1 University of Belgrade-Faculty of Chemistry, 11000 Belgrade, Serbia
2 University of Belgrade-Institute of Chemistry, Technology and Metallurgy, 11000 Belgrade, Serbia
3 Laboratory of Bioinformatics and Computational Chemistry, Institute of Nuclear Sciences Vinca, National Institute of the Republic of Serbia, University of Belgrade, 11001 Belgrade, Serbia
4 Department of Molecular Biology and Endocrinology, VINCA Institute of Nuclear Sciences, National Institute of the Republic of Serbia, University of Belgrade, 11001 Belgrade, Serbia
5 Department of Pathology, University of Texas Medical Branch, Galveston, TX 77555, USA
6 Institute for Human Infections and Immunity, University of Texas Medical Branch, Galveston, TX 77555, USA
Academic Editor: Paramjit S. Tappia
Abstract
Background: Drug resistance is a critical problem in health care that
affects therapy outcomes and requires new approaches to drug design. SARS-CoV-2
M
Keywords
- anti SARS-CoV-2
- Mpro
- COVID-19
- arginine
- vitamin C/arginine combination
- Mpro candidate inhibitors
The SARS-CoV-2 virus quickly spread around the world and was classified by the World Health Organization on March 11, 2020 as the second pandemic of the 21st century [1]. SARS-CoV-2 illness is frequently accompanied by unremitting fever, hypoxemic respiratory failure, systemic complications, encephalopathy, delirium, and thromboembolic events [2, 3, 4]. Following infection with SARS-CoV-2, patients with severe and critical illnesses suffer frequent neurological complications [4]. The blood-brain barrier (BBB) is a critical interface that regulates the entry of circulating molecules into the central nervous system (CNS). The BBB is therefore essential for the treatment of viruses that can infect the CNS, such as SARS-CoV-2 [5].
Nutraceuticals, phytochemicals from medicinal plants, and dietary supplements have been used as adjunct therapies for many diseases, including viral infections. The use of adjunct antiviral therapy may be beneficial in the treatment and prophylaxis of COVID-19 [6].
Arginine is a natural molecule that crosses the BBB through a transporter with specificity for amino acid analogs that possess cationic terminal guanidine groups, such as those contained in L-arginine [7]. Recently, it was shown that amino acids can improve immunity and shorten disease length in patients with COVID-19 [8]. In a randomized clinical trial of adults with severe COVID-19, L-arginine plus standard care significantly reduced the need for respiratory support and reduced the length of hospitalization. Large doses (1.66 g) of L-arginine were given orally twice per day for the entire hospitalization period [8]. The molecular mechanisms that underlie the significant modulating effect of arginine are still to be clarified.
The main protease of SARS-CoV-2, M
We previously proposed a simple theoretical criterion for rapid virtual
screening of molecular libraries for candidate inhibitors of M
Some of the important effects of vitamin C following viral infection are reduced pro-inflammatory response, improved epithelial barrier function, enhanced alveolar fluid clearance, antiviral activity, and immune system stimulation. Vitamin C is also a crucial factor in the production of type I interferons during the antiviral immune response, and acts as an inactivating agent for RNA and DNA viruses [15].
Due to its antioxidant, anti-inflammatory, and immunomodulatory properties, vitamin C is a possible therapeutic option for the prevention and treatment of COVID-19 infection, as well as a possible adjuvant therapy for COVID-19 critical care [16].
The results of the present study showed that arginine has inhibitory activity
against M
Microorganisms were grown using the thermostat-controlled “Environmental Shaker-Incubator ES-20” and the shaker “Thermo-shaker TS-100 Biosan” (Ratsupites iela 7 k-2, Riga, Latvia). The “Consort E122” system was used for protein electrophoresis and the HPLC AKTA (Emeryville, Cytivia, CA, USA) system for enzyme purification. A “Thermo Scientific Appliscan” (ThermoFischer Scientific, Waltham, MA, USA) device was used to measure enzyme activity by fluorescence.
The antibiotic Kanamycin was purchased from Invitrogen, catalog number: 11815024, Carlsbad, CA, USA. Various components for media preparation (agar, peptone and tryptone) were purchased from Torlak, Belgrade, Serbia. Other substances were ordered from Centrohem, Belgrade, Serbia.
The gene for M
The M
DGSGFRKMAFPSGKVEGCMVQVTCGTTTLNGLWLDDVVYCPRHVICTSEDMLNPNYEDLLIRKSNHNFLVQAGNVQLRVIGHSMQNCVLKLKVDTANPKTPKYKFVRIQPGQTFSVLACYNGSPSGVGSVGFNIDYDCVSFCYMHHMELPTGVHAGTDLEGNFYGPFVDRQTAQAAGTDTTITVNVLAWLYAAVINGDRWFLNRFTTTLNDFNLVAMKYNYEPLTQDHVDILGPLSAQTGIAVLDMCASLKELLQNGMNGRTILGSALLEDEFTPFDVVRQCSGVTFQ.
Collected cells were resuspended in 5 mL of lysis buffer composed of 50 mM
Na-phosphate buffer, 300 mM NaCl and 10 mM imidazole (pH 7.5). Samples were
sonicated on ice. Aliquots were taken before induction (0 h) and after expression
(24 h). Sonication was performed using an ultrasound probe, 10 times for 10
seconds each, with a 20 second pause in between. After lysis, the mix was
centrifuged for 5 min at 13,000 rpm and the supernatant then passed through a
sterile 0.22
M
Changes in fluorescence were monitored every 135 s for 45 min at an excitation
wavelength of 485 nm and an emission wavelength of 535 nm. The total volume of
reaction mixture was 200
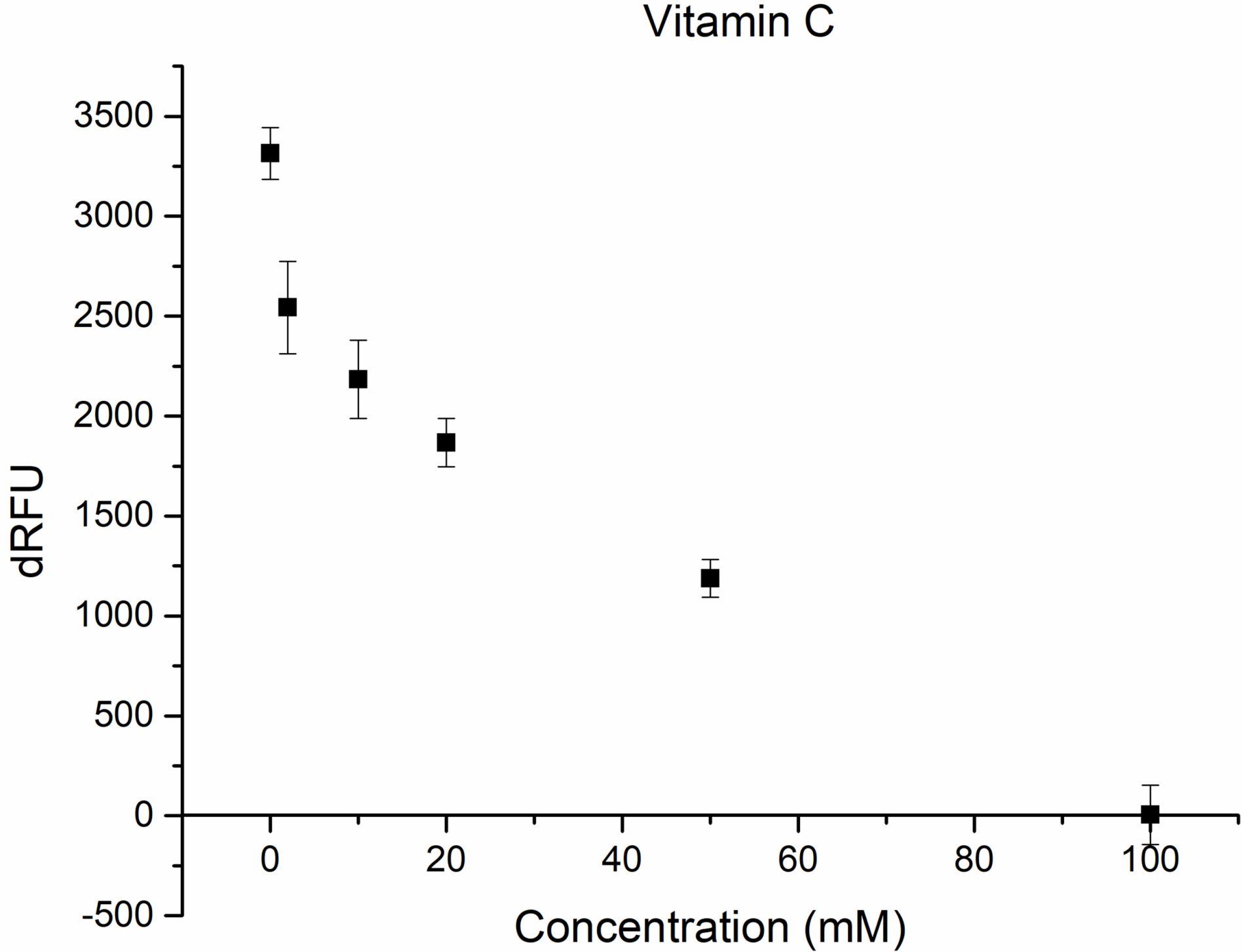
Figs. 2,3 show the inhibition of M
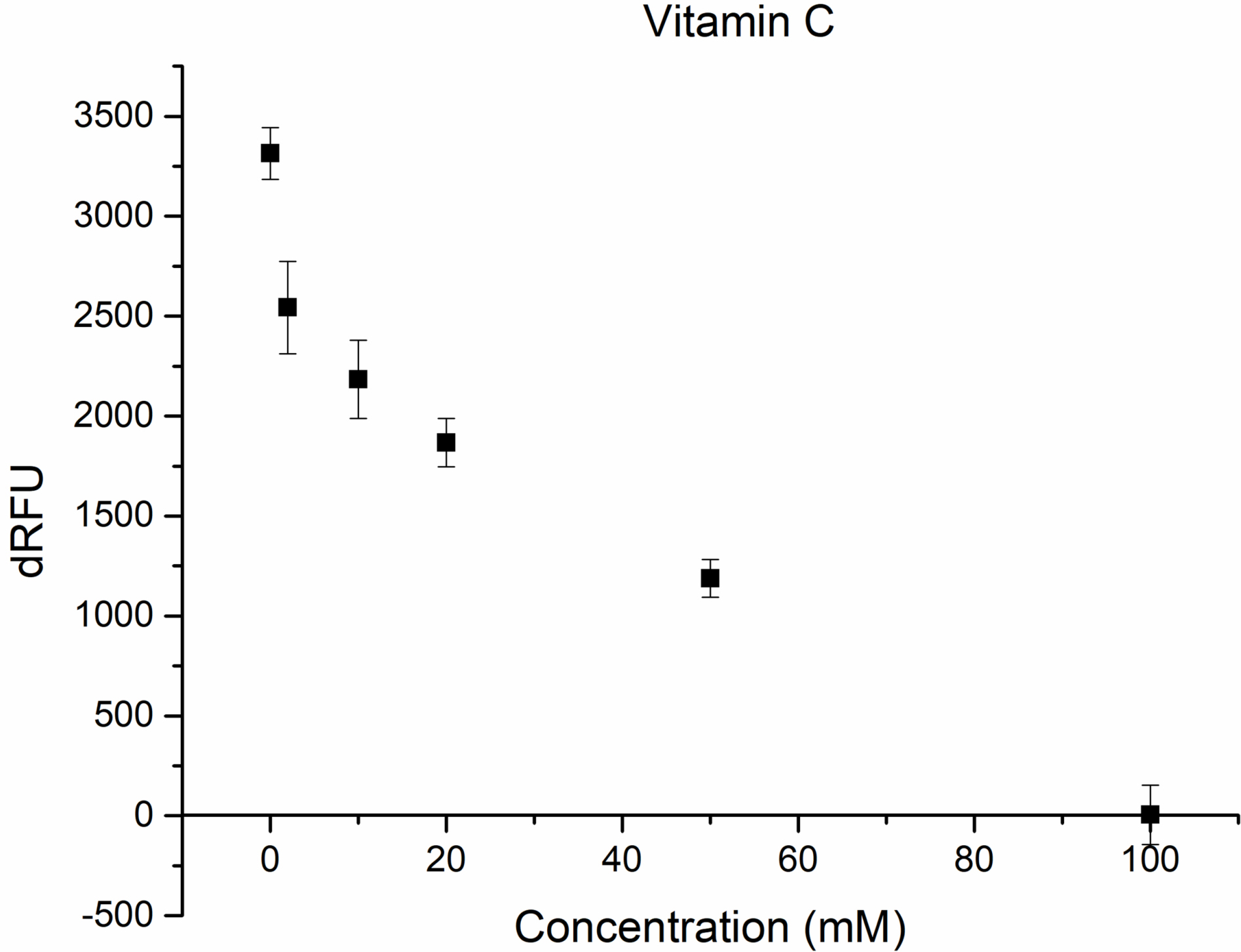 Fig. 2.
Fig. 2.
Inhibition of M
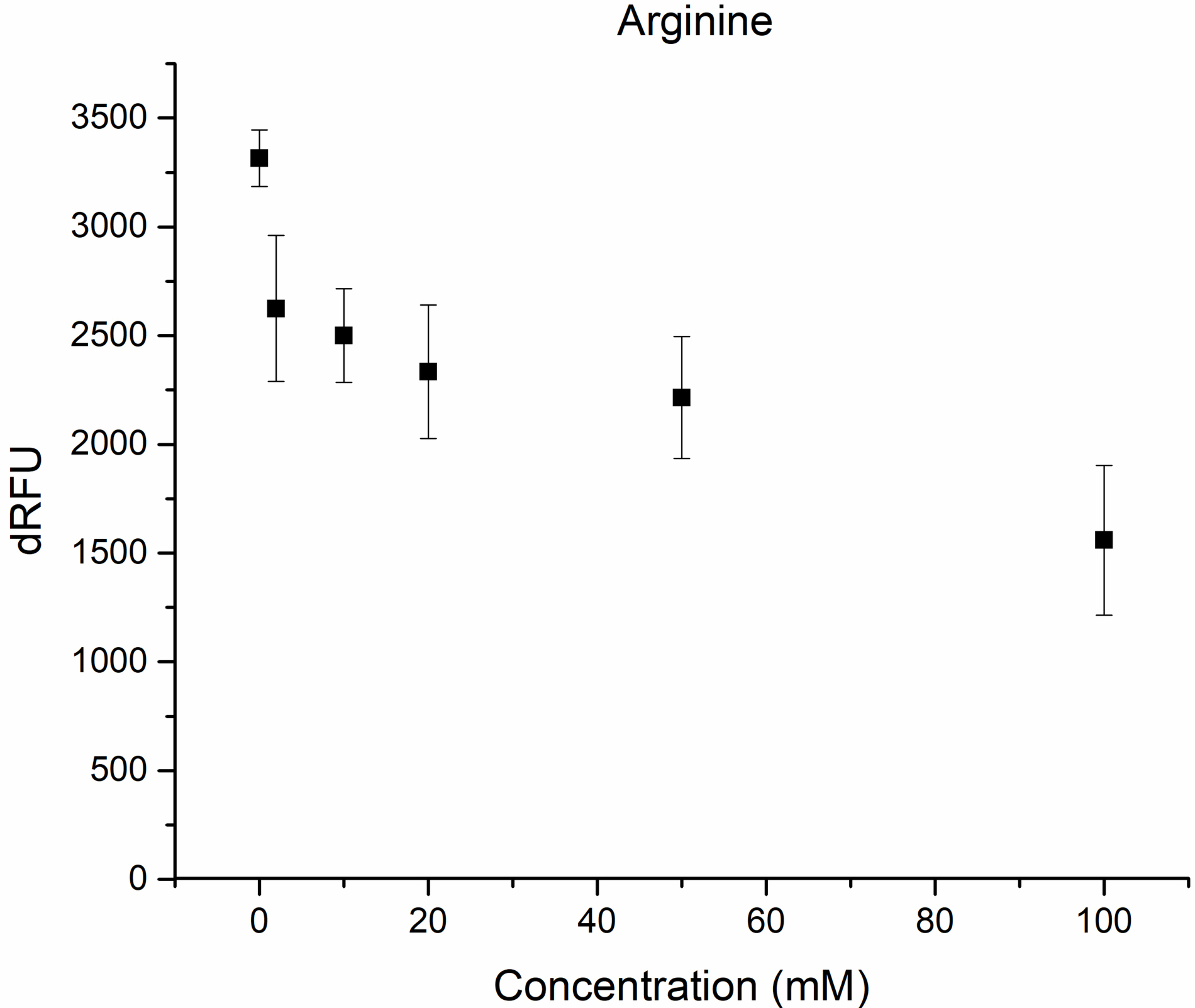
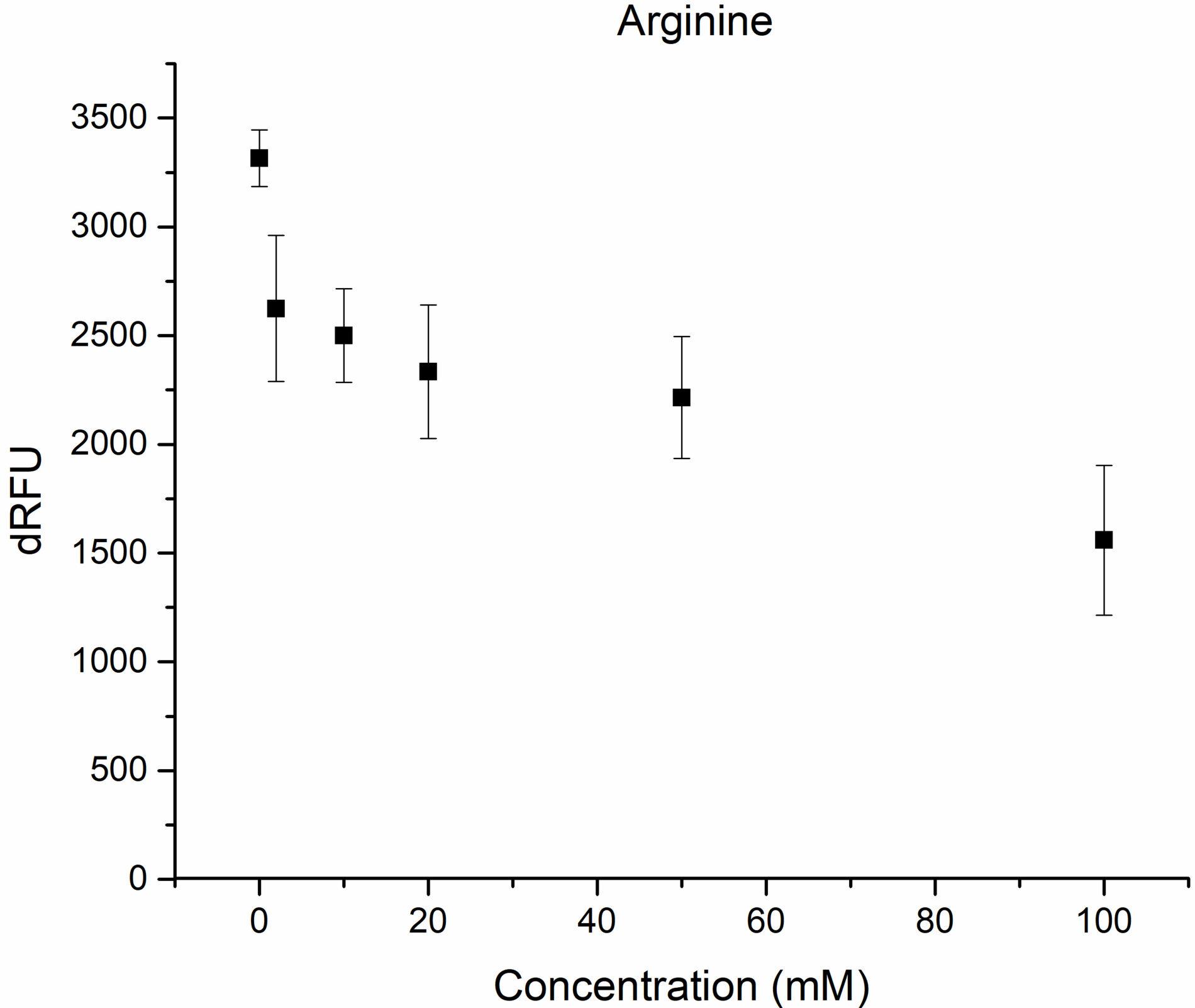 Fig. 3.
Fig. 3.
Inhibition of M
| Inhibitor | Concentration (mM) | Inhibition (%) | Relative error |
|---|---|---|---|
| Water | 0 | 0 | 0 |
| Vitamin C | 20 | 43.66 | |
| Vitamin C | 50 | 67.29 | |
| Arginine | 75 | 39.48 | |
| Vitamin C + Arginine | 20 + 75 | 60.97 | |
| Vitamin C + Arginine | 50 + 75 | 82.23 |
It could be seen from the obtained results that around 44% inhibition was achieved using 20 mM vitamin C concentration (Fig. 2), while in the case of arginin 39% of inhibition was achieved at 75 mM concentration (Fig. 3).
When both compounds were added at the same concentration’s inhibition was
increased to 61% showing an additive inhibitory effect on the in vitro
proteolytic activity of the M
Drug resistance is a critical problem in health care that affects therapy
outcomes and requires new drug design approaches. SARS-CoV-2 mutations are
therefore of great concern as they could lead to drug resistance. The SARS-CoV-2
main protease (M
Nutraceuticals are defined as any food or food component that provides medical or health benefits, including the prevention and treatment of disease [19]. These may have potential therapeutic efficacy in the fight against the SARS-CoV-2/COVID-19 pandemic. In a randomized clinical trial of adults with severe COVID-19, L-arginine given with standard care was found to significantly reduce the need for respiratory support and the length of hospitalization (NCT04637906, registration date November 20, 2020; [8]). Recently, the L-Arginine and Vitamin C improves Long-COVID (LINCOLN) survey found that L-arginine when taken with vitamin C improved long-COVID, thus demonstrating for the first time the beneficial effect of this combination. The survey was completed by 1390 patients who were divided into two groups: those who received L-arginine plus vitamin C, and those who received a multivitamin combination. According to the survey, supplementation with L-arginine plus vitamin C had beneficial effects for long-COVID patients, with less symptoms and significantly lower effort perception [20].
The important roles of amino acids, including arginine, in immune responses were recently reviewed [21]. L-arginine is converted in the body to nitric oxide, which has been suggested as a therapeutic option for COVID-19. Nitric oxide was shown to be an effective antiviral against SARS-CoV in vitro, as well as in vivo by inhalation of very low concentrations in a small clinical trial [22]. Although some published studies have linked arginine supplementation with increased nitric oxide production, other reports claim that acute L-arginine supplementation does not increase nitric oxide production in healthy subjects. The molecular mechanisms that underlie this important modulating effect of arginine therefore remain to be clarified [23].
The focus of the current study was to investigate the action of a non-toxic,
natural amino acid against the M
We examined the inhibition of M
The synergistic antiviral action of arginine/vitamin C against SARS-CoV-2
M
The inflammation triggered by oxidative stress is the cause of many chronic diseases. Oxidative stress is characterized by increased production of free oxygen radicals and represents one of the basic pathological processes of atherosclerosis. It is also closely related to endothelial dysfunction and promotes a vascular inflammatory response [32]. The correlation observed between COVID-19 and atherosclerosis suggests that effort should be directed towards cardioprotection [33]. A previous study reported that supplemental L-arginine and vitamin C could be anti-atherogenic, as observed by the modulation of endothelial dysfunction biomarkers [34]. Based on these findings, the vitamin C/arginine combination could also have a cardioprotective effect in COVID patients, in addition to the direct antiviral effect suggested by our study.
The findings of our study are significant in at least two respects. First, we
demonstrated that arginine exerts inhibitory action against SARS-CoV-2 M
The results of the current study are important in the search for effective, safe and affordable therapeutics against COVID-19. Our findings could also help to develop an effective nutritional strategy to fight infectious diseases.
Drug resistance is an important issue in health care that affects therapeutic
results and necessitates novel drug design approaches. Nutraceuticals are an
interesting treatment option for COVID-19. This study examined the potential
antiviral activity of L-arginine and vitamin C in vitro. The
experimental results showed that arginine inhibits SARS-CoV-2 M
The results of the current study suggest a potential dietary approach to COVID-19, in addition to pharmaceutical treatments. This is important because it is vital to develop COVID-19 therapies that are effective, affordable, and have a favorable safety profile. Furthermore, our findings might help to develop an effective nutritional approach for the prevention and treatment of infectious diseases, thereby reducing the burden on communities and healthcare systems.
Not applicable.
Conceptualization—RP, SP and SG; performed the experiments—RP, NK, and IĐ; validation—NK, and RP; analyzed the data—NK, IĐ, MS, and RP; investigation—NK, IĐ, and MS; resources—RP; writing, original draft preparation—RP, SG, MS, SBP, JM and JP; writing, review and editing—SG, SBP, MS, SP and RP; visualization—IĐ, NK, RP; supervision—RP; project administration—RP, SG, JM, and MS. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicable.
Science Fund of the Republic of Serbia, Special research program on COVID-19.
This research was funded by Science Fund of the Republic of Serbia, grant number 7551100 - COVIDTARGET – Repurposing of drugs for prevention and treatment of COVID-19, Funded under Special research program on COVID-19.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.