1 Department of Biochemistry and Molecular Biology, Harbin Medical University, 150081 Harbin, Heilongjiang, China
2 Translational Medicine Research and Cooperation Center of Northern China, 150081 Harbin, Heilongjiang, China
3 Department of Biochemistry and Biotechnology, Technical University of Kenya, 999070 Nairobi, Kenya
4 Department of Biochemistry and Molecular Biology, Hainan Medical University, 571199 Haikou, Hainan, China
5 Academy of Medicine Sciences, 150081 Harbin, Heilongjiang, China
†These authors contributed equally.
Abstract
Background: The stemness characteristics of cancer cells, such as self-renewal and tumorigenicity, are considered to be responsible, in part, for tumor metastasis. Epithelial-to-mesenchymal transition (EMT) plays an important role in promoting both stemness and tumor metastasis. Although the traditional medicine juglone is thought to play an anticancer role by affecting cell cycle arrest, induction of apoptosis, and immune regulation, a potential function of juglone in regulating cancer cell stemness characteristics remains unknown. Methods: In the present study, tumor sphere formation assay and limiting dilution cell transplantation assays were performed to assess the function of juglone in regulating maintenance of cancer cell stemness characteristics. EMT of cancer cells was assessed by western blot and transwell assay in vitro, and a liver metastasis model was also performed to demonstrate the effect of juglone on colorectal cancer cells in vivo. Results: Data gathered indicates juglone inhibits stemness characteristics and EMT in cancer cells. Furthermore, we verified that metastasis was suppressed by juglone treatment. We also observed that these effects were, in part, achieved by inhibiting Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Pin1). Conclusions: These results indicate that juglone inhibits maintenance of stemness characteristics and metastasis in cancer cells.
Keywords
- juglone
- stemness
- epithelial-to-mesenchymal transition (EMT)
- Pin1
- metastasis
- tumorigenicity
Metastasis is the principal cause of cancer-related death [1]. Despite the development of therapeutic methods, such as radiotherapy and chemotherapy, many patients with advanced cancer still have a poor prognosis and low overall survival because of cancer metastasis and recurrence [2, 3]. The theory of cancer stem cells (CSCs) suggests that CSCs are one subpopulation of cancer cell which are generally considered to be responsible for high cancer metastasis and recurrence due to their stemness characteristics. CSCs is characterized by a slower proliferative rate, which allows it to escape the cytotoxic effects of radiotherapy and chemotherapy. CSCs also has other properties of stem cells, such as self-renewal, which increases the tumorigenicity of CSCs, helping to form spheroids or even tumors with fewer cells [4, 5]. A variety of transcription factors play important roles in maintaining CSCs stemness, including Nanog, OCT4 and SOX2 [6]. The elevated expression of Nanog can independently maintain embryonic stem (ES) cell self-renewal [7]. Nanog also works together with other transcription factors, such as Oct4 and Sox2, to control a set of target genes that have important functions in maintaining the stemness characteristics of cells [6, 8]. Furthermore, the epithelial-to-mesenchymal transition (EMT) can promote maintenance of stemness and metastasis in cancer cells. SOX2 also plays a key role in suppressing EMT and inhibiting cancer metastasis [9]. Therefore, interfering with the cancer cell stemness characteristics may be an effective strategy to inhibit cancer metastasis and recurrence.
Juglone is the main component of Juglans mandshurica peel, a traditional antitumor Chinese herbal formula that can prevent tumor metastasis and achieve long-term tumor-free survival [10]. It has been reported that juglone can block the cell cycle in the G0/G1 phase and inhibit the proliferation of glioblastoma cells [11]. Furthermore, juglone was shown to prevent tumor angiogenesis and induce cell apoptosis by regulating Bcl-2/Bax in breast cancer cells [11, 12]. Our previous work found that juglone could participate in tumor immune regulation and play an anticancer role [13]. However, the role of juglone in regulating cancer cell stemness characteristics, which are closely related to tumor metastasis and recurrence, has not been clarified.
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (Pin1) is a unique enzyme that specifically catalyzes the cis-trans isomerization of phosphorylated serine/threonine-proline (pSer/Thr-Pro) motif [14]. Juglone is a small molecular inhibitor of Pin1 and can bind to Cys113 of Pin1, which could reduce the stability of Pin1 and make it rapidly ubiquitylated and degraded [15]. Many evidences suggest that Pin1 is widely overexpressed in cancer and has an important effect on tumor initiation and progression by regulating biological activity.
In this study, we report that juglone inhibits cancer cell stemness characteristics, including the self-renewal and tumorigenicity, in both breast and colorectal cancer cells. We also confirmed that juglone could significantly inhibit EMT and the migratory ability of various cancer cells. Further, we demonstrated that this effect of juglone on stemness characteristics may be achieved, in part, by downregulating Pin1 in cancer cells. Our present data elucidate a novel mechanism of the antitumor effect of juglone by inhibiting stemness characteristics and EMT in cancer cells, an affect that is, in part, induced by suppressing the expression of Pin 1.
The human breast cancer cell line MCF-7, the mouse breast cancer cell line 4T1
and the mouse colorectal cancer cell line CT26 used in this study were
purchased from the China Center for Type Culture Collection (Shanghai, China).
The human colorectal cancer cell line HCT116 was purchased from China Center for
Type Culture Collection (Beijing, China). HCT116 and CT26 were cultured in
RPMI-1640 medium (Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum
(FBS, Gibco, Carlsbad, CA, USA). 4T1 and MCF7 were maintained in Dulbecco’s
modified Eagle’s medium (DMEM, Gibco, Carlsbad, CA, USA) containing 10% FBS. All
cells were maintained in a humidified incubator containing 5% CO
Cell viability was tested using the cell
counting kit-8 (CCK8, Dojindo, Japan). Briefly, the cells were seeded at a
density of 1
Full-length human PIN1 (NM006221) cDNA was amplified using the primers Pin1-Forward (EcoRI): 5′-CTGAATTCGCCACCATGGCGGACGAGGAGAAG-3′ and Pin1-Reverse (BamHI): 5′-CGGATCCCTCAGTGCGGAGGATGATG-3′. Total RNA used in this amplification step was isolated from HCT116. RNA was isolated as previously describe [16]. Resultant cDNA was subsequently subcloned into pLVX-DsRed vector (donated by Dr. Jianfeng Jin) using the primer-encoded BamHI and EcoRI restriction sites. Lentivirus was subsequently packaged as previously described [16].
According to previously obtained IC50 values, various doses of juglone were
selected to treat different cancer cells. The dose gradient for 4T1 was 0
500 cells/well of each line were seeded in a 24-well plate (Corning Life
Science, MA, USA) and cultured in serum free DMEM supplemented with 20 ng/mL
basic fibroblast growth factor (bFGF, Sigma, Darmstadt, Germany), 20 ng/mL
epidermal growth factor (EGF, Sigma, Darmstadt, Germany) and 20 ng/mL B27 (Gibco,
Carlsbad, CA, USA). 200
Cultured cells were treated with differing doses of juglone for 24 hours as indicated above. Then the cells were subsequently seeded in 35 mm culture dishes (Corning Life Science, MA, USA) at a density of 500 cells/dish and incubated for 7 days. Following this, colonies were fixed and stained with 0.1% crystal violet (Sigma, Darmstadt, Germany) and counted.
A transwell chamber (8
Twenty-one six-week-old BALB/c female mice were randomly divided into 3 groups.
4T1 cells were first treated with differing doses of juglone (0
Each tumor injection was prepared using a 50
Western blot analysis was conducted as previously described
[16]. Briefly, 30–50
All experiments were performed in, at least, triplicate, data
are presented as the mean (
Tumor sphere formation assays are a widely employed approach to assess cell capability of self-renewal [7]. Human breast and colorectal cancer cells were treated with differing concentrations of juglone for 24 hours, and a tumor sphere formation assay was subsequently performed. The appropriate concentrations of juglone used in different cell lines were selected according to an empirically obtained 50% inhibition concentration (IC50) (see Supplementary Fig. 1). The numbers and diameters of tumor spheres were counted after 7 days. Notably, juglone treatment significantly reduced the number and diameter of tumor spheres compared with the control (Fig. 1A,B). In a separate set of experiments, a colony formation assay also showed that juglone treatment reduced the number of colonies in a dose-dependent manner in both breast and colorectal cancer cells (Fig. 1C,D).
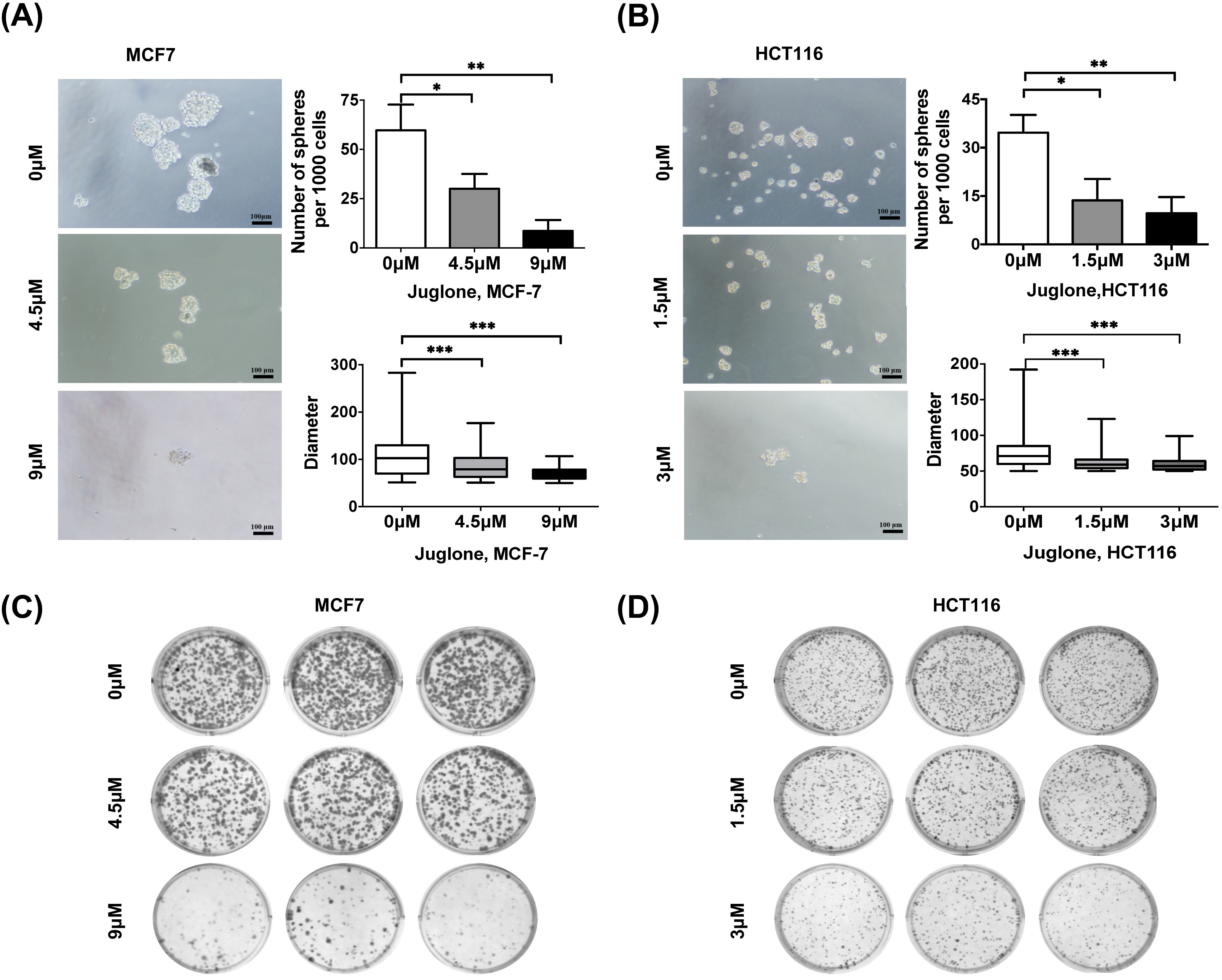 Fig. 1.
Fig. 1.Juglone suppresses the self-renewal ability of different cancer
cells. (A,B) MCF7 and HCT116 cells were treated with indicated doses of juglone
and cultured in serum-free media under non-adherent conditions for 7 days. Scale
bar, 100
To further determine the role of juglone in maintaining cancer cell stemness
characteristics, we investigated the effect of juglone on the CSC signaling
network. The Wnt/
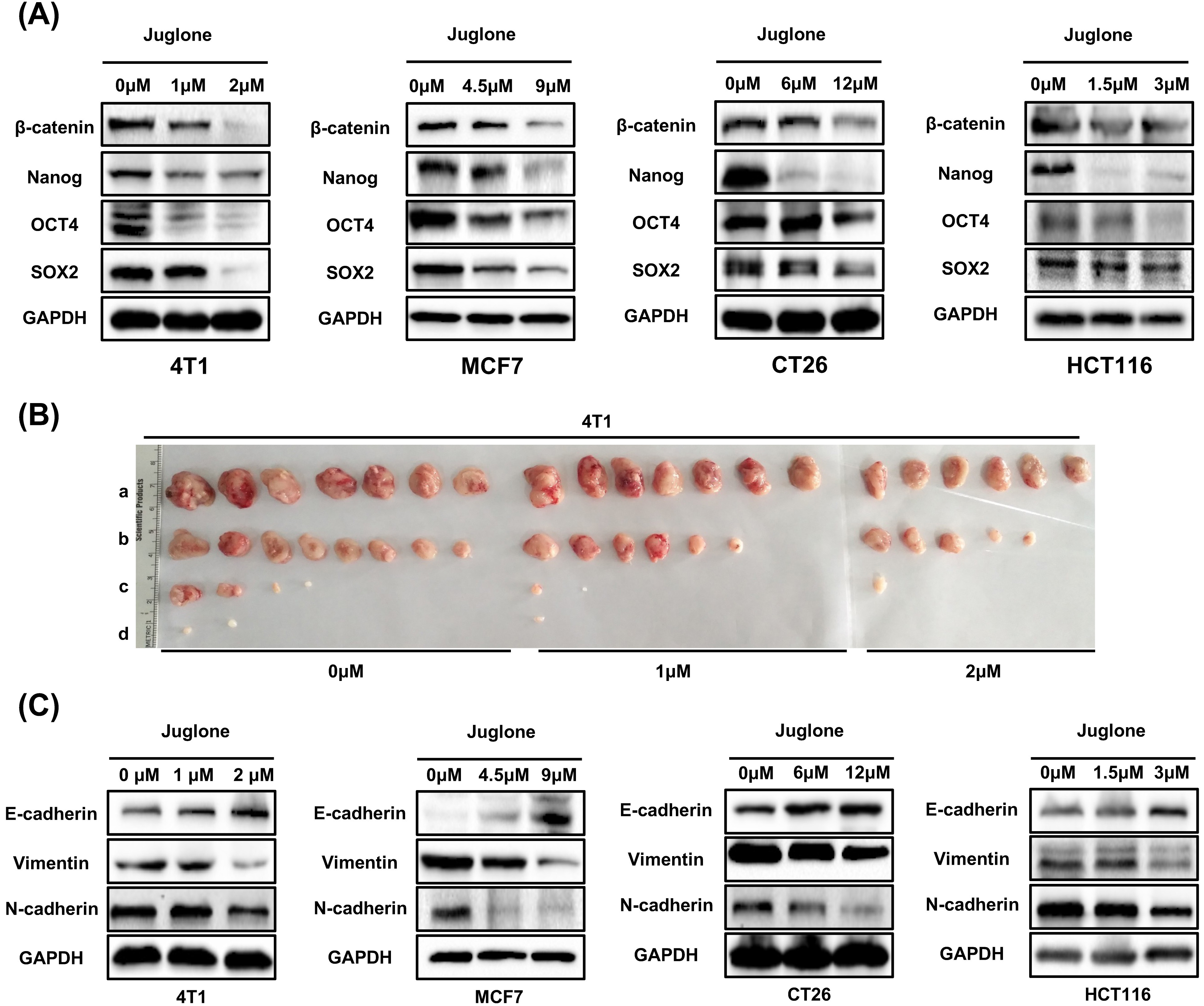 Fig. 2.
Fig. 2.Juglone suppresses stemness characteristics and EMT in cancer
cells. (A) The expression of
To examine the effect of juglone on cancer cell tumorigenicity in vivo,
a limiting dilution cell transplantation assay was performed in BALB/c mice
[19]. 4T1 breast cancer cells were treated
with indicated concentrations of juglone (0
| Group | Inoculation (cells/100 |
Tumor formation (Juglone treatment) | ||
|---|---|---|---|---|
| 0 |
1 |
2 | ||
| a | 1 |
7/7 | 7/7 | 6/7 |
| b | 1 |
7/7 | 6/7 | 5/7 |
| c | 1 |
4/7 | 2/7 | 1/7 |
| d | 1 |
2/7 | 1/7 | 0/7 |
EMT plays an important role in stemness maintenance and metastasis in cancer cells [17]. To further examine the effect of juglone on EMT, some typical EMT markers were assayed in cancer cells after juglone treatment. The results showed juglone treatment increased E-cadherin expression and decreased the vimentin and N-cadherin (Fig. 2C). These results are consistent with juglone downregulating EMT [9]. Taken together, these findings suggested that juglone could inhibit the self-renewal, tumorigenicity, and EMT of cancer cells in vitro and in vivo.
The transwell migration assay is a commonly used test to study the migratory response of cancer cells, a key characteristic for metastatic tumor spread [20]. To evaluate the role of juglone in downregulating tumor cell mobility, we performed a transwell assay with breast and colorectal cancer cells. The results showed juglone treatment could effectively reduce the mobility of both breast and colorectal cancer cells (Fig. 3A–D).
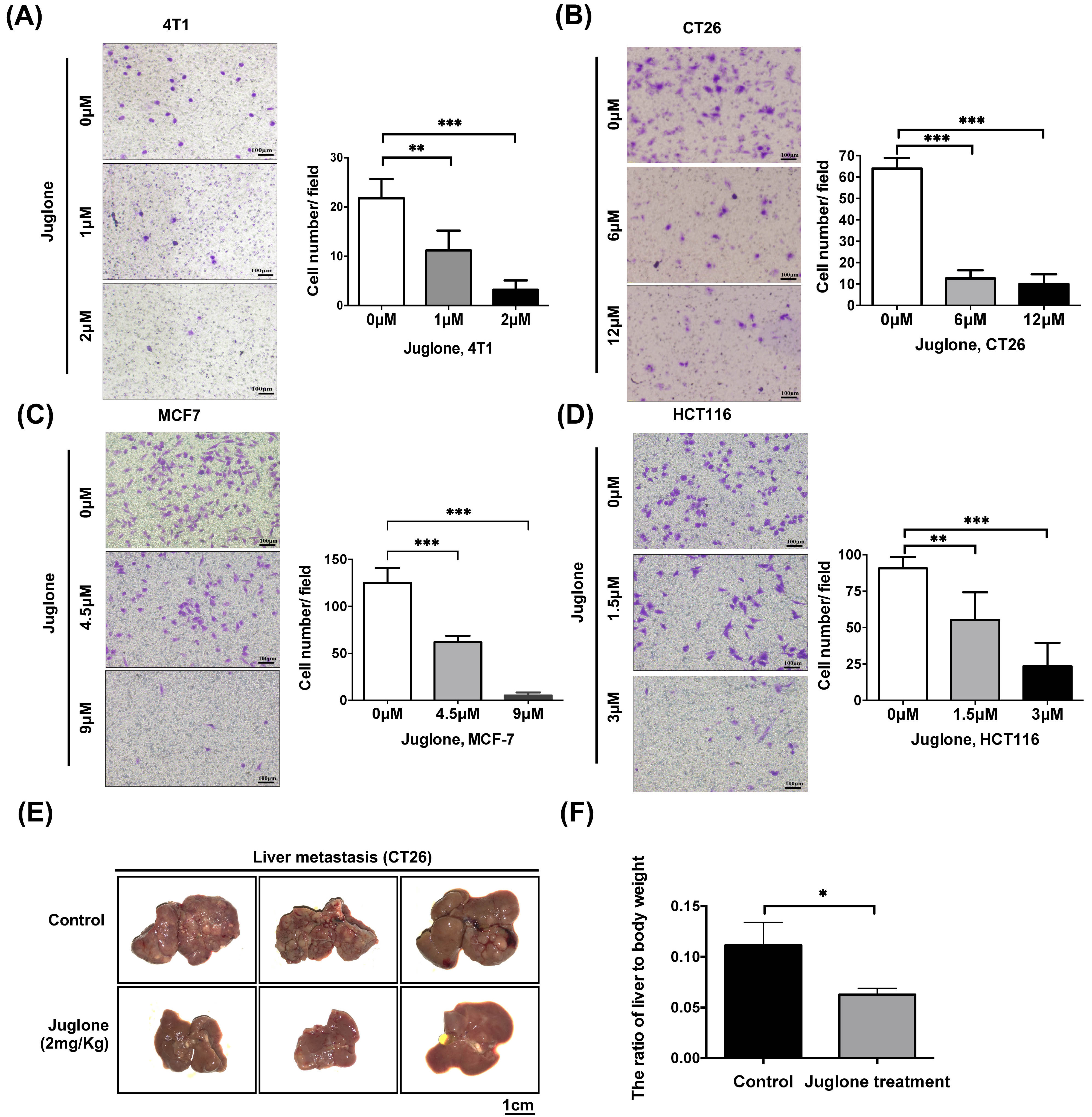 Fig. 3.
Fig. 3.Juglone inhibits cancer cells metastasis both in vitro
and in vivo. (A–D) Breast cancer cells (4T1, MCF7) and
colorectal cancer cells (CT26, HCT116) were treated with indicated doses of
juglone for 24 hours and subsequently counted and seeded into the chambers (1
Next, we established a mouse model of
colorectal cancer liver metastasis using a splenic injection model. 1
Juglone is a small molecule inhibitor of Pin1 [15] and
it has been reported that Pin1 could play a role in the maintenance of stemness and
induction of metastasis in breast cancer [21, 22]. To clarify whether Pin1 has the
same function in colorectal cancer cells, we first verified the inhibitory effect
of juglone on Pin1 expression in HCT116 cells (Fig. 4A).
We subsequently tested
the expression of
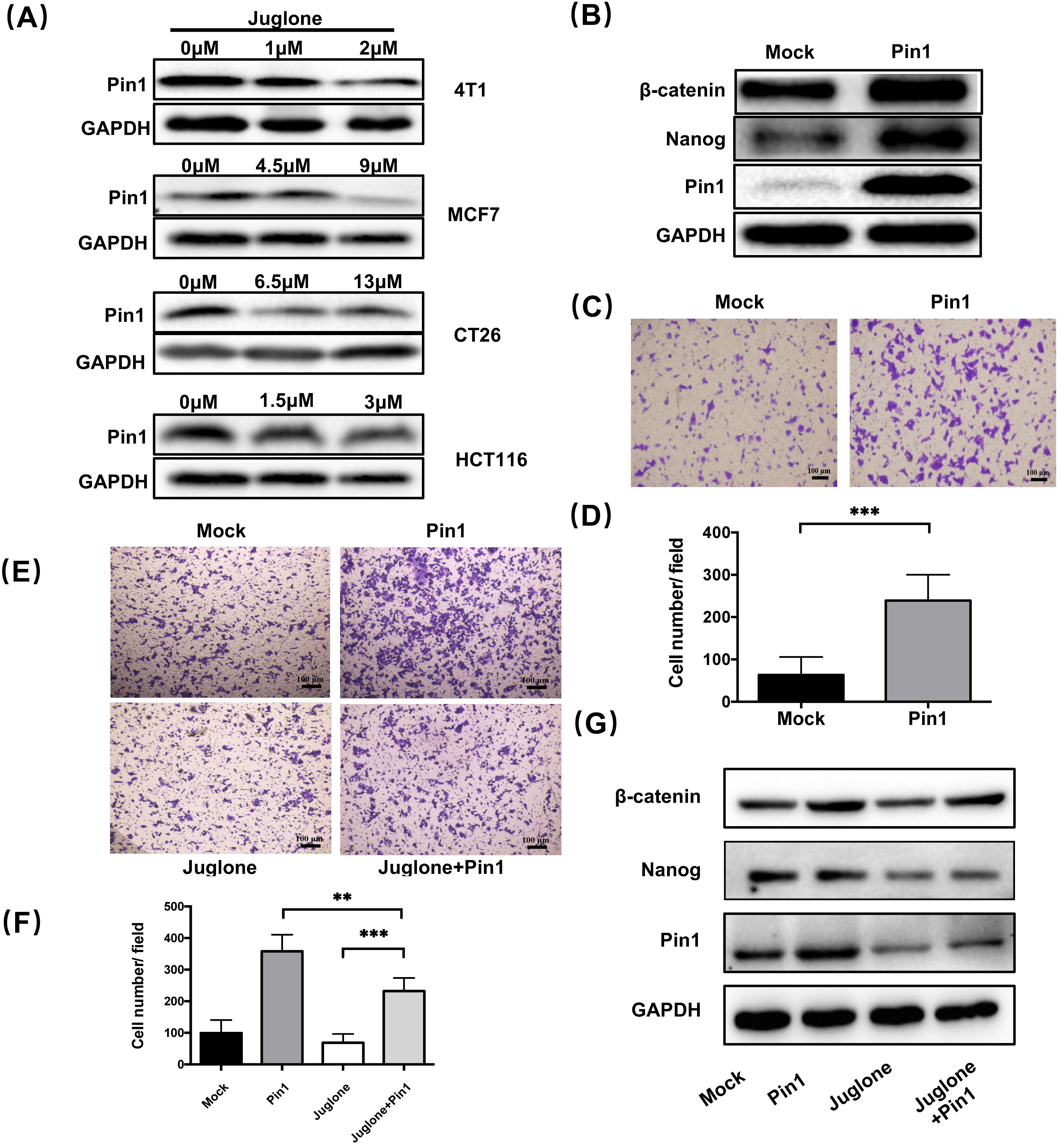 Fig. 4.
Fig. 4.Juglone suppresses the stemness characteristics of cancer cells
through inhibiting Pin1. (A)
The expression of Pin1 in four cell lines
treated with different concentrations of juglone was assayed
by western blot analysis. (B) HCT116 was
transfected with either pLVX-DsRed vector (Mock group) or pLVX-Pin1 vector (Pin1
group). The expression of
To clarify whether the
effect of juglone on cancer cell stemness characteristics is related to Pin1
inhibition, we next performed a rescue test. Specifically,
Pin1-overexpressing HCT116 cells were
treated with or without juglone (3
Cancer stem cells (CSCs) play a vital role in tumor recurrence and metastasis. Traditional treatments used to prevent tumor metastasis and recurrence, including radiotherapy and chemotherapy, kill tumor cells by interfering with DNA replication or cell cycle advance [5, 23]. However, due to their feature of inactive or very slow proliferation, CSCs can successfully escape radiotherapy and chemotherapy and play an important role in maintaining minimal residual disease which may result in metastatic tumor formation [24]. It is widely accepted that targeting CSCs may provide a promising therapeutic approach to reduce the risk of cancer recurrence and metastasis [25].
Juglone has been shown to have inhibitory
effects on tumor progression in various cancer types. Juglone inhibits the
proliferation of cancer cells by blocking cell cycle advance [11] and inducing
apoptosis through mitochondrial-dependent pathways [12]. In addition, juglone
suppressed tumor progression in mice by increasing oxidative stress, which led to
apoptosis and cell cycle blockade [21, 26]. Our previous study also found that
juglone could eliminate myeloid-derived suppressor cell accumulation and enhance
tumor immunity [13]. Besides, juglone can inhibit angiogenesis and metastasis in
pancreatic cancer cells by targeting Wnt/
Pin1 has been found to promote oncogenesis by upregulating more than 40 oncogenes in different types of cancers [22]. Pin1 plays a vital role in stemness maintenance, while inactivation of Pin1 function curbs cancer stem cell expansion and restores chemosensitivity [30]. It has been reported that Pin1 can promote the proliferation of breast CSCs by upregulating Rab2A transcription or targeting miR-200c [31]. Juglone is a small molecule inhibitor of Pin1 [15]. To clarify whether the regulatory role of juglone in the stemness characteristics of cancer cells is related to Pin1, we performed a rescue test combining juglone treatment and Pin1 overexpression. The results showed that Pin1 overexpression could block the suppressive effect of juglone on the stemness features displayed by cancer cells. Although current evidence does not allow us to conclude that juglone relies on Pin1 to inhibit cancer cell stemness, our findings suggest that juglone inhibits the maintenance of stemness characteristics and EMT through Pin1 inhibition as its mechanism of suppressing tumor metastasis.
Metastasis and recurrence are leading causes of cancer-related death. Stemness characteristics, including self-renewal and increased tumorigenicity, play important roles in resistance to cancer therapy and promote cancer metastasis. Our study reported a novel role of juglone in suppression of maintenance of stemness and EMT of cancer cells. Furthermore, we verified that cancer cell metastasis was suppressed by juglone treatment using an in vivo model. Finally, we document that these effects of juglone were likely achieved, at least in part, by inhibiting Pin1. In sum, these results indicate that juglone plays an inhibitory role in the maintenance of stemness and metastatic activity of cancer cells.
All data generated or analyzed during this study are included in this published article.
CXZ and XG designed the research study. CDZ, YY and HW performed the research. CM, SD and JJ provided help and advice on conception, acquisition of data and supervision. XC, YL and LW analyzed the data. CDZ and CXZ wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
The animal experiments were approved by the local ethics committee of Harbin Medical University (KY-2018-071) and were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Thanks to He Zhang for the technical support of data analysis.
This research was funded by Heilongjiang Touyan Innovation Team Program, HMU Marshal Initiative Funding [HMUMIF-21025]; Heilongjiang Postdoctoral Science Foundation [LBH-Z21178]; Open Project Program of Key Laboratory of Preservation of Human Genetic Resources and Disease Control in China (Harbin Medical University), Ministry of Education [LPHGRD2022-004] and Hainan Provincial Natural Science Foundation of China [No.2019RC203].
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.




