD↑, glutathione peroxidase (GSP-PX)↑ | Nrf2 antioxidant pathway | [21] |
| Aqueous and ethanol extracts of the L. barbarum fruit | Ultraviolet | Arising retinal pigment epithelia cell line-19 (ARPE-19) | 0–200 |
Incubate, 2 h | - | ROS↓, |
antioxidant and prevent DNA damage and cell apoptosis | [24] |
| LBP | Ultraviolet | Rat corneal epithelial (RCE) cells | 0, 0.05, 0.1, 0.5, 1, 5, or 10 mg/mL, 24 h before irradiation and 0–6 h after irradiation | Incubate, 24 h before irradiation and 0–6 h after irradiation | - | Bcl-2 mRNA↑, Bax mRNA↓, caspase-3 mRNA↓, caspase-3 protein↓, p-JNK/JNK↓ | Attenuate the mitochondrial pathway and inhibit JNK phosphorylation | [89] |
| Lycium ruthenicum Murr | X-ray (whole body), 5 Gy | Kunming mice, male, 4–6 week old, 25 |
2, 4, 8 g/kg, 3, 7, 14 days after irradiation | Oral administration, 14 days | - | caspase-3↓, caspase-6↓, P53↓ | Inhibit apoptosis | [82] |
| LBP | Wistar rats, male, 160–200 g | 10 mg/kg, 6 h before irradiation | Once daily, intragastric administration, 4 weeks | - | Bcl-2↑, Bax↓ | Inhibit apoptosis | [84] | |
| Lycium barbarum polysaccharide-rich hydrogel formulation | Ultraviolet | HRS/J hairless mice, female, 6-weeks -old | 5%, 6 weeks after irradiation | Three times a week, apply, 3 weeks | - | c-Fos↓, c-Jun↓, MMP-1↓, MMP-2↓, MMP-9↓, collagen I↑, collagen III↑, fibroblast growth factor-2 (FGF2)↑ | MAPK signal pathway | [87] |
| Black wolfberry water extracts | Ultraviolet | HaCaT cells | 2 mg/mL, 12 h before irradiation | Incubate, 12 h | - | P38 MAPK↓, P53↓, caspase-8↓, caspase-3↓, Bcl-2↑ | Promote cell proliferation and prevent cell apoptosis | [88] |
Brain tissue has a higher oxidative metabolism and fewer antioxidases, and is more sensitive to oxidative stress than any other organ [90]. Oxidative stress is very important in the development of many diseases, especially in ischemic strokes [91, 92, 93]. During normal conditions, the body can produce reactive oxygen species (ROS) during aerobic metabolism and there is a balance between the production and elimination of ROS. As a subtype of nicotinamide adenine dinucleotide phosphate hydride (NADPH), the expression of NADPH oxidase 4 (Nox4) significantly increases during cerebral ischemia, leading to increased production of ROS [94], and reduced activity of endogenous antioxidant enzymes, such as superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) [95], which results in decreased cellular structure and enzymatic activity [96, 97]. Excessive ROS-induced lipid peroxidation can produce large amounts of malondialdehyde (MDA), which further increases brain damage [48, 67]. LBP treatment reduced ROS and MDA, but increased SOD, GSH-Px and CAT production in the mouse brain with ischemic stroke [67, 68, 69]. Western blotting (WB) results showed that LBP reduced Nox4 protein levels in the ischemic side of the cerebral cortex [67], and decreased cerebral ischemia-reperfusion injury (Fig. 1, Table 2).
As an important barrier between the brain and peripheral circulation, BBB maintains the homeostasis of the central nervous system. It has been reported that the BBB is destroyed during ischemic stroke resulting in edema formation and hemorrhagic transformation [98, 99], which further exacerbates the ischemic injury [100]. As a key protein responsible for water transport in brain, aquaporin-4 (AQP4) mRNA expression is increased after middle cerebral artery occlusion, which is consistent with the exacerbation of brain edema monitored by magnetic resonance imaging, indicating that the up regulation of AQP4 expression is involved in the formation of brain edema [101, 102]. Matrix metalloproteinases (MMPs) are mainly expressed in the ipsilateral ischemic penumbra vascular endothelial cells, and activated by brain ischemia. The upregulation of matrix metalloproteinase-9 (MMP-9) exacerbates BBB damage [103]. In the mouse MCAO model, LBP treatment reduced brain edema and Evans Blue (EB) extravasation in the ipsilateral hemisphere, and vascular luminal leakage of immunoglobulin G (IgG). The down-regulation of AQP4 and MMP-9 further supports the protective effects of LBP [70]. A recent study showed that pretreatment with LBP protected the BBB by significantly reducing the cerebral infarct volume, cell apoptosis, and IgG leakage, which resulted in decreased hyperglycemia-exacerbated cerebral ischemia/reperfusion injury [71] (Fig. 1, Table 2).
Glycogen synthase kinase-3 (GSK-3) is a multifunctional serine/threonine protein
kinase, belonging to the glycogen synthase kinase family [104]. Its dysfunction
induces a variety of diseases. GSK-3
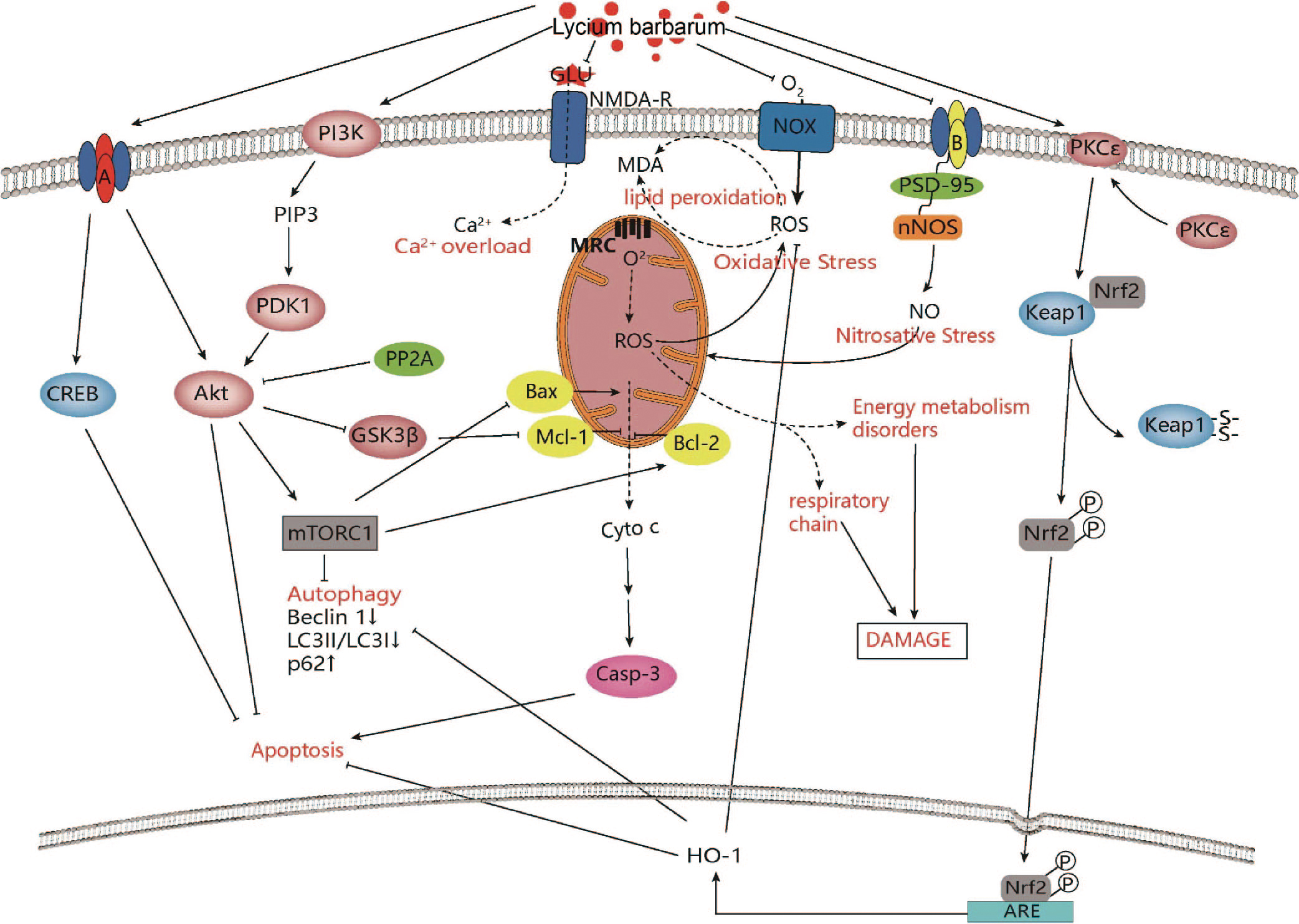 Fig. 2.
Fig. 2.Intracellular mechanisms of Lycium barbarum in improving
ischemic stroke. Lycium barbarum shows neuroprotective effects against ischemic
stroke through modulation of PI3K/AKT-GSK3
Activation and modulation of the PI3K/Akt/mammalian target of rapamycin (mTOR) signal pathway has been shown to regulate apoptosis and autophagy [111]. The PI3K/Akt/mTOR signal pathway has been found to play an important role in angiogenesis, including endothelial cell survival, migration and tube formation [112, 113], which suggests that it may become a promising therapeutic target in ischemic stroke [114, 115]. It has been reported that ginsenoside Rg1 treatment activated the PI3K/Akt/mTOR signal pathway in cerebral cortical ischemia after strokes [116]. LBP inhibited neuronal apoptosis by reversing cleaved-caspase 3/caspase 3 and Bax/Bcl-2. In addition, Beclin 1 expression and microtubule-associated protein 1 light chain 3 II (LC3-II)/microtubule-associated protein 1 light chain 3 I (LC3-I) were decreased in the LBP-treated group, but p62 expression was increased, indicating that LBP suppressed neuronal autophagy, which was further supported by transmission electron microscopy. A mechanistic study revealed that LBP treatment increased the expression of p-Akt and p-mTOR proteins, confirming that LBP may exert neuroprotective effects through the PI3K/Akt/mTOR signal pathway [62] (Fig. 2).
Protein kinase C (PKC) belongs to the serine/threonine kinase family and mediates the phosphorylation of nuclear factor E2-related factor 2 (Nrf2) at ser-40 site and its antioxidant response [117, 118]. Nrf2 is one of the key regulators of endogenous antioxidant molecules, its nuclear translocation induces the expression of the cytoprotective gene heme oxygenase 1 (HO-1) [119]. It has been reported that HO-1 is an effective target for the protection of cerebral ischemia [120]. The cytoplasmic activity of PKC was decreased and membrane activity of PKC was increased during cerebral ischemia [121]. Reperfusion injury consistently reduced the levels of Nrf2 and HO-1 [122].
In the Sprague-Dawley (SD) rat model, Lycium barbarum treatment improved the
neurological deficit score and brain infarct volume. Furthermore, it reduced
oxidative stress, partly and significantly increased the expression of nuclear
Nrf2 and cytoplasmic HO-1 in ischemic cerebral cortex; as knocking out either
Nrf2 or HO-1 reduces the protective effect of lyciumamide A (lyA). In addition,
lyA-induced Nrf2 nuclear translocation and the up regulation of HO-1 expression
were inhibited by the knockout of PKC
An in-vitro experiment showed that LyA treatment reduced lactate dehydrogenase (LDH) leakage, cleaved caspase-3 levels, Bax/Bcl-2 levels, and the number of apoptotic cells [73] (Fig. 2).
Kelch-like ECH-associated protein 1 (Keap1) is localized to the actin cytoskeleton [123]. The Neh2 structural domain of Nrf2 can be bound to Keap1 in the cytoplasm under physiological conditions and subsequently degraded by ubiquitination, to maintain dynamic stability [124]. Pathological conditions can induce Nrf2 phosphorylation, dissociation from Keap1 and nuclear translocation, and binds to the nuclear antioxidant response element (ARE) to promote the expression of antioxidant genes such as HO-1 [125, 126], enhancing the antioxidant capacity of the organism.
Experiments in mice revealed that ischemic injury disrupted neurological function and cortical brain electrical activity and reduced cortical blood perfusion on the ischemic side, while LBP treatment reversed these abnormal changes. The expression of Nrf2, HO-1 and Keap-1 were increased, while the expression of LC3-II and Beclin-1 was decreased after LBP treatment. Cell experiments revealed that LBP increased cell survival, reduced intracellular ROS levels and intracellular free calcium ion content, and stabilized mitochondrial membrane potential. Nrf2 inhibitor significantly reduced the Nrf2 and HO-1 protein content and increased the expression of Keap-1, LC3-II and Beclin-1 protein on the ischemic side compared to the LBP group, which was consistent in mouse and cell experiments. These studies suggest that LBP may protect ischemic brain injury by regulating the Keap1-Nrf2/HO-1 signal pathway [74] (Fig. 2).
The activation of different subunits of the glutamate receptor/N-methyl-D-aspartate receptor (NMDAR) effects several pathways. For instance, activation of intrasynaptic NMDAR 2A (NR2A) stimulates the survival signaling pathway, while the activation of the extrasynaptic NMDAR 2B (NR2B) triggers the apoptotic pathway. In models of cerebral ischemia and other neurodegenerative diseases, the inhibition of NR2B-containing NMDARs are neuroprotective, while the inhibition of NR2A-containing NMDARs results in neural death [127, 128]. The inhibition of postsynaptic density-95 (PSD-95) expression selectively attenuates excitotoxicity triggered by NMDAR, demonstrating the importance of PSD-95 in effectively coupling NMDAR activity to NO toxicity [129]. Once ischemic injury occurs, neuronal nitric oxide synthase (nNOS) can be transferred to the cell membrane to form the NR2B-PSD-95-nNOS complex which subsequently produces a large amount of nitric oxide (NO) resulting in neuronal damage.
In the early ischemic phase, LBP attenuates neuronal damage by preventing the upregulation of NR2B and nNOS. In the late ischemic phase, LBP reduces calcium influx and mitochondrial permeability, preventing the overexpression of nNOS, the Bcl-2 associated agonist of cell death (Bad), Cyt-C, and cleaved-caspase-3 in the NR2B signal pathway. LBP increased the expression of NR2A, p-Akt, and the phosphorylated cAMP response to element binding (p-CREB). These results suggest that LBP may attenuate ischemic damage to hippocampal neurons by the NR2A and NR2B signal pathway containing NMDA receptors [64] (Fig. 2).
Several studies have shown that Lycium barbarum or Lycium barbarum extract play
a vital role against radiation-induced damage [26, 49, 81, 82]. In the mouse
X-ray radiation model, Lycium ruthenicum Murr reversed radiation-induced decrease
of the body weight, alterations in hematology, thymus and spleen indexes, and
reduced the expression of caspase-3, caspase-6 and P53 [82]. LBP inhibited
X-ray-induced apoptosis in bone marrow mononuclear cells (BMNC), reduced
oxidative damage and decreased the expression of adhesion molecules CD44 and
CD49d [83]. In the
In the brain, Lycium barbarum berry extraction prevented radiation-induced hippocampal neuron loss, and alleviated radiation-induced spatial memory and emotional impairment, and improved the behavioral performance of BALB/c mice exposed to acute 5.5 Gy X-ray [49]. LBP pretreatment for 2 weeks reduced brain MDA, but enhanced SOD and GSH-Px expression. It shortened the escape latency period and space exploration time of SD rats exposed to a single 20 Gy X-ray during the Morris water maze test. LBP pretreatment for 1 hour significantly inhibited the apoptosis of hippocampal neurons, increased the expression of Bcl-2 protein, and decreased the expression of Bax protein and capsase-3 protein. Furthermore, the protein expression of PI3K, Akt, and mTOR increased, indicating that LBP may prevent the radiation-induced apoptosis of hippocampal neurons through the PI3K/Akt/mTOR signal pathway [130]. Furthermore, in in-vitro studies, LBP improved cell viability, and had a neuroprotective role in spinal cord neurons exposed to 10Gy X-ray radiation by up-regulating the expression of LC3II/I and Beclin-1 [131, 132] (Table 3, Ref. [49, 130, 131, 132]).
| Compounds | Radiation source and dose | Strain, sex, age, and weight | Dosage and time | Administration and end point | Behavioral change | Brain effect at cellular level | Molecular mechanism | References |
|---|---|---|---|---|---|---|---|---|
| Lycium barbarum berry extract | X-ray (whole body), 5.5 Gy | BALB/c mice, male, 8-week-old, 22 |
10 g/kg, 2 h after irradiation | Once daily, oral administration, 4 weeks | Tail suspension immobility times↓, forced swimming immobility times↓, the average total travelling distance↑, the average central area staying time↓, the average escape latency↓, the average platform crossing time↑, platform quadrant resident time↑ | NeuN immunopositive neurons in the hilus↑, CB immunopositive interneurons in the the strata radiatum lacunosum, moleculare (SRLM) and stratum oriens (SO)↑, PV positive interneurons in the CA1 stratum pyramidum (CA1-SP), and the stratum granulosum of the dentate gyrus (DG-SG)↑ | Improves Radiation-Induced hippocampal neuron loss, improvement of spatial memory and depression | [49] |
| LBP | X-ray (Head), 20 Gy | SD rat, male, -, 180–200 g | 50 mg/kg, before irradiation | Once daily, intragastric administration, 2 weeks | The escape latency period↓, space exploration time↓ | MDA↓, SOD activity↑, GSH-Px activity↑ | PI3K/Akt/mTOR signaling pathway and oxidative stress | [130] |
| X-ray, 30 Gy | Primary hippocampal neurons from fetus of SD rats | 50 |
Incubate, 1 h | Phosphatidylinositol-3-kinase (PI3K)↑, protein kinase B (Akt)↑, mammalian target of rapamycin (mTOR)↑, B-cell lymphoma/leukemia 2 (Bcl-2)↑, Bcl-2 associated X protein (Bax)↓, capsase-3↓ | ||||
| LBP | X-ray, 10 Gy | Primary spinal cord neurons from rats | 10, 25, 40 mg/L | Incubate, 24 h | Cell viability↑ | LC3II/LC3I↑ | Promote autophagy | [131] |
| LBP | X-ray, 10 Gy | Spinal cord nerve cells | 10, 25, 40 mg/L | Incubate, 24 h | Cell viability↑ | LC3II/LC3I↑, Beclin-1↑, Number of autophagy lysosomes ↑ | Promote autophagy | [132] |
Based on the molecular mechanism of radiation-induced brain damage seen in these studies [133, 134, 135, 136, 137], the neuroprotective effect of Lycium barbarum in the ischemic stroke model and other diseases may also apply to radiation-induce brain damage (Fig. 3).
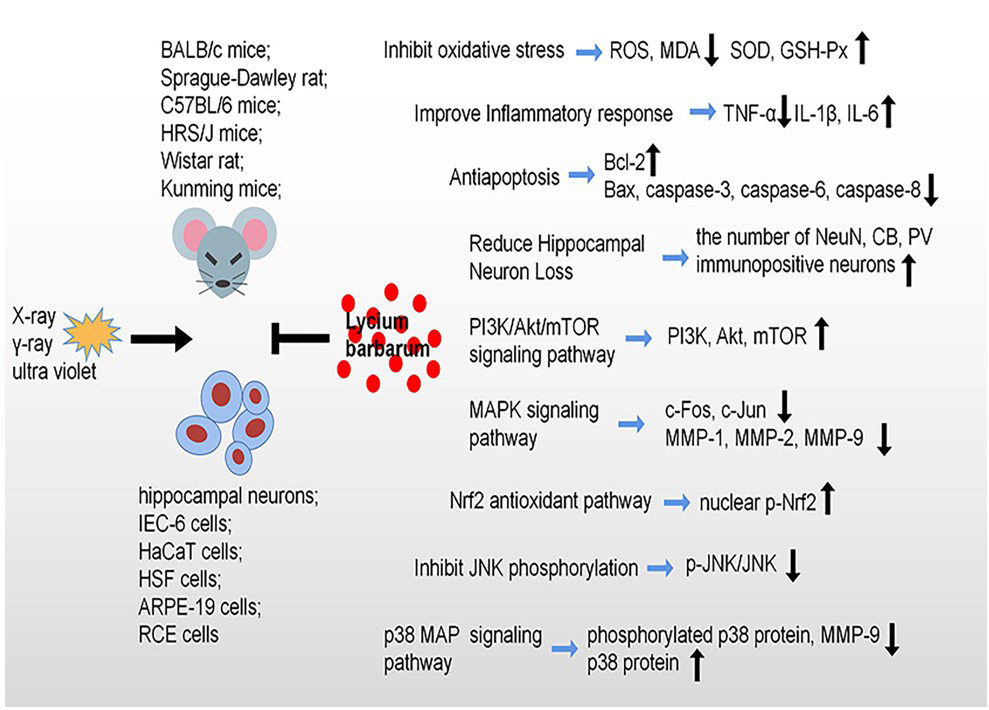 Fig. 3.
Fig. 3.Radioprotective effects and molecular mechanisms of Lycium barbarum against radiation-induce damage. Lycium barbarum shows protective effects against radiation through inhibiting oxidative stress, cell apoptosis, reduce hippocampal neuron loss and improve inflammatory response, specially, modulation of PI3K/Akt/mTOR signaling pathway, MAPK signaling pathway, Nrf2 signaling pathway, JNK signaling pathway and p38 MAP signaling pathway.
Ischemic stroke, caused by a decrease of blood supply to a certain region of the brain due to obstruction of a blood vessel, is the second leading cause of death worldwide [11]. In addition, there is a shift in the ischemic stroke burden to younger individuals from elderly groups [137]. Despite progress in the understanding of the pathophysiological mechanisms in stroke over the past 30 years, cognitive impairment and depression, the common complications of stroke [138, 139], remains difficult for treatment and rehabilitation. In this paper, we reviewed the molecular mechanism of ischemic stroke, including apoptosis, inflammation, oxidative stress, and various signal pathways. A recent study found that the RANKL genetic variation played an important role in ischemic stroke [140]. Recombinant tissue plasminogen activator, the only Food and Drug Administration approved therapy for ischemic stroke, has important limitations in the treatment of ischemic stroke because of the narrow therapeutic time window time of 4.5 h and the potential risk of hemorrhagic transformation [141, 142]. In recent decades, many studies have focused on the pathophysiology and mechanisms of stroke and found that neuroprotection is a promising strategy for the treatment of stroke [143]. Unfortunately, most neuroprotective drugs have failed to translate to clinical practice [144, 145]. However, the potential impact of sulfonylures in the outcome of type 2 diabetic patients with ischemic stroke has suggested that it can decrease ischemic stroke induced damage [146]. Recently, Traditional Chinese Medicines have attracted more attention due to its effective neuroprotective roles. Unlike Western medicine, the Traditional Chinese Medicines have unique advantages due to their regulatory effects at multiple targets and organs. LBP, as the main active ingredient of Lycium barbarum, has been used for traditional herbal and food supplements for many years in Asiatic countries. Many studies indicated that LBP play an anti-oxidative role in oxidative liver injury [147], and it can protect ganglion cells against retinal ischemic/reperfusion injury [148]. Song et al. [149] found that LBP could reduce glucose deprivation-induced injury in PC-12 cells. Similarly, several studies have suggested that it can limit ischemic stroke injury [64, 149]. Considering the neuroprotective effect of Lycium barbarum on ischemia stroke injury, Lycium barbarum may be used as a potential therapeutic agent for ischemic injury in the future clinical trials.
Radiotherapy kills the tumor cells, but also affects the adjacent normal cells [150], which greatly increases the risk of radiation-induced brain damage. In addition, radiodiagnosis, accidental or environmental high dose/dose rate radiation contamination can cause biological effects, such as behavioral disorders, apoptosis, inflammation, neuronal injury, and oxidative stress [151, 152, 153, 154]. Amifostine, a radioprotective drug used with radiotherapy for patients with head and neck cancer, is limited in its wide application due to its relatively large side effects. Therefore, it is urgent to develop radioprotective drugs with low side effects. Many Traditional Chinese Medicine can improve radiation-induced damage [155]. The neuroprotective effect of LBP has been reported in both in vivo and in-vitro models [43]. LBP improves microglia damage induced by bipolar pulse current by regulating autophagy [156], and Lycium barbarum water extract improves brain trauma induced cognitive impairment by prevention of neuronal apoptosis and promotion of regeneration of hippocampal neurons [157].
Due to many shared neuropathological changes between ischemic stroke and radiation exposure such as neuro-inflammation, neuronal damage, apoptosis, oxidative stress, BBB damage, and impaired neurogenesis, the neuroprotective effect of Lycium barbarum in ischemic stroke may be applied to radiation-induced brain damage. Additional extensive research on the radio-neuroprotective effect of Lycium barbarum will be necessary to determine its role in clinical practice.
Although more and more attention has been paid to ischemic stroke, therapeutic treatments are limited to intravenous thrombolysis with recombinant tissue plasminogen activator [158, 159]. The combined complexity of the brain structure and the timeliness with which patients receive treatment compromise clinical treatment of ischemic stroke [11]. Similarly, the mechanism of radiation-induced brain damage is still unclear and there is no ideal radioprotective drug in clinical practice. Many experimental studies have strongly suggested that Lycium barbarum produces neuroprotective effects on neurological and neuropsychiatric diseases, ischemic stroke and injury induced by radiation exposure. These protective effects are mainly achieved by regulating brain oxidative stress, inflammation and neuronal apoptosis. By preventing neuronal loss, less neural pathway will be destroyed, and more newly generated neurons are integrated into the dentate gyrus-related afferent and efferent pathways which will reduce ischemic stroke- and radiation-induced impairment of learning and memory and patients’ symptoms.
The molecular mechanisms and neuroprotective effect of Lyceum barbarum in lacunar versus non-lacunar acute ischemic stroke will need further study, since the pathophysiology, prognosis and clinical features of acute small-vessel ischemic strokes are different from other types of cerebral infarcts [160]. This review provides evidence that Lycium barbarum treatment might be a promising treatment for the protection of the brain against acute ischemic stroke injury in humans. The radioprotective effect of Lycium barbarum on other organs such as skin, eye, reproductive systems and the intestine may also apply to the brain. Further studies on the radio-neuroprotective effect of Lycium barbarum on other neurodegenerative disorders may provide the evidence needed to use Lycium barbarum as a supplementary treatment after radiotherapy to prevent acute radiation exposure-induced chronic brain damage.
In this review paper, only limited numbers of studies on the radio-neuroprotective role of Lycium barbarum were available. Since ischemic stroke and radiation exposure share many similar neuropathological changes, it is predicted that further study will provide promising results to confirm the radio-neuro-protective role of Lycium barbarum to translate it for clinical use.
CT, computed tomography; PET, positron emission tomography; RPT,
radiopharmaceutical therapy; TCMs, Traditional Chinese Medicines; LBP, Lycium
barbarum polysaccharide; Cyt-C, cytochrome C; AIF,apoptosis inducing factor;
PARP, poly (ADP-ribose) polymerase; MCAO, middle cerebral artery occlusion; LBP,
Lycium barbarum polysaccharide; Bcl-2, B-cell lymphoma-2; Bax, Bcl-2-associated
X; NF-
YH, XZ and LC contributed to the acquisition and interpretation of data, they contributed equally. BXR and FRT contributed to the conception of the research and the revision of key elements.
Not applicable.
Not applicable.
This research was funded by the following research grants from the National Natural Science Foundation of China (Grant no. 81772223) to BXR. Grants from National Research Foundation of Singapore to Singapore Nuclear Research and Safety Initiative (FRT).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.



