1 Department of Anesthesiology, The Affiliated Hospital of North Sichuan Medical College, 637000 Nanchong, Sichuan, China
Abstract
Neuropathic pain is a chronic secondary pain condition resulting from lesions or diseases of the peripheral or central nervous system (CNS). Neuropathic pain is closely related to edema, inflammation, increased neuronal excitability, and central sensitization caused by glutamate accumulation. Aquaporins (AQPs), mainly responsible for the transport and clearance of water and solute, play important roles in developing CNS diseases, especially neuropathic pain. This review focuses on the interaction of AQPs with neuropathic pain, and the potential of AQPs, especially aquaporins 4, as therapeutic targets.
Keywords
- aquaporins
- neuropathic pain
- glymphatic system
- review
Aquaporins (AQPs) are water channel proteins essential to life and expressed in
all kingdoms [1]. To date, thirteen AQPs were identified in human body and found
widely distributed in specific cell types in various organs and tissues [2]. Four
AQP monomers, each consisting of six transmembrane
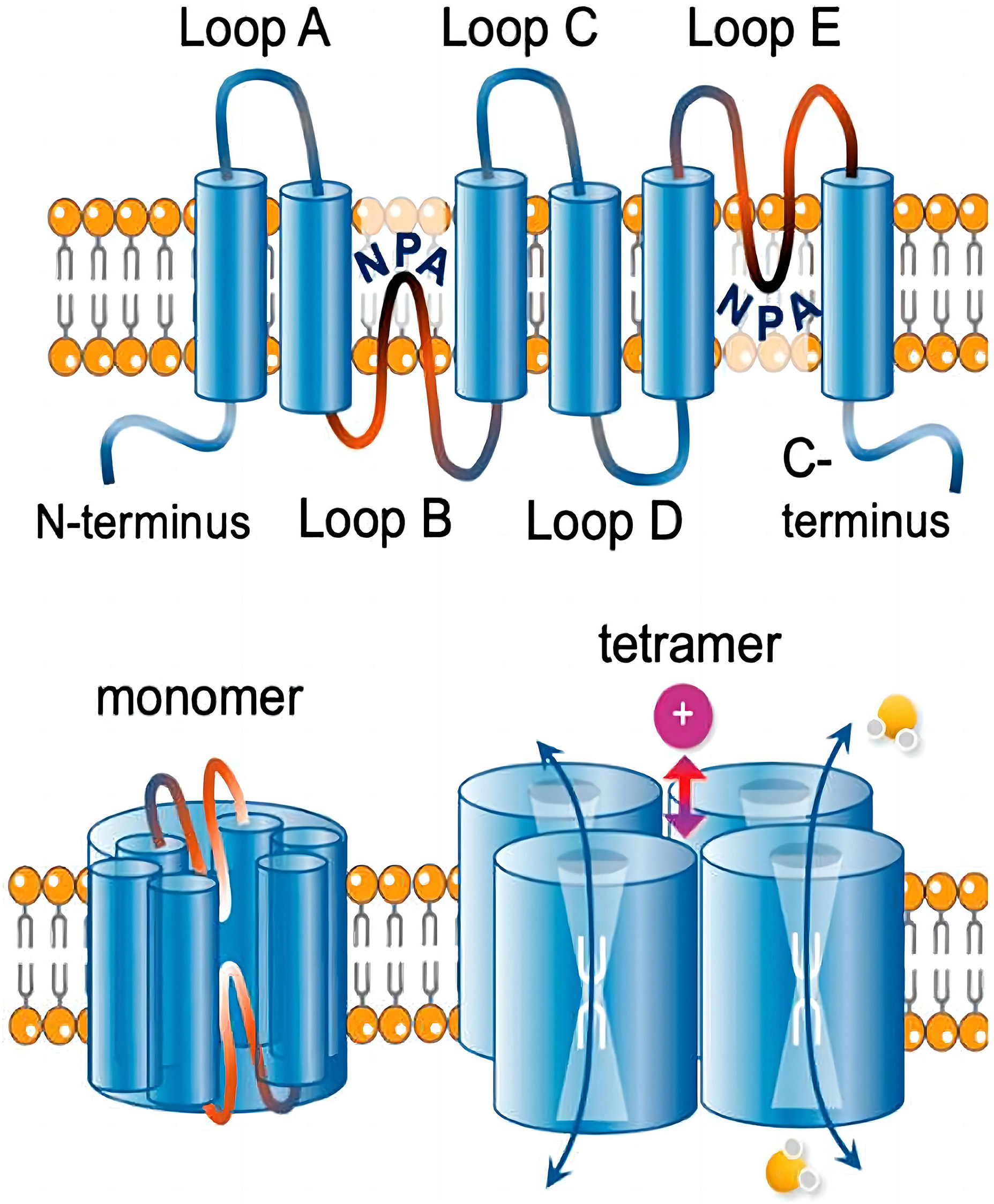
 Fig. 1.
Fig. 1.Transmembrane topology of an aquaporin. Reproduced from [6] under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/). Copyright 2020 Khan, Ricciardelli and Yool. Each monomer consists of six transmembrane helices connected by loops A to E. Loops B and E typically carry signature NPA (Asn-Pro-Ala) motifs that fold together within each subunit to form a water pore. Four monomers form a tetramer (the functional channel). A subset of AQPs uses the central pore as a gated ion channel. AQPs, aquaporins.
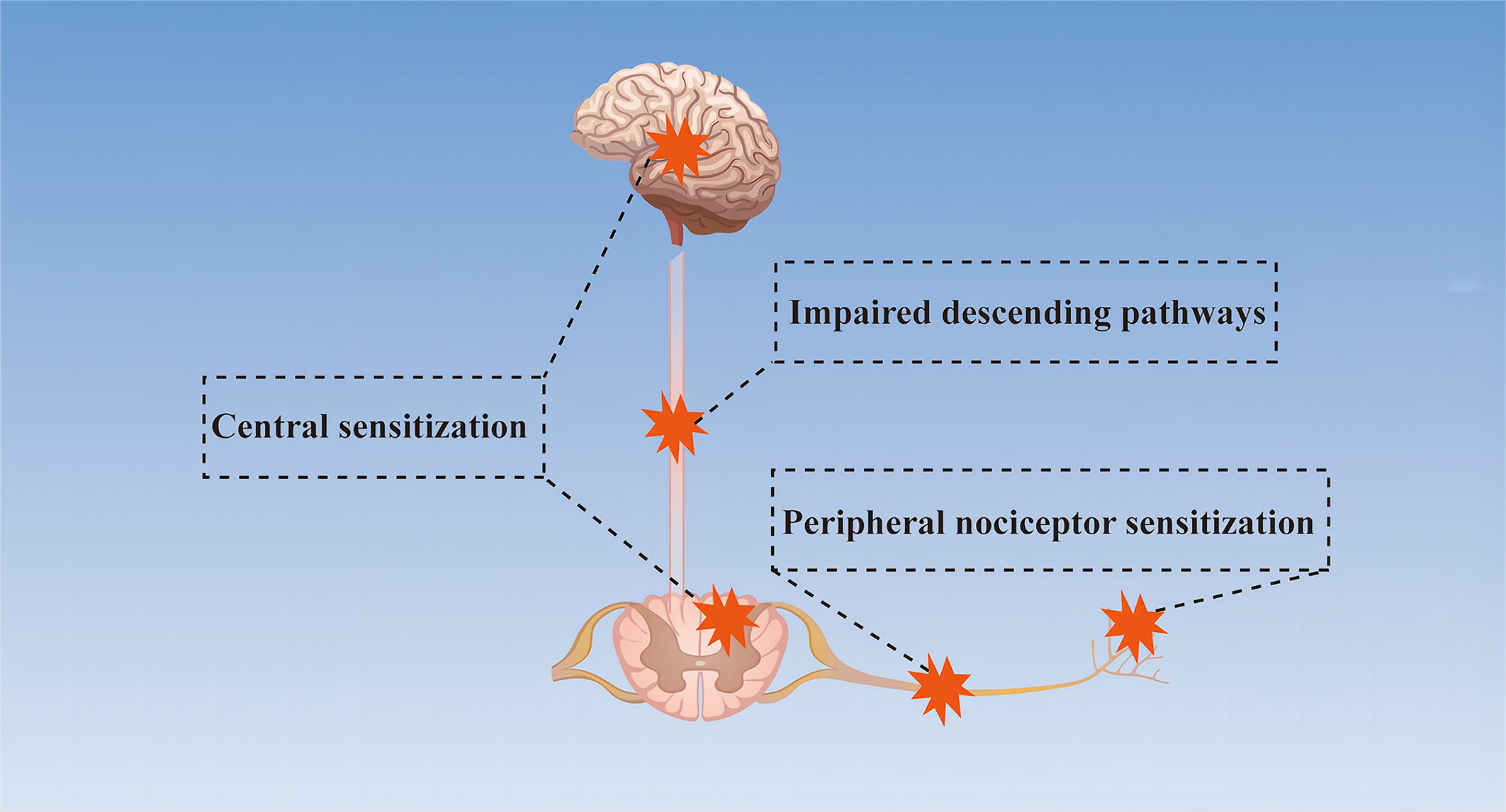
Neuropathic pain is a chronic secondary pain condition resulting from lesions or diseases of the peripheral or central nervous system (CNS) [8, 9, 10]. It is estimated that around 5% of the population suffers from neuropathic pain [11]. Most patients complain of an ongoing or intermittent spontaneous pain of, for example, burning, pricking, or squeezing quality, which may be accompanied by evoked pain, particularly to light touch and cold [12]. Studies have indicated that the ectopic activity in damaged or adjacent nerves, dorsal root ganglia or central pathways, peripheral and central sensitization and a range of molecular mechanisms are involved in the development of neuropathic pain [11, 12] (Fig. 2).
 Fig. 2.
Fig. 2.Simplified mechanism diagram of neuropathic pain. Peripheral nociceptor sensitization due to abnormal nociceptors and primary afferents, impaired descending pathways between the brain and spinal cord, and central sensitization caused by the imbalance between excitatory and inhibitory neurotransmitters are the mechanisms of neuropathic pain.
Studies have shown that several aquaporins are closely related to neuropathic pain, including aquaporin 1 (AQP1), aquaporin 2 (AQP2), aquaporin 4 (AQP4), aquaporin 5 (AQP5) and aquaporin 9 (AQP9) [13, 14, 15, 16, 17]. Since AQPs promote water and solute transport and are involved in the occurrence and development of many diseases, it is logical that AQPs are involved in the occurrence and development of neuropathic pain. This review focuses on our current understanding of AQPs and neuropathic pain and the potential of these AQPs as therapeutic targets.
AQP1, the first water channel being identified, functions as a gas channel and
can increase CO
Interestingly, in addition to regulating water and gas function, AQP1 has also
been implicated in pain. Studies have indicated that AQP1
In a clinical study on leprosy, Salgado et al. [28] found that AQP1 may be involved in the loss of sensation or leprosy neuropathic pain. Moreover, animal experiments have shown that inhibition of AQP1 expression alleviated neuropathic pain caused by chronic dorsal root ganglion compression and melatonin decreased mechanical allodynia by regulating AQP1 [14, 29]. In the treatment of a series of diseases targeting AQP1, many traditional Chinese medicines were found to play therapeutic effects by regulating AQP1, such as alpinetin, dachengqi, and shenmai injection [30, 31, 32]. Interestingly, AQP1 was shown to increase the sensitivity of acetazolamide and cisplatin in the treatment of cancer, suggesting a positive potential antitumor role of AQP1 [33, 34]. Overall, AQP1 may be involved in pain perception through ion channels and, in this way, in the development of neuropathic pain.
Water balance plays an important role in maintaining the normal function of the body, and water reabsorption by the collecting duct of the kidney is one of its key factors, which is regulated by AQP2 [35]. AQP2, a channel that is selective only for water molecules and impermeable to ions or other small molecules, is an aquaporin regulated by arginine vasopressin [2, 35]. Normal functioning of AQP2 depends on multiple posttranslational modifications, and aberrant arginine vasopressin signaling and altered AQP2 expression or transport can lead to diseases characterized by dysregulation of mechanisms controlling water homeostasis [36]. Impairment of AQP2 results in various water balance disorders, including disorders associated with polyuria (urinary tract obstruction, hypokalemia, inflammation, and lithium intoxication), dilutive hyponatremia (improper antidiuretic syndrome, congestive heart failure, cirrhosis syndrome), and Ménière’s Disease [37, 38].
In addition to its role in transporting water, mainly to the kidneys, AQP2 also appears to be involved in pain. In an acute inflammatory pain animal model, expression of AQP2 in the trigeminal ganglia was increased, and the redistribution of AQP2 was mainly identified in small-sized neurons and Schwann cells. The above results indicated the involvement of AQP2 in pain transmission in the peripheral nervous system [39]. Sciatic nerve injury, a classic pain model, is widely used in the study of neuropathic pain. The expression of AQP2 was increased in the small dorsal root ganglion neurons of rats with chronic contraction injury and in sciatic nerve crush injury models [15, 40]. Moreover, it is worth mentioning that erythropoietin protects against neuropathic pain by regulating AQP2 expression in a rat model of chronic contractile injury in vivo [41]. These results suggest that AQP2 may be involved in inflammatory nerve injury and may play a role in promoting regeneration after nerve injury as well as in the treatment of neuropathic pain.
Since AQP2 is highly involved in the water transportation of the kidneys, the research on renal diseases targeting AQP2 has become one of the current hot spots. For example, statins ameliorate cholesterol-induced inflammation and improve AQP2 expression by inhibiting NOD-like receptor thermal protein domain associated protein 3 (NLRP3) activation in the kidney, thus contributing to the treatment of chronic kidney disease [41]. Protein kinase A may treat congenital nephrogenic diabetes insipidus by activating AQP2 [42]. Zhen-wu-tang reduces renal edema induced by doxorubicin by regulating AQP2 and Mir-92b [43]. Steviol slows renal cyst growth by reducing AQP2 expression and promoting AQP2 degradation [44]. In addition, electroacupuncture alleviates arginine vasopressin-induced endolymphatic hydrops by regulating AQP2 and vincamine is capable of suppressing endolymphatic hydrops formation by down-regulating the VAP/AQP2 signaling pathway [45, 46]. These results suggest that AQP2 can be used as a target for the treatment of renal diseases and Ménière’s Disease. However, the role of AQP2 in neuropathic pain and its potential as a therapeutic target of neuropathic pain needs to be further studied.
Among the 13 identified AQPs, AQP4 has become an interesting therapeutic target in various neurological disorders, due to its variety of functions and widespread expression in the CNS [47]. AQP4 is the most abundant water channel in the brain, spinal cord, and optic nerve. It controls brain water homeostasis [48]. AQP4 monomers have a molecular size of ~30 kDa and contain six membrane-spanning helical segments and two shorter helical segments that only partly span the membrane [49]. According to the translation start sites, AQP4 can be divided into 2 major isoforms, M1 and M23 [48, 49]. AQP4 can form crystal-like supramolecular assemblies in the plasma membrane, which are called orthogonal arrays of particles (OAPs) [50, 51]. Moreover, OAPs have been identified as the target of anti-AQP4 antibodies in neuromyelitis optica spectrum disorder (NMOSD) [50, 51, 52].
In addition to its primary water transport function, AQP4 also regulates
astrocyte migration, participates in neural signal transduction, and regulates
neuroinflammation [49]. It is worth mentioning that the increased AQP4 expression
and the redistribution/surface localization are two different concepts. Previous
studies have shown an increase in AQP4 membrane localization in primary human
astrocytes which wasn’t accompanied by a change in AQP4 protein expression. This
mislocalization was supposed to be a potential therapeutic target [53, 54, 55]. Many
diseases such as hypothermia, painful diabetic neuropathy, Alzheimer’s disease
and epilepsy have been manifested to be associated with impaired expression or
mislocalization of AQP4 [12, 17, 56, 57, 58]. Interestingly, recent studies have
demonstrated the existence of the glymphatic system, which consists of AQP4 and
astrocytes and is responsible for removing metabolic waste products from the CNS
[17, 59] (Fig. 3, Ref. [6]). Currently, there are many studies on the glymphatic system, and
MRI is the most commonly used device. In terms of contrast agent selection, the
newly discovered contrast agent that can be used to observe the paracellular flow
and diffusive transcellular exchange of water is H
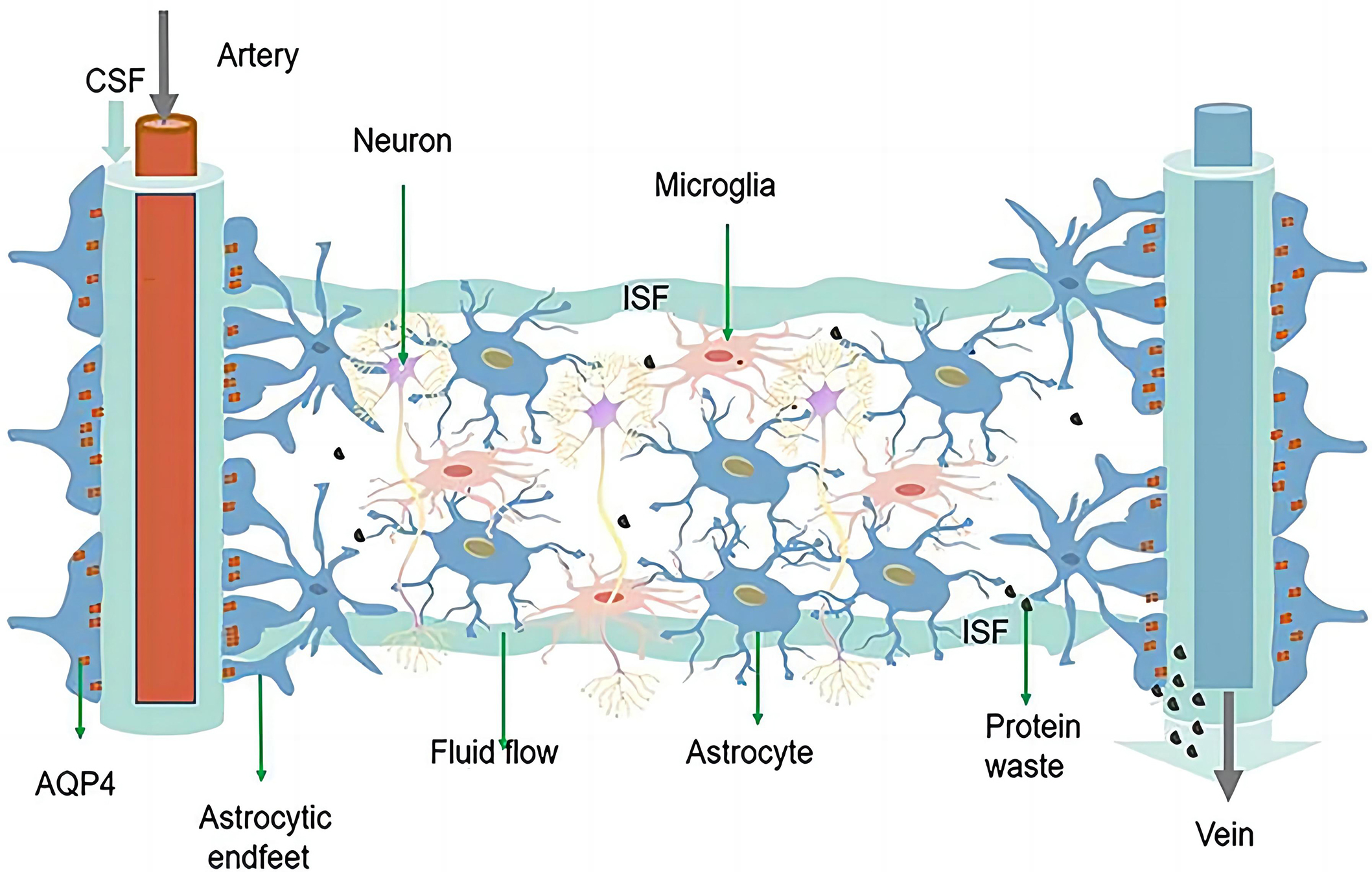
 Fig. 3.
Fig. 3.The schematic diagram of the glymphatic system. Reproduced from [6] under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/). Copyright 2021 Ren, Liu, Lian, Li, Li, Li and Zhao. The glymphatic system is composed of astrocytes and AQP4 located at the endfeet of astrocytes. CSF enters the brain parenchyma through the periarterial space, exchanges with ISF, and finally exits through the perivenous space. AQP4 can facilitate this fluid exchange, through which solutes and metabolic waste products (e.g., glutamate) from the interstitium are exchanged, and finally expelled from the brain by the meninges and cervical lymphatics. AQP4, aquaporin 4; CSF, Cerebrospinal fluid; ISF, interstitial fluid.
Neuromyelitis Optica (NMO) is a clinical syndrome characterized by attacks of acute optic neuritis and transverse myelitis [51, 52, 68]. In most patients, NMO is caused by pathogenetic serum IgG autoantibodies to AQP4 (AQP4-IgG) and IgG autoantibodies to myelin oligodendrocyte glycoprotein (MOG-IgG), and the term NMOSD is used to refer to NMO and its formes frustes [68]. It has been reported that NMO occurs in all ethnicities around the world, with significant regional differences, and non-white individuals have higher incidence and prevalence rates [71]. AQP4-IgG associated NMO is characterized by IgG and complement deposition occurring at the endfeet of astrocytes, often accompanied by loss of astrocytes, oligodendrocytes, and neurons, whereas MOG-IgG associated NMO is characterized by demyelination with dominant loss of MOG and relative preservation of axons and oligodendrocytes [68].
Previous studies have shown a high negative correlation between pain severity and quality of life in NMO patients [72]. At present, the mechanism of neuropathic pain associated with NMO is still unclear. The loss of excitatory amino acid transporter 2 mediated by AQP4-IgG leads to the imbalance between excitation and inhibition in the nociceptive pathway caused by extracellular glutamate accumulation, the increase of local nerve growth factor concentration mediated by MOG-IgG, abnormal sprouting of injurious spinal cord fibers and inflammatory lesions have been reported to be involved in the occurrence and development of NMO [68, 73, 74]. The AQP4-based glymphatic system is responsible for the transport of glutamate and other metabolic wastes and has been reported to be a therapeutic target for Alzheimer’s Disease [75]. Since NMO mostly manifests as AQP4-IgG, its pathogenesis is mainly central sensitization caused by glutamate transport disorder, and the AQP4-based glymphatic system can promote glutamate transport [68, 75]. Therefore, there must be a close relationship between NMO and the glymphatic system based on AQP4.
There are several treatments available for NMOSD, such as conventional immunosuppressants, B cell-depleting agents, interleukin-6 signaling blocking agents, complement blocking agents, and intravenous immunoglobulins [76]. It is worth noting that the research on the interleukin-6 (IL-6) signaling pathway is hot at present. The plasmablasts in the peripheral blood of NMOSD patients with positive AQP4-IgG are increased, IL-6 drives these plasma cells to produce and secrete AQP4-IgG, whereas an IL-6 blocking agent reduces the production of AQP4-IgG [77]. For example, two IL-6 blocking agents, tocilizumab and satralizumab, have been shown to have good therapeutic effects on NMOSD and are potentially effective and safe therapeutic methods for relapse prevention in NMOSD [76].
Spinal cord injury (SCI) refers to the direct or indirect spinal cord injury caused by external factors and accompanied by anatomical structure or functional changes [78, 79, 80]. It has been reported that SCI affects about 250,000–500,000 people every year, with road traffic accidents as the main cause and young males as the main population, which can seriously affect the quality of life of patients and even cause death [80]. Clinical and animal studies have shown that many factors are involved in the pathogenesis of SCI, including changes in the expression of glutamate, Gamma-aminobutyric acid (GABA), serotonin, reactive oxygen species (ROS), and proinflammatory cytokines, as well as the neuroanatomical shifts of receptors/ion channels, budding/denervation of primary afferent fibers, and activation of glial cells [80]. Due to the difficulty in conducting clinical studies and the fact that animal studies mainly focus on the use of drugs or injuries to build rodent models, the mechanism of SCI is still not clear [78, 79, 80, 81, 82].
Pain is one of the main symptoms of SCI patients, which can be roughly divided into nociceptive pain (pain caused by nociceptive receptors) or neuropathic pain (pain caused by damage to the somatosensory nervous system), and more than half of the patients will be accompanied by neuropathic pain [79, 83, 84]. Neuropathic SCI pain can arise as a consequence of altered sensory processing at any point along the path from peripheral sensation to conscious perception either at or below the level of injury [79]. Abnormal activation of nociceptors and its increased excitability, changes in the expression of ion channels, abnormal activation of dorsal root ganglion neurons and their associated cells, and changes in the expression of connexin-43 are all components of the peripheral mechanism of SCI, whereas reactive astrocytosis, decreased glutamate reuptake caused by decreased expression of glutamate transporter glutamate transporter 1 (GLT-1), and increased AQP4 expression are all components of the central mechanism of SCI [80, 85, 86, 87, 88, 89]. In addition, the reorganization of the neural axis as well as the de-afferentation of different regions have also been confirmed as one of the pathogenesis of neuropathic pain [90].
Electrical stimulation, cell transplantation, drugs, and stem cell therapy have certain therapeutic effects on spinal cord injury, and the most important thing to promote the recovery of neurological function is to restore good blood perfusion [79]. Moreover, studies have reported that AQP4 increases in the chronic post-injury phase are associated with the development of pain-like behavior in SCI rats, while possible mechanisms underlying pain development may involve astrocytic swelling-induced glutamate release [91]. Studies have indicated that inhibition of AQP4 expression can reduce edema and improve neuropathic pain after SCI [92, 93]. In combination with the aforementioned findings and the roles of AQP4 such as water transport, astrocyte migration, and inflammation regulation, AQP4 must be closely related to SCI and the so associated neuropathic pain, and thus plays an important role in the secondary pathological process (spinal cord edema, glial scar formation, and inflammation) after SCI [49, 94].
Peripheral neuropathy is one of the most common complications of type 1 and type 2 diabetes, with up to half of the patients with diabetes developing neuropathy during the course of their disease, of which 30%–40% are accompanied by neuropathic pain [95]. Painful diabetic neuropathy (PDN) is a frequent subtype of peripheral neuropathic pain and is defined as pain as a direct consequence of abnormalities in the peripheral somatosensory system in people with diabetes [10]. PDN usually manifests as spontaneous (that is, stimulus-independent) burning pain of the feet or other positive sensory symptoms, such as brush-evoked allodynia (when a normally non-noxious stimulus evokes pain) and paresthesias, which can result in sleep disturbances, fatigue, decreased activity, reduced quality of life, and high medical costs [96, 97, 98]. So far, the pathogenesis of PDN is not clear, and the treatment is mainly symptomatic, which is not ideal [98, 99, 100].
Studies have shown that the hyperexcitability of primary afferents, the local
factors around the dorsal root ganglia, such as tumor necrosis factor and
IL-1
Currently, there are three main treatment modalities for diabetic neuropathy
(glycemic control, foot care, and symptomatic treatment). In addition, for PDN,
Calcium channel a2
AQP5 was first cloned from the rat submandibular gland [124]. It is mainly
permeable to water and CO
Given the close relationship between AQP5 and tumors, AQP5 has been used as one of the biomarkers of some tumors and has been involved in some experiments on drug resistance and drug screening against cancer cells [129, 130, 131]. In addition, since its wide distribution in various systems of the body, AQP5 can be found to be involved in many glandular secretion-related diseases, such as poor salivary gland secretion in patients with Sjogren’s syndrome, salivary gland dysfunction induced by ovariectomy in rats, and pulmonary edema in mice with allergic asthma induced by ovalbumin [128, 132, 133].
Studies have found that AQP5 is also expressed in the CNS, suggesting that AQP5 may be involved in the development of some neurological diseases caused by water imbalance [40, 134]. In a study of rats and mice, the expression of AQP5 was up-regulated after crushed sciatic nerve injury, and neuropathic pain is one of the main symptoms of sciatic nerve injury, suggesting that AQP5 may be related to neuropathic pain [40]. In addition, topical administration of gabapentin was found to alleviate neuropathic ocular pain by inducing AQP5 expression in the lacrimal gland, and resveratrol was found to mitigate neuropathic pain by inhibiting AQP5 activation caused by chronic contractile injury [16, 135]. Moreover, studies have indicated that AQP5 colocalizes with AQP2 in the kidney, and AQP5 can also regulate AQP2 expression, which is associated with neuropathic pain [15, 41, 136]. These pieces of evidence suggest a direct or indirect link between AQP5 and neuropathic pain, and further studies are needed to confirm the specific signaling pathways.
AQP9 is expressed in multiple organs and systems, such as the liver, epididymis,
testis, spleen, and brain [137]. AQP9 is permeable to water, glycerol, urea,
carbides, CO
Due to its wide distribution, studies have shown that AQP9 can be used as a therapeutic target for some diseases. For example, RG100204, a novel AQP9 inhibitor, mitigated septic cardiomyopathy and multiple organ failure in septic mice [148]. Curcumin attenuated cerebral edema in mice with cerebral hemorrhage by inhibiting the expression of AQP4 and AQP9 [149]. The herbal medicines Inchinkoto and Saireito improved hepatic fibrosis by regulating AQP9 in the liver of a rat bile duct ligation model [150]. Leptin administration ameliorated nonalcoholic fatty liver in ob/ob mice by coordination regulation of liver-specific AQP9 [151]. Of course, the effect of drugs targeting AQP9 to play a role in other diseases needs to be further investigated.
In addition to its involvement in the previously described diseases, AQP9 also appears to be associated with pain. Studies have shown that the expression of AQP9 increased after crushed sciatic nerve injury in rats, and AQP9 was proved to be one of the biomarkers selected for the diagnosis of lumbar disc herniation [40, 152]. AQP9 also promotes astrocytoma invasion and motility while astrocyte activation has been proven to be a high-risk factor for neuropathic pain [153, 154, 155]. On the contrary, other studies have shown that AQP9 knockdown promoted the occurrence of neuropathic pain in rat chronic constriction injury model [13]. These results indicate that the effect of AQP9 on neuropathic pain is still inconclusive at present, and the link and signaling pathways between AQP9 and neuropathic pain are not clear, but it can be confirmed that AQP9 and neuropathic pain are indeed correlated, and more experiments are needed to explore it.
The incidence of neurological diseases is high, and most neurological diseases are usually accompanied by neuropathic pain, making the study of neuropathic pain the current research hotspot and has profound significance. Neuropathic pain is closely related to edema, inflammation, increased neuronal excitability, and central sensitization caused by glutamate accumulation [66, 67, 156]. Aquaporins, mainly responsible for water transport and clearance, play an important role in the development of CNS diseases. Studies have shown that AQP1, AQP2, AQP4, AQP5, and AQP9 are directly or indirectly related to neuropathic pain, and among them, AQP4 is closely related to neuropathic pain [13, 14, 15, 16, 17]. The CNS is sensitive to changes in the surrounding environment and lacks traditional lymphatic pathways. The newly discovered glymphatic system promotes the elimination of metabolic wastes and maintains water balance in the CNS, which provides a new direction for further understanding the pathogenesis of nervous system diseases [64, 65]. It is reported that many diseases are associated with the impaired glymphatic system, such as Alzheimer’s disease, traumatic brain injury, and cognitive deficiency associated with diabetes [157, 158, 159].
As components of the glymphatic system, astrocytes and AQP4 have been proven to be closely related to neuropathic pain. Reducing the expression of AQP4, restoring the polarization of AQP4, and inhibiting reactive astrogliosis can alleviate neuropathic pain [17, 59, 69, 154, 155]. AQP4 not only serves as the structural basis of the glymphatic system, but also regulates astrocyte migration, participates in nerve signal transduction, and regulates neuroinflammation. Hereby it has undoubtedly become a new therapeutic target for neuropathic pain [44, 66, 67]. In recent years, on the basis that the glymphatic system is involved in improving the effectiveness of intrathecal drug delivery, the research and development of drugs targeting AQPs for the treatment of a variety of diseases have become a hot topic [1, 160, 161, 162]. TGN020 is the most effective AQP4 inhibitor found in current studies and has been proven to have therapeutic effects on diabetic retinopathy, cerebral ischemia edema, Alzheimer’s disease, etc. [163, 164, 165, 166]. In addition, trifluoperazine has been confirmed to play a therapeutic role in CNS edema by regulating the expression and localization of AQP4 [167, 168]. The interaction between AQPs, NLRP3 inflammasome and Sigma1 receptor points out the way to develop new drugs targeting oxidative stress [169, 170]. These pieces of evidence suggest that the direct or indirect interactions between AQPs and intermediate proteins and ion channels will be the focus of future drug development.
Studies have demonstrated that AQP plays a key role in fluid homeostasis, glandular secretions, signal transduction and sensation, barrier function, immunity and inflammation, cell migration, and angiogenesis [171]. Recent hot studies have shown that targeting the trafficking of AQP proteins to the plasma membrane is a viable alternative drug target to direct inhibition of the water-conducting pore [161]. Hence, AQPs have been validated as an important drug target but there is no single drug that has yet been approved to successfully target it, given the close link between AQPs and many diseases, drug development targeting aquaporins will meet the urgent, unmet clinical need of millions of patients for whom no pharmacological interventions are available [61, 172]. Interestingly, the advent of human-scale self-organizing models, organoids, 3D cultures, human microvascular platforms on a chip and calcein fluorescent dye, as well as more advanced systems capable of real-time imaging, have provided great help to our further understanding of AQPs [173, 174, 175, 176]. Moreover, the emergence of high-throughput screening platforms and computer-aided drug design provides new insights into drug development and supports AQP4 target validation in future studies [177, 178].
In general, water balance is essential for maintaining the normal function of the CNS, which is further confirmed by neuropathic pain caused by the imbalance of water and solute. It also further demonstrates the importance of AQPs for CNS disorders such as neuropathic pain. Since the glymphatic system has been manifested to improve the effectiveness of intrathecal drug delivery and several AQPs are involved in neuropathic pain, we expect more and more new treatments or drugs based on AQPs especially AQP4 to appear with the deepening of studies, aiming to improve treatments and prognosis of the millions suffering from neuropathic pain.
AQPs, aquaporins; CNS, central nervous system; AQP1, aquaporin 1; AQP2, aquaporin 2; AQP4, aquaporin 4; AQP5, aquaporin 5; AQP9, aquaporin 9; NLRP3, NOD-like receptor thermal protein domain associated protein 3; OAPs, orthogonal arrays of particles; NMOSD, neuromyelitis optica spectrum disorder; NMO, Neuromyelitis Optica; AQP4-IgG, IgG autoantibodies to AQP4; MOG-IgG, IgG autoantibodies to myelin oligodendrocyte glycoprotein; SCI, spinal cord injury; GABA, Gamma-aminobutyric acid; ROS, reactive oxygen species; GLT-1, glutamate transporter 1; PDN, painful diabetic neuropathy; SNRIs, serotonin and noradrenaline reuptake inhibitors; TCA, tricyclic antidepressants.
FW and JYL designed the research study. FW and WX performed the research. CX and JL provided help and advice. FW and WX analyzed the data. FW wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicable.
We acknowledge the support of the Nanchong Science and Technology Bureau.
This research was funded by the Grant of the Nanchong Science and Technology Bureau, grant number 22SXQT0125.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.