1 Key Laboratory of Tropical Translational Medicine of Ministry of Education, College of Pharmaceutical, Hainan Medical University, 571199 Haikou, Hainan, China
2 Department of Anesthesiology, Hainan Medical University First Affiliated Hospital, 570102 Haikou, Hainan, China
3 Department of Anesthesiology, The Second Affiliated Hospital of Harbin Medical University, 150086 Harbin, Heilongjiang, China
4 Department of Otolaryngology, Hainan Medical University First Affiliated Hospital, 570102 Haikou, Hainan, China
†These authors contributed equally.
Abstract
Heart failure (HF) is a cardiovascular disease with an extremely high mortality rate. However, Morinda officinalis How (MO) has not been studied for cardiovascular purposes at this time, the aim of this study was to find new mechanism for the MO of treatment of HF through a bioinformatics and experimental validation. The present study also aimed to establish a link between the basic and clinical applications of this medicinal herb. MO compounds and targets were obtained by traditional Chinese medicine systems pharmacology (TCMSP) and Pubchem. Subsequently, HF targets were acquired from DisGeNET and the interactions of all the targets and other human proteins were obtained via String so as to establish a component-target interaction network by Cytoscape 3.7.2. All the targets of clusters were inserted into Database for Annotation, Visualization and Integrated Discovery (DAVID) to perform GO (gene ontology) enrichment analysis. Molecular docking was adopted to predict the targets of MO relevant to the treatment of HF and to further explore the associated pharmacological mechanisms. Subsequently, a series of in vitro experiments, including histopathological staining, immunohistochemical and immunofluorescence analyses were conducted for further verification. Moreover, western blot analysis and in vivo experiments were performed. The results indicated that MO alleviated apoptosis, regulated cholesterol metabolism and transport function, and reduced inflammation, which resulted in the successful treatment of HF. Beta-sitosterol, Asperuloside tetraacetate and americanin A were the key bioactive components of MO. ALB, AKT1, INS, STAT3, IL-6, TNF, CCND1, CTNNB1, CAT, and TP53 were the core potential targets, which were significantly associated with multiple pathways, namely the FoxO signaling pathway, the AMPK signaling pathway, and the HIF-1 signaling pathway. In vivo experiments validated that MO may protect against heart failure or treat this disease by increasing the levels of autophagy via the FoxO3 signaling pathway in rats. The present study suggested that a combination of network pharmacology prediction with experimental validation may offer a useful tool to characterize the molecular mechanism of action of the traditional Chinese medicine (TCM) MO in the treatment of HF.
Keywords
- traditional Chinese medicine
- mechanism of action
- heart failure
- Morinda officinalis How
Heart failure (HF) is the leading cause of cardiovascular death, it is not
considered a single disease based on a specific etiology and causes a clinical
syndrome that includes symptoms, such as dyspnea and weariness. Markers are high
jugular venous pressure, tachycardia, and peripheral edema [1]. The prevalence of
heart failure has been projected to increase to 46% between 2012 and 2030 [2],
causing a serious financial and social burden in the world. At present, the
remedy of heart failure is still a conservative cure containing organic
chemicals. However, the continuous use of modern medicine may result in dizziness
and fainting due to excessively low blood pressure, which can be life-threatening
in severe circumstances. Using traditional Chinese medicine (TCM) for to
treatment cardiovascular diseases has last for thousands of years. It has been
used in combination with various clinical drug regimens and diagnostic
guidelines, which can greatly promote the development of local medical care. TCM
has been widely utilized to treatment heart failure [3], which reduces the side
effects caused by excessive modern medicine. Therefore, TCM is likely to become
mainstream drugs in the future. Morinda officinalis (MO) is a
traditional Chinese medicinal herb used in southeastern China that has shown
various pharmacological activities. The Nrf2 is a crucial transcription factor
that controls the intracellular antioxidant response and is an arising focus for
the treatment and prevention of illnesses caused by oxidative stress [4]. MO
could reduce H
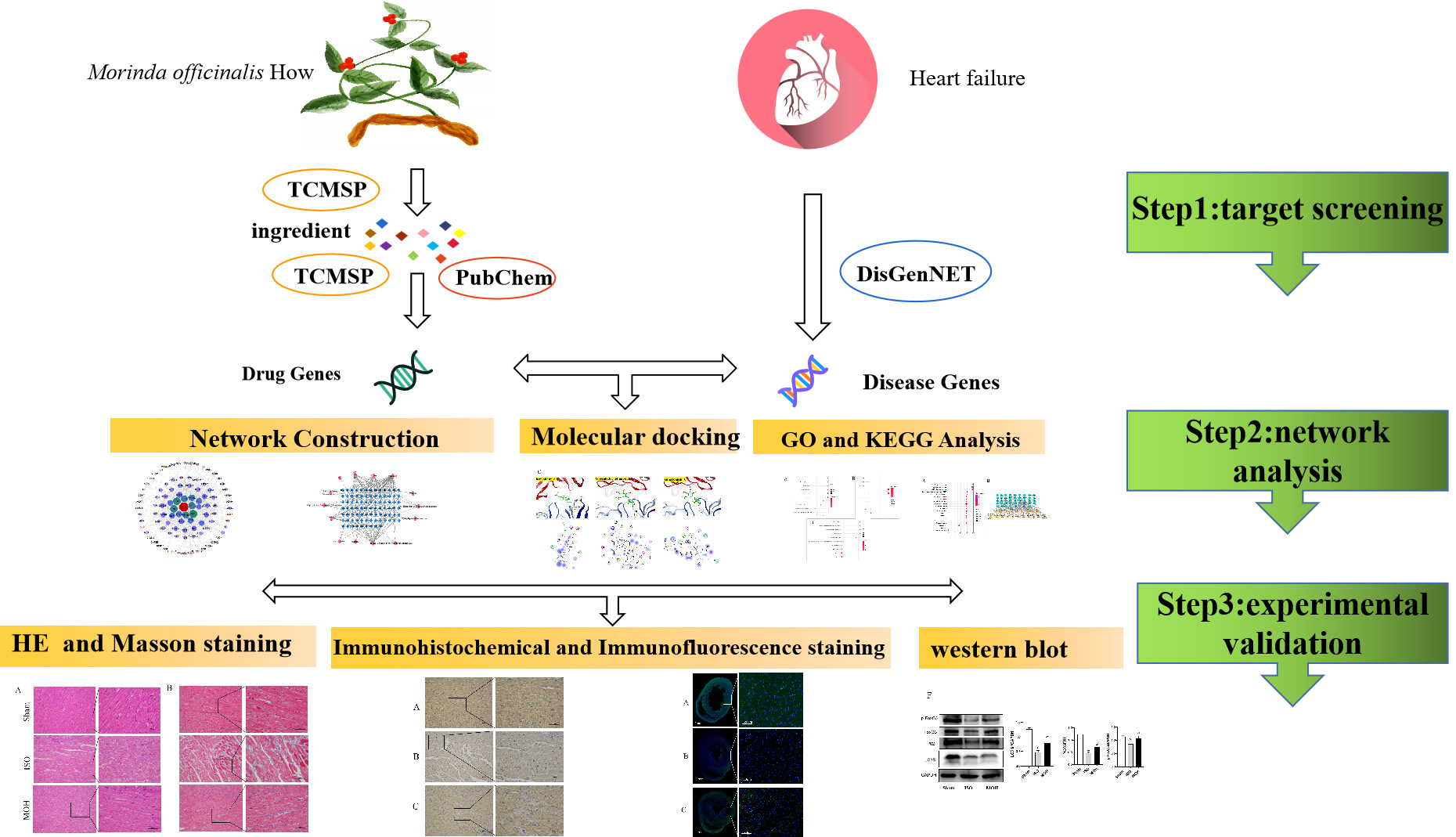 Fig. 1.
Fig. 1.Detailed design flow chart of the current study.
All components of the MO were searched from the traditional Chinese medicine
systems pharmacology (TCMSP) database (http://tcmspw.com/). The
exploration of the pharmacokinetic characteristics was performed using the TCMSP
database, which is a unique pharmacological platform for TCMs or compounds. It
can offer systematic information on the Absorbsion Distribution Metabolism and Excretion (ADME) characteristics of a drug with
potential biological function such as, oral bioavailability (OB) and
drug-likeness (DL). The values set as cut-off for the evaluation of the bioactive
components were OB
DisGeNET (http://www.disgenet.org/) serves a wide range of users and
purposes by providing one of the most comprehensive repositories on the genetic
origins of human illnesses [15], we collected HF-related targets with “heart
failure” as the search term, and screened the targets with Score_gda
Based on the Venny 2.1.0 (https://bioinfogp.cnb.csic.es/tools/venny/index.html) was analyzed to intersect the MO active component targets with the HF targets, and obtain information on potential targets for MO for HF. And then, the target information of the active component compounds of MO and the target information of Heart failure were categorized and saved, and a Venn diagram was created.
Establishment of STRING Network and Module Construction of heart failure
targets. The database STRING has collected and integreate a large number of
organisms PPI networks, including physical and functional relationship [16, 17].
The PPI network construction data were exported from STRING. The term “multiple
proteins” was selected in the module area, overlapping genes were input, and
“Homo sapiens” was used as the study object with a confidence level of
Based on above results, a compound-target-disease network composed of compounds, Compounds, genes, and proteins are nodes, while the interactions between compounds and targets are denoted as edges [20]. The molecular mechanism of MO in the treatment of HF can be further expressed to generate a network map of targeting HF and an interactive map of core gene-related pathways.
The DAVID functional annotation bioinformatics microarray analysis
bioinformatics resources (https://david.ncifcrf.gov/) are a set of integrated
biological knowledge repository and analytic website targeted at systematically
retrieving biological meaning from humongous gene/protein lists [21], The core
targets collected were imported into the GO enrichment and KEGG pathway analyses
were performed with p
Based on the aforementioned study, the integration of the core targets of MO-HF was performed by selecting the key targets from the Cytohubba plug-in gene network map and conducting molecular docking analysis with active compounds. The 3D structures of the core active ingredients were obtained by PubChem database search, the file was saved in SDF format, and MOE software was used to convert it to PDB by clicking “quickprep”. The protein crystal structures of the kernel targets were obtained from the PDB database (http://www.rcsb.org/), and PyMOL software was used to remove waters and ligands. Finally, the molecular structure protein was docked to the ligand using MOE software.
MO pellet herbs were produced in Guangdong Party Pharmaceutical Co., Ltd (Foshan, Kwangtung, China) purchased from Hainan Hospital of Traditional Chinese Medicine; ISO (Isoprenaline Hydrochloride) was purchased from Macklin Co. (Shanghai, China). The antibodies against FoxO3, p-FoxO3, P62, and GAPDH were from Proteintech, and LC3B was from Cell Signaling Technolog (Danvers, MA, USA), whereas HRP-labeled Goat Anti-Rabbit IgG, HRP-labeled Goat Anti-Mouse IgGand and BeyoECL Star from Beyotime (Shanghai, China). Hematoxylin-eosin and DAPI staining solutions were from Thermo Fisher Scientific (Waltham, MA, USA). A fully automated chemiluminescence image analysis system (978978, Tanon, Shanghai, China); Embedding Machine (EG1150C, Leica, Wetzlar, ED); tabletop high-speed frozen centrifuge (5424R, Eppendorf, Hamburg, Germany) and a multifunctional Enzyme Labeler (SynergyHTX, BioTek, Vermont, USA).
A total of 18 SPF Wistar male rats (200
SpecificpathogenFree (SFP) rats were anesthetized with 1% pentobarbital sodium intraperitoneally and perfused with 4% paraformaldehyde after rapid perfusion of heart with prechilled sodium chloride solution via the aorta to fix. Subsequently, it was dehydrated and paraffin-embedded blocks were prepared. The cardiac histology and myocardial fibrosis were observed using haematoxylin and eosin (H & E) and Masson staining heart slices.
Microwave heating in citrate buffer for 20 min was used to perform antigen unmasking. At 4 °C overnight, the sections were immunostained with a primary antiFoxO3 antibody. The sections were stained with diaminobenzidine following incubation with the secondary antibody. The purpose of this study was to investigate the mechanism of action of MO in the treatment of heart failure by assessing the protein expression of FoxO3 in the myocardium.
The heart tissue sections were incubated with blocking buffer at room temperature for 30 min and antiFoxO3 was added (1:100). The samples were incubated overnight. The antibody was discarded and the samples were washed three times with PBS the following day. Subsequently, they were incubated with a goat antirabbit antibody (1:500) for 1 h at 37 °C and washed three times with PBS. Subsequently, they were covered with 75% glycerol containing 1% DAPI. Finally, they were examined on a fluorescence microscope.
The proteins were extracted from the left ventricular myocardial tissue of each group of rats. Total protein was extracted and analyzed by western blot analysis. Protein separation was performed using SDS-PAGE and subsequently, the samples were transferred on polyvinylidene fluoride (PVDF) membranes. The membranes were blocked in a western blot blocking buffer for 15 min at room temperature and incubated with a primary antibody. The bands were visualized using horseradish peroxidase-coupled secondary antibody and BeyoECL Star. The final analysis was performed in a chemiluminescence imaging system.
The data are shown as mean
By using the aforementioned database, the potential active ingredients of MO were screened and the basic information of the relative active compound of MO is shown in (Table 1). A total of 176 drug targets and 2454 HF-related targets were mapped by applying a Venn diagram. A total of 101 overlapped targets were obtained (Fig. 2A).
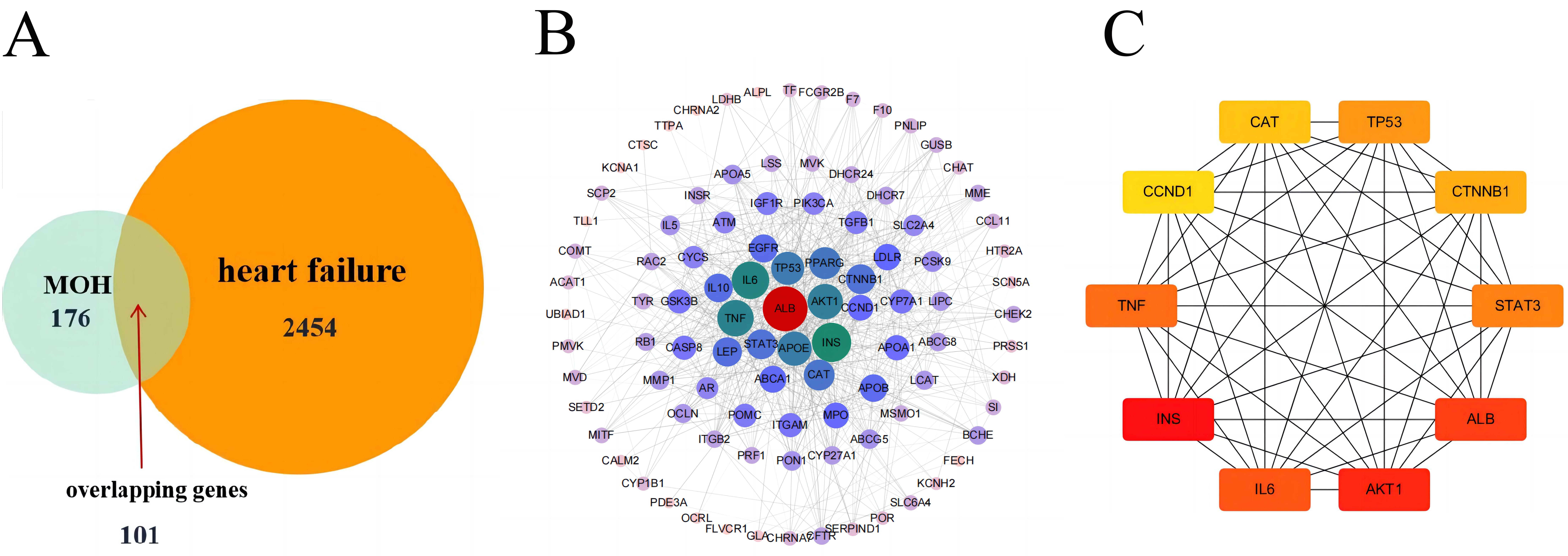 Fig. 2.
Fig. 2.Active Compounds and Targets of MO. (A) Venny diagram of Morinda officinalis How and heart failure targets. (B) Morinda officinalis How-Heart failure core target PPI network. (C) The MCC algorithm was used to analyze the top 10 hub genes network of MO for HF treatment.
| Molecule Name | Chemical structures | OB (30%) | DL (0.18%) |
|---|---|---|---|
| Ethyl oleate (NF) | 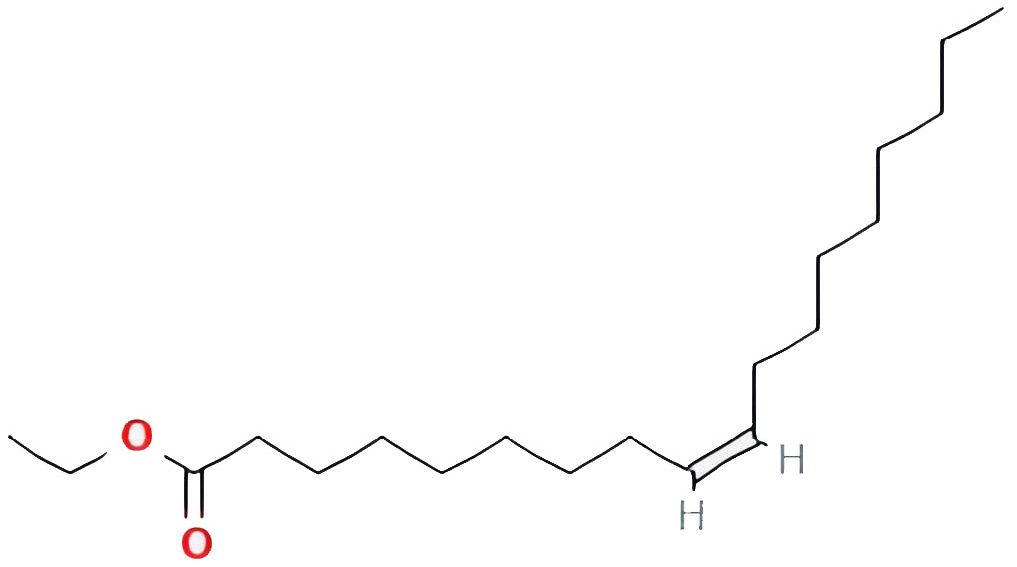 |
32.39738821 | 0.19061 |
| Alizarin-2-methyl ether | 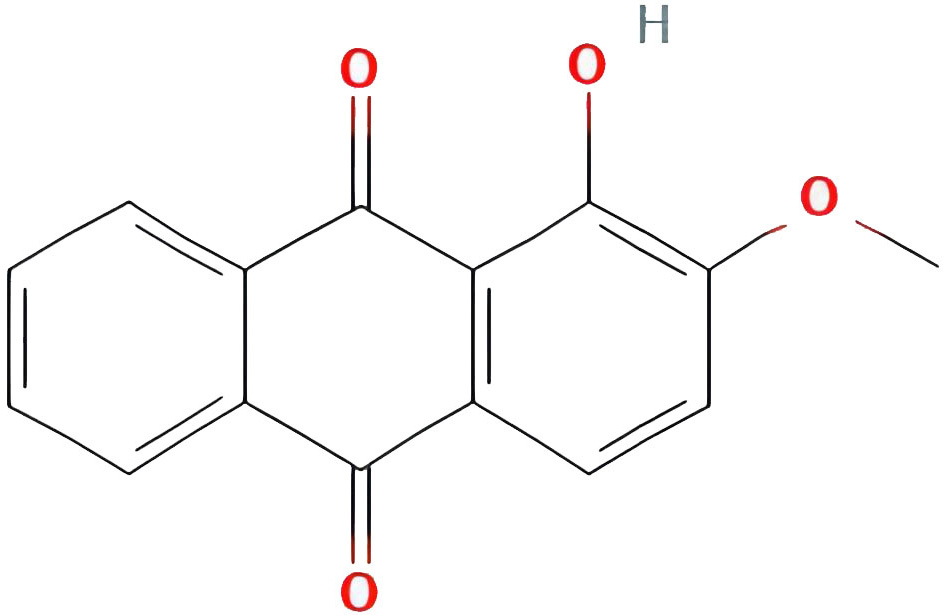 |
32.80877385 | 0.20971 |
| Supraene |  |
33.54594264 | 0.42161 |
| 3beta-24S(R)-butyl-5-alkenyl-cholestol | NA | 35.35248934 | 0.82221 |
| sitosterol | NA | 36.91390583 | 0.7512 |
| beta-sitosterol |  |
36.91390583 | 0.7512 |
| 3beta,20(R),5-alkenyl-stigmastol | NA | 36.91390583 | 0.75074 |
| Ohioensin-A |  |
38.13467065 | 0.75842 |
| Diop |  |
43.59332547 | 0.39247 |
| Asperuloside tetraacetate | 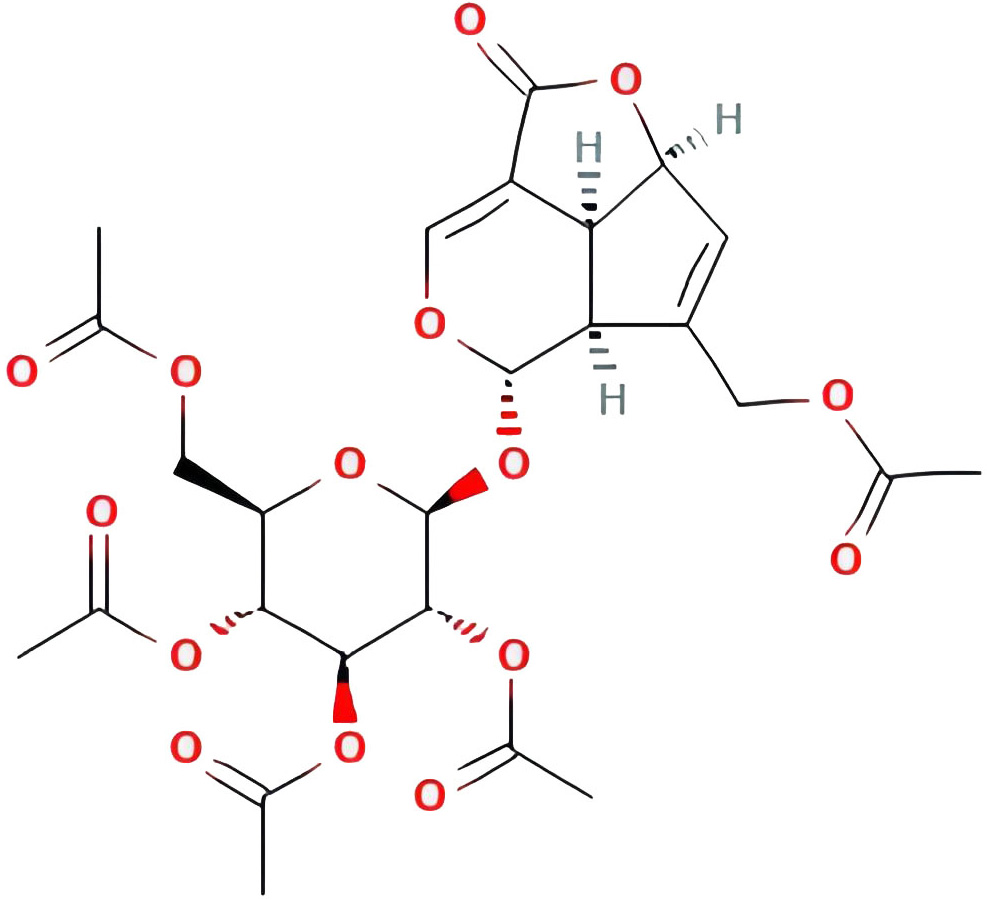 |
45.47262233 | 0.81584 |
| americanin A | 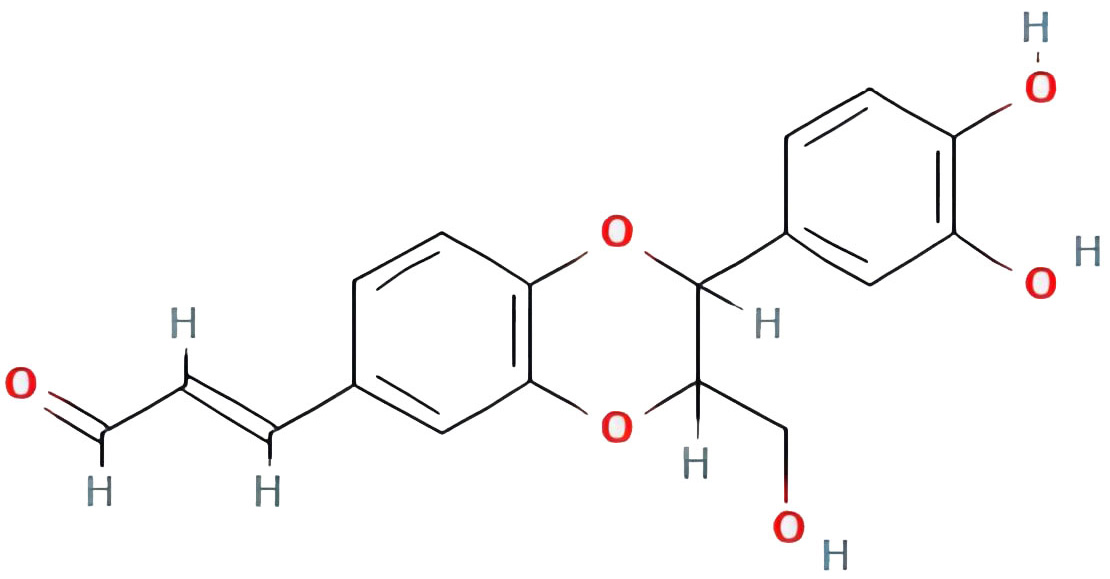 |
46.70571232 | 0.34901 |
| isoprincepin | 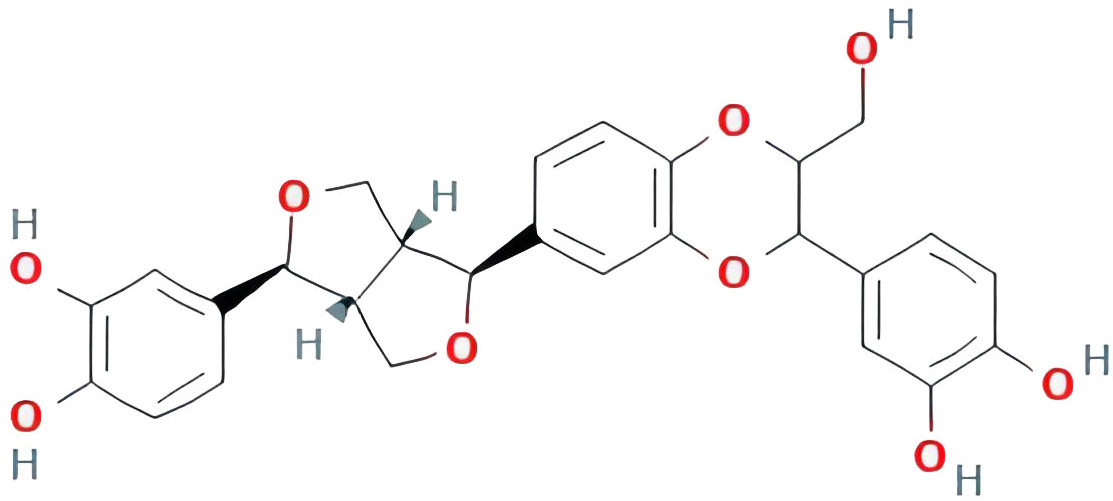 |
49.12131675 | 0.77375 |
| (2R,3S)-(+)-3’,5-Dihydroxy-4 ,7-dimethoxydihydroflavonol | NA | 77.23781866 | 0.33461 |
| 1,5,7-trihydroxy-6-methoxy-2-methoxymethylanthracenequinone | NA | 80.4229501 | 0.37789 |
| 1-hydroxy-6-hydroxymethylanthracenequinone | NA | 81.76548177 | 0.2115 |
| 2-hydroxy-1,5-dimethoxy-6-(methoxymethyl)-9,10-anthraquinone | 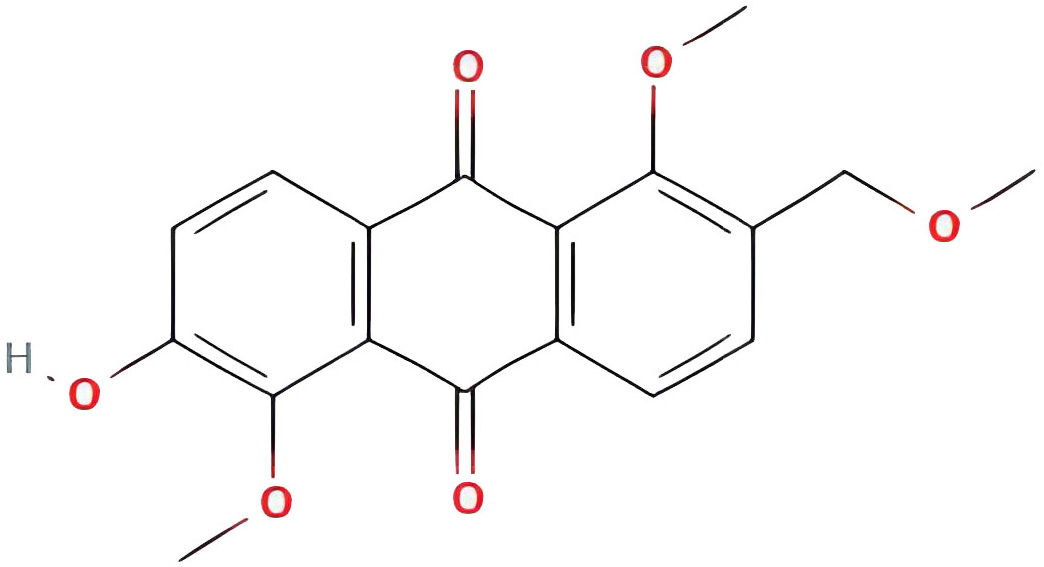 |
95.85173977 | 0.37249 |
| 1,6-dihydroxy-5-methoxy-2-(methoxymethyl)-9,10-anthraquinone |  |
104.5393992 | 0.33917 |
| 2-hydroxy-1,8-dimethoxy-7-methoxymethylanthracenequinone | NA | 112.3026489 | 0.37164 |
In Cytoscape 3.7.2 software, the PPI network of the 101 targets was established (Fig. 2B). A total of 101 nodes and 832 edges were involved in the PPI network. These targets with higher values of “Degree” were classified as the core targets for HF (Fig. 2C).
Firstly, the no-target and repeat active compounds were removed, resulting in 14 compounds. The “active compounds-disease targets” network was built in Cytoscape 3.7.2 using overlapping targets and related active components (Fig. 3). A total of 101 nodes and 175 edges were found in this network. The compounds with the dense connectivity maybe the main compound components of MO used in the treatment of HF.
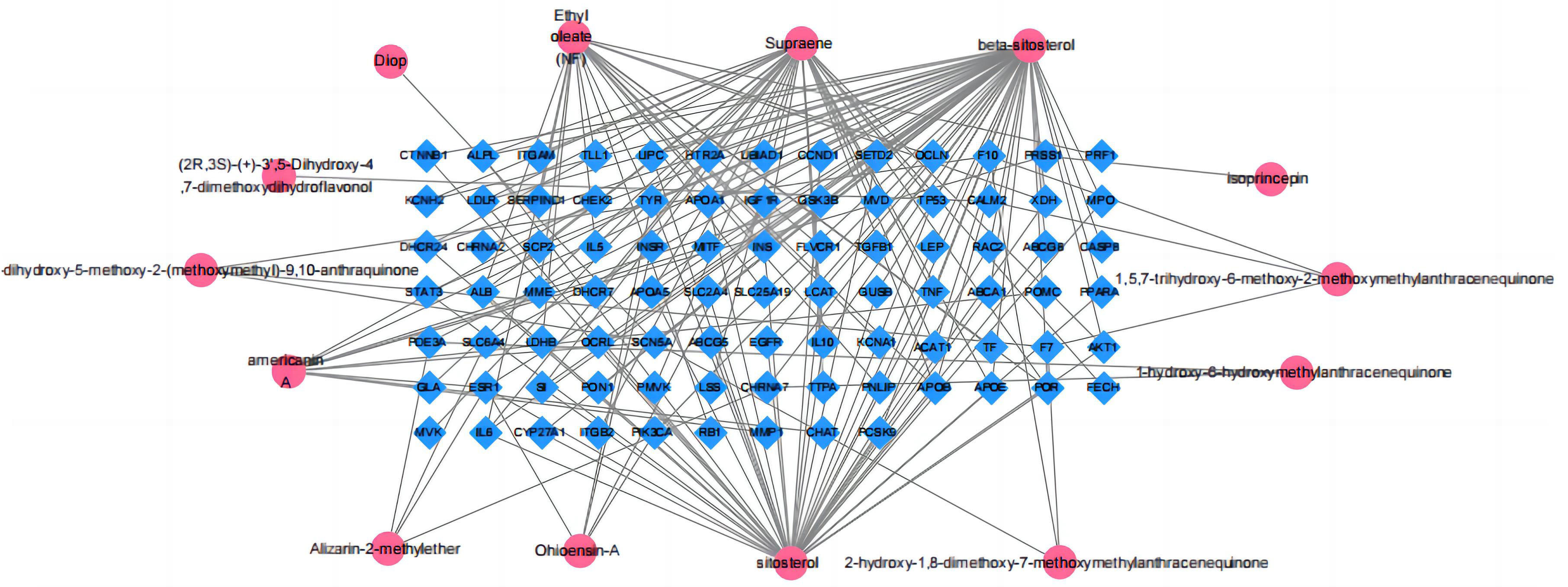 Fig. 3.
Fig. 3.Morinda officinalis How active ingredients-potential targets network.
According to GO and KEGG enrichment analyses, the mechanism of action of MO in the treatment of heart failure was mainly related to the role of nitric oxide biosynthesis in regulating the synthesis of secondary metabolites in cells. Biological process (BP) was mainly involved in the positive regulation of transcription, DNA-template synthesis, positive regulation of protein kinase B signaling and regulation of cholesterol metabolism and transport; the cellular component (CC) mode mainly involved extracellular space, lipoprotein particles, and receptor complexes in the lumen of the endoplasmic reticulum lumen; the molecular function categories (MF) mainly involved binding of enzymes, cholesterol, and phospholipids. The graphs were formed using the bioinformatics (http://www.bioinformatics.com.cn/) platform (Fig. 4A–C). The top 20 pathways and the number of targets involved were screened (Fig. 5A), including cancer (prostate, colorectal, and pancreatic), inflammation (nonalcoholic fatty liver, hepatitis B), apoptosis, receptor-conduction (FoxO, AMPK, Rap1, and HIF-1 signaling pathway, and blood glucose (insulin resistance). The results indicated that the mechanism of action of MO in the treatment of HF involved AKT1, STAT3, IL-6, and other targets, which affected cancer, inflammation, regulation of blood glucose levels, and receptor functions. The target-pathway network comprised 64 nodes and 202 edges (Fig. 5B).
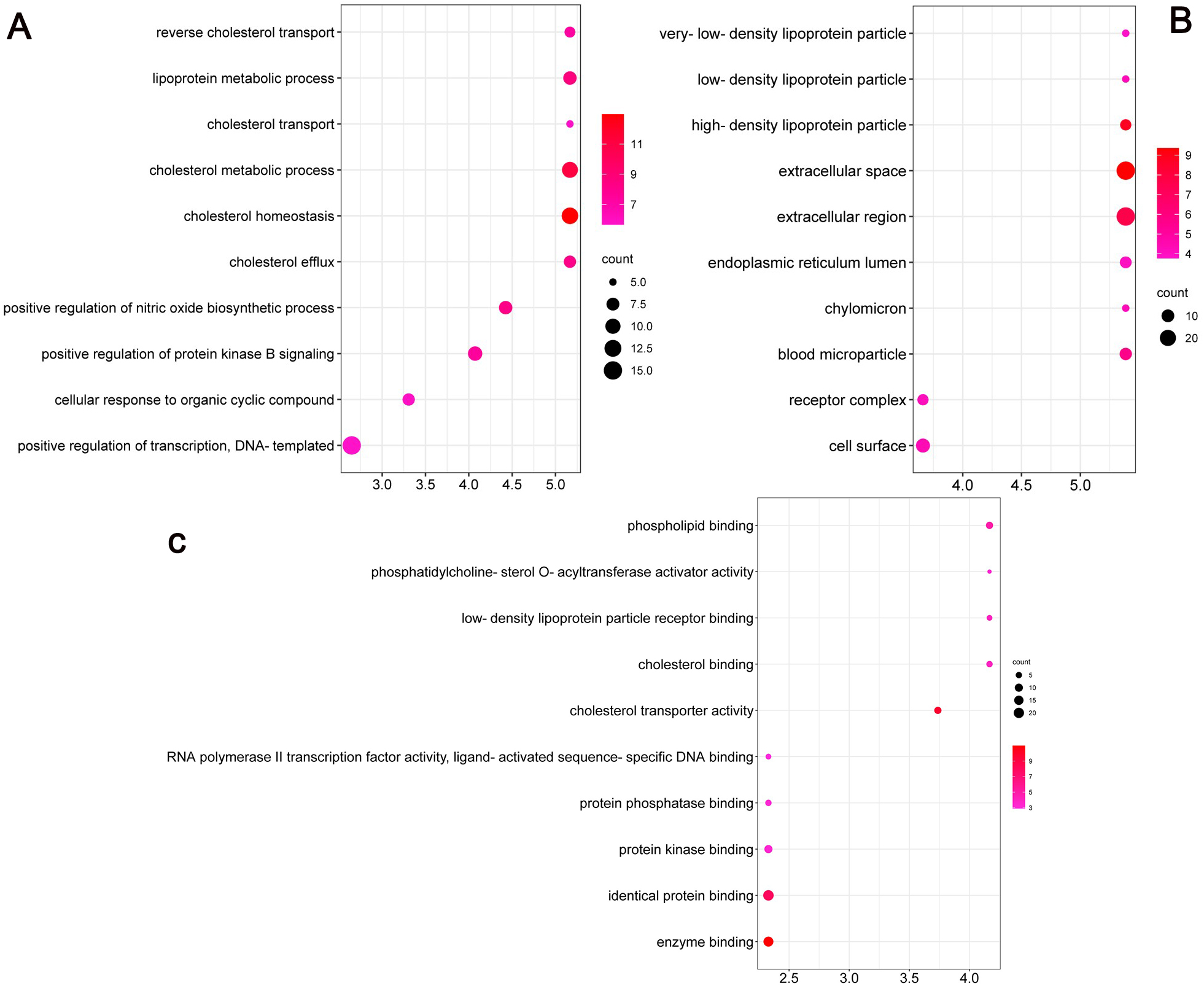 Fig. 4.
Fig. 4.GO enrichment analysis of the antiheart failure targets of Morinda officinalis How. (A) Biological Processes, (B) Cellular Components and (C) Molecular Function.
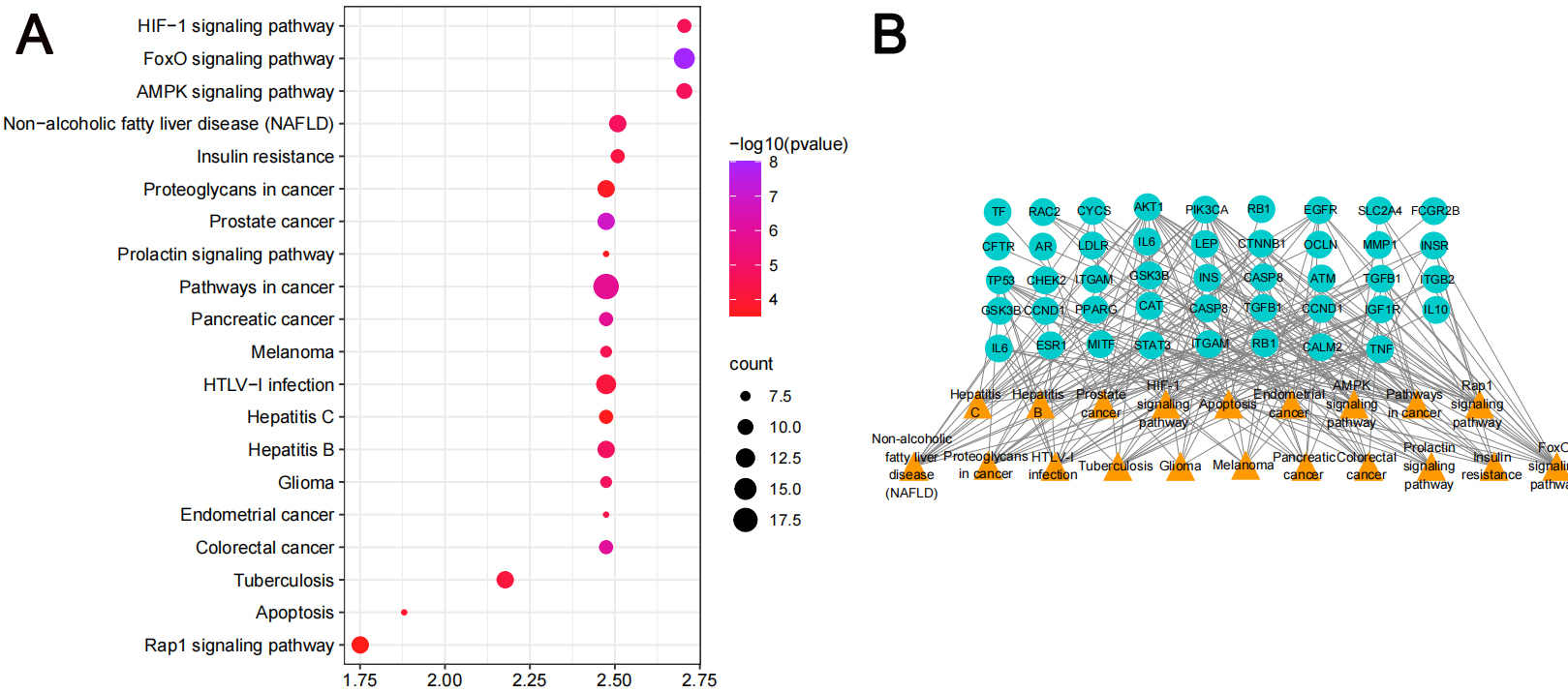 Fig. 5.
Fig. 5.KEGG pathway analyses of the antiheart failure targets of Morinda officinalis How. (A) KEGG enrichment analysis (B) The target-pathway network implicated in the mechanism of Morinda officinalis How in heart failure treatment.
MOE software was used to dock the MO active components (beta-sitosterol, americanin A, Asperuloside tetraacetate) with the core targets of ALB (PDB code: 6O69), AKT1(PDB code: ZUZW), STAT3 (PDB code: 4Z1A), IL6 (PDB code: 4O9H). These receptor proteins play a significant role in the KEGG signaling pathways and in the critical nodes of the PPI network and clusters. Most of the docking interactions between MO active ingredients and protein are hydrophobic interactions and hydrogen bonding effects. The lower the intermolecular binding energy, the better the docking strength. When the binding energy was less than 5 kcal/mol, the receptors and ligands had optimal binding properties. The core targets and their corresponding compounds demonstrated binding energies of almost –5 kcal/mol, which suggested that the core targets and their matching molecules exhibited a high affinity (Fig. 6A–C, Table 2). By using molecular docking, it was shown that the core targets of MO responsible for its action on HF and the relevant active constituents had a strong binding ability, which confirmed the reliability of the network pharmacological model used.
| Ligand receptors | beta-sitosterol | Asperuloside tetraacetate | americanin A |
|---|---|---|---|
| AKT | –6.4641 | –7.0476 | –6.6326 |
| STAT3 | –6.3519 | –6.5650 | –5.0980 |
| IL-6 | –5.3850 | –6.9994 | –5.2379 |
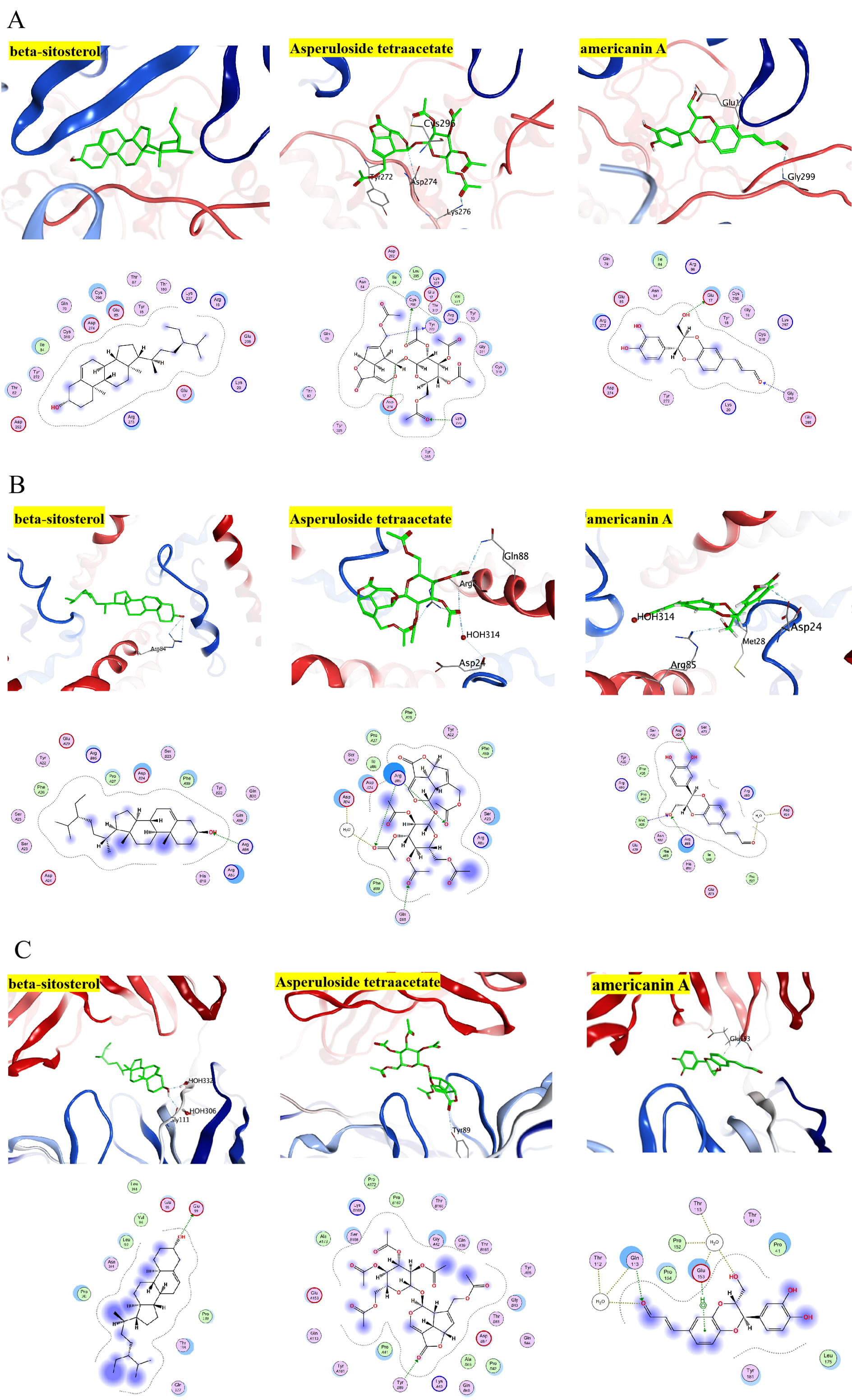 Fig. 6.
Fig. 6.Diagrammatic 3D and 2D representation that the molecular docking model. (A) The docking result of compounds with AKT. (B) The docking result of compounds with STAT3. (C) The docking result of compounds with IL-6.
H & E and Masson staining were used to estimate whether MO could be used to treat interstitial fibrosis in HF rats. The results indicated that the cardiomyocytes in the Sham group were normal in shape without inflammatory infiltration. The cardiac muscle fibers were ordered neatly. In the ISO group, the myocardial cells were associated with interstitial edema, which is a disorder of the cardiac muscle fibers; they were also arranged in a disordered manner and were accompanied by a high number of infiltrating inflammatory cells. Such histological changes were obviously alleviated in the MO groups compared with the ISO group (Fig. 7A,B).
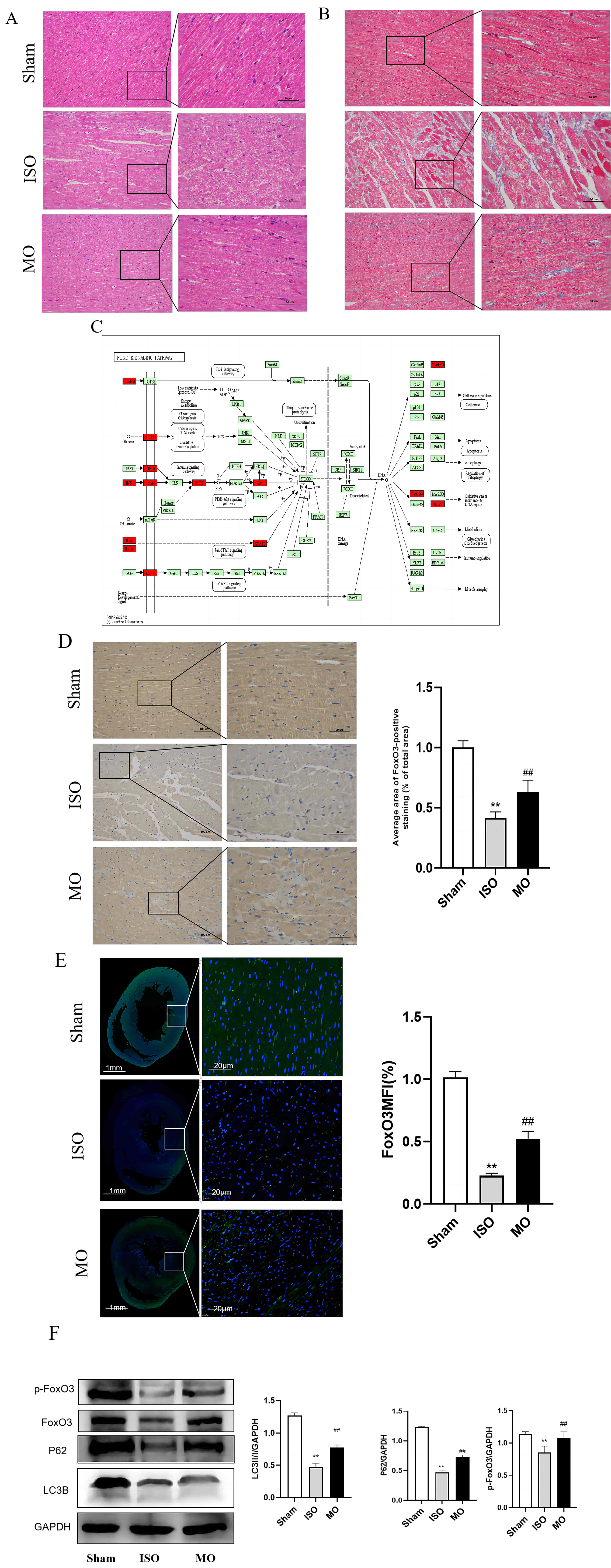 Fig. 7.
Fig. 7.In vivo study. (A,B) Pathological changes of myocardial
tissue in rat. H&E and masson staining. (C) The regulation of FoxO signaling
pathway in heart failure. (D,E) FoxO3 expression in rat myocardial tissue,
Immunohistochemical and immunofluorescence staining. ** p
KEGG enrichment analysis indicated that the FoxO signaling pathway was widely active in the cells investigated and could regulate the cell cycle, metabolism, autophagy, and other functions (Fig. 7C), to investigate whether MO could modulate the FoxO3 signaling pathway for the treatment of HF, the role of this pathway was investigated in myocardial tissues. The results indicated that the FoxO3 of the ISO group exhibited a dramatic reduction compared with the Sham group. Moreover, the FoxO3 of the MO group was apparently increased in comparison with the ISO group (Fig. 7D,E). Based on network pharmacology analysis and literature research, the exploration of the underlying mechanism of MO was assessed in the treatment of HF. FoxO3 is a master regulator of protein catabolism in the heart, orchestrating an atrophy response via both autophagy-lysosomal and proteasomal degradation pathways [24]. The expression levels of LC3B, P62 (autophagy markers), p-FoxO3 proteins were analyzed in tissues following ISO injury. The results demonstrated that the expression levels of LC3B and P62 in the ISO group indicated an apparent decrease compared with those of the Sham group, whereas significant recovery of these protein levels were achieved following MO group intervention. In addition, the expression levels of p-FoxO3 in the ISO group were reduced compared with the Sham group, which were significantly recovered by MO treatment. These results were in accordance with the immunohistochemical and immunofluorescence analysis (Fig. 7F).
Adjuvant therapy with TCM may be helpful to patients with HF, and its mechanism
of action may be related to the regulation of angiogenesis, apoptosis, oxidative
stress, and to the attenuation of inflammation [25]. Network pharmacology can
provide additional insight into a better understanding of the pharmacological
mechanisms of TCM and may also offer a reference for interdisciplinary research
[26]. The active ingredient of MO, beta-sitosterol (BS) causing an upregulation
of cellular glutathione redox cycling, restored the red blood cell membrane
mobility, and reduced oxidative damage to cardiac muscle cells [27]. During
inflammation, BS can be imported by calcium uptake in activated neutrophils and
inhibits IL-1
The top 10 important key targets related to the development of HF were identified based on CytoHubba analysis, including AKT1, ALB, INS, IL-6, STAT3, TNF, CCND1, CTNNB1, TP53, and CAT. Loss of the Akt protein could lead to early contractile dysfunction, which could in turn lead to heart failure [36]. By activating the Akt protein, cardiac insulin sensitivity is improved, which prevents the reduction of myocardial contraction caused by aging [37]. In conclusion, AKT regulates cardiac growth, heart aging, glucose metabolism, and lipid metabolism disorders. STAT3 is a transduction protein and activity factor that plays a key function that affects the heart from myocardial infarction, hypertrophy, and cardiomyopathy [38]. Likewise, previous studies have shown damage angiogenesis and aggravated HF via suppressing STAT3 [39, 40]. IL-6 is an inflammatory cytokine, which exerts different effects on the cardiovascular system. During the development of ventricular hypertrophy, IL-6 knockout mice alleviated LV hypertrophy, and well preservation of LV function, reducing myocardial fibrosis after TAC, leading to the reduction of fibrosis and apoptosis [41]. Molecular docking results also strongly suggest that active MO compounds can effectively treat HF by binding to the above core genes. The extracellular and intracellular signals play a crucial role in cardiac homeostasis [42]. KEGG pathway analysis indicated that the targets influenced by HF included primarily the cancer-related, receptor-conduction, and insulin signaling pathways. HIF-1 is a key regulator of the hypoxia response, stimulating the transcription of target genes [43], regulating mitochondrial ROS to prevent excessive postischemic cardiac fibroblasts activation and proliferation [44]. Energy deficiency in cardiomyocytes is a dominant cause of heart failure. AMPK can sense the energy state of the cell and orchestrate a global metabolic response to energy deprivation [45]. FoxO has the most primitive antistress signaling molecule, and as a transcription factor that regulation the transcription of several target genes. In cardiomyocytes, it is mainly involved in cellular resistance to oxidative stress, cellular autophagy, mitochondrial regulation, and inflammatory response. These processes can regulate the function of heart cells following HF [46]. There are many isoforms of the Forkhead Box O (FoxO) gene in mammals, FoxO3 is the most widely expressed isotype in the heart [47]. Under starvation or myocardial ischemia conditions, FoxO3 increases the levels of autophagy. The increased FoxO3 activity following starvation or myocardial ischemia reduces cardiac apoptosis and fibrosis [48], and prevents ISO-induced myocardial fibrosis by increasing autophagy. Therefore, the regulation of FoxO3 can improve myocardial remodeling in atrial fibrillation [49].
In vivo studies combined with network pharmacology to provide more information and discuss the therapeutic applications of FoxO3 in the treatment of HF. In the present study, we found the expression levels of the autophagy markers downregulation in cardiac tissues of the ISO group. However, the autophagy markers significantly increased following MO pretreatment. The histopathology showed that decreased inflammatory cell infiltration and improved cellular fibrosis, indicating that MO may increase the immune function of myocardial cells; the associated mechanism of action may be mediated via the regulation of FoxO3 (Fig. 8), which in turn increases autophagy. These findings are consistent with the aforementioned research study.
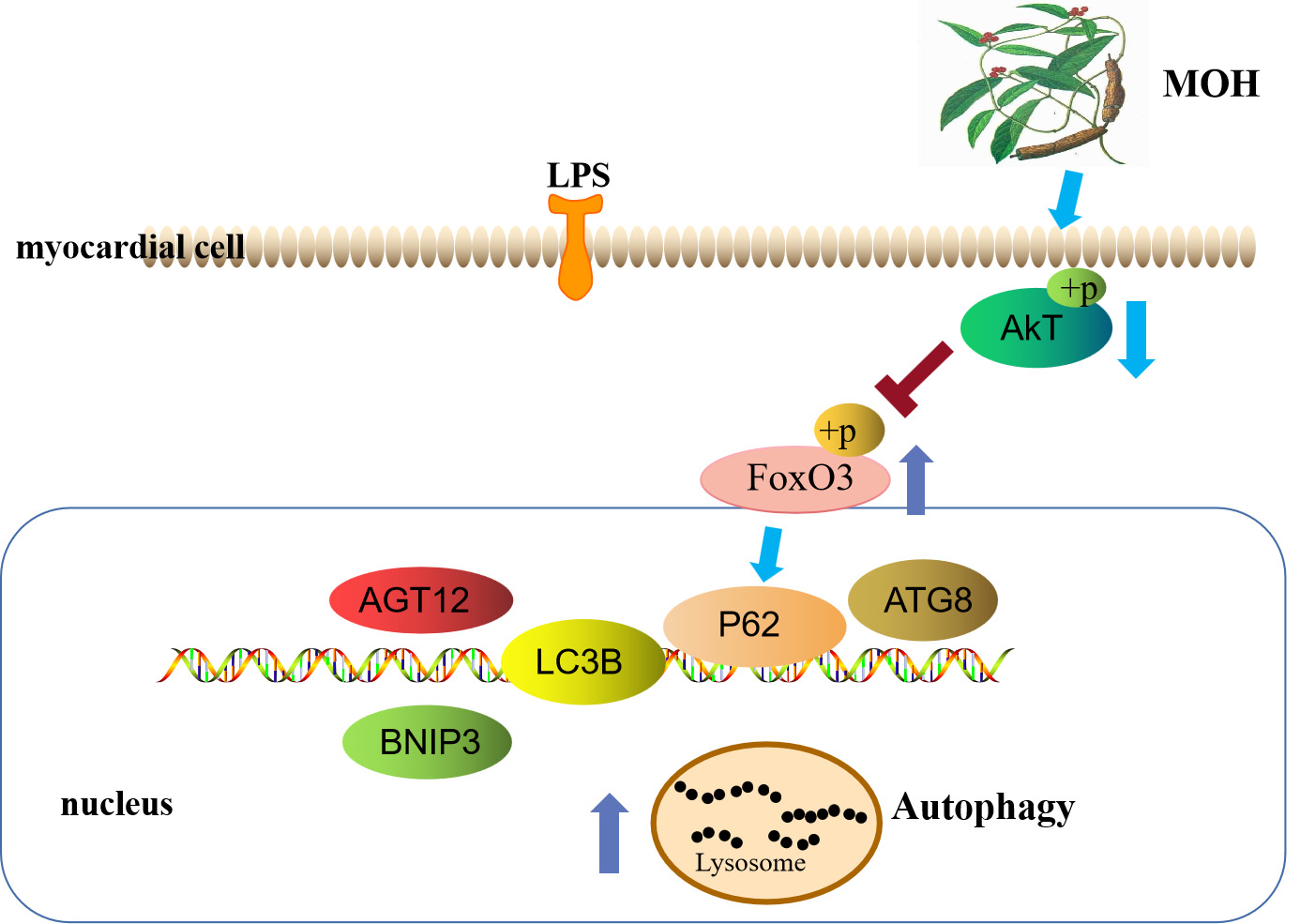 Fig. 8.
Fig. 8.Morinda officinalis How activates autophagy through FoxO3 Signaling Pathways to inhibit heart failure.
In conclusion, our results preliminarily revealed the pharmacological effects of MO against HF through bioinformatics and experimental investigations. The results showed that MO is a potential source that can be used in HF treatment, and it will contribute to validating the herbal medicine for treating cardiovascular disease. The data provided a theoretical basis for the subsequent studies that can be conducted to explore the mechanism of action of MO in the treatment of HF. However, the present study did not assess the clinical indicators which MO treated cardiac function. Therefore, follow-up experiments are further required to confirm these preliminary findings.
The data used to support the findings of this study are available from the corresponding author upon request.
YL and HZ—conceived and designed the study and experiments. AW and SD—drafted the experiments and manuscript. YG—analyzed interpreted the data. YY and ZY—help with the experiment and data collection. YL were responsible for supervision of the work. All authors reviewed the final manuscript. AW, SD and YG—have contributed equally to this work and share the first authorship.
Not applicable.
The authors thank Key Laboratory of Tropical Translational Medicine of Ministry of Education and Clinical Skills Experimental Teaching Center of Hainan Medical University gave support to the lab research.
The authors thank the members of their laboratory and their collaborators for their research work. This work was supported by High Level Talent fund project of Hainan Province (No. 2019RC376), and Project supported by the Education Department of Hainan Province (Hnky2022-37).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.








