1 1st Department of Internal Medicine and Oncology, Semmelweis University, 1083 Budapest, Hungary
2 1st Department of Internal Medicine and Oncology, Oncology Profile, Semmelweis University, 1083 Budapest, Hungary
Academic Editors: Josef Jampílek and Graham Pawelec
Abstract
SIRT1 was discovered in 1979 but growing interest in this protein occurred only 20 years later when its overexpression was reported to prolong the lifespan of yeast. Since then, several studies have shown the benefits of its increased expression in preventing or delaying of many diseases. SIRT1, as a histone deacetylase, is an epigenetic regulator but it has wide range of non-histone targets which are involved in metabolism, energy sensing pathways, circadian machinery and in inflammatory regulation. Disturbances in these interconnected processes cause different diseases, however it seems they have common roots in unbalanced inflammatory processes and lower level or inactivation of SIRT1. SIRT1 inactivation was implicated in coronavirus disease (COVID-19) severity as well and its low level counted as a predictor of uncontrolled COVID-19. Several other diseases such as metabolic disease, obesity, diabetes, Alzheimer’s disease, cardiovascular disease or depression are related to chronic inflammation and similarly show decreased SIRT1 level. It has recently been known that SIRT1 is inducible by calorie restriction/proper diet, physical activity and appropriate emotional state. Indeed, a healthier metabolic state belongs to higher level of SIRT1 expression. These suggest that appropriate lifestyle as non-pharmacological treatment may be a beneficial tool in the prevention of inflammation or metabolic disturbance-related diseases as well as could be a part of the complementary therapy in medical practice to reach better therapeutic response and quality of life. We aimed in this review to link the beneficial effect of SIRT1 with those diseases, where its level decreased. Moreover, we aimed to collect evidences of interventions or treatments, which increase SIRT1 expression and thus, open the possibility to use them as preventive or complementary therapies in medical practice.
Keywords
- health
- lifestyle
- SIRT1
- methylation
- metabolism
- cancer prevention
Discovery of SIRT1 goes back to 1979 when it was reported as mating-type regulator protein in Saccharomyces cerevisiae [1]. Emerging interest in this protein occurred 20 years later when its overexpression was reported to extend yeast lifespan [2]. SIRT1 is a conserved protein but its increased complexity of function was described in mammals where seven enzymes are belonging to the sirtuin family [3]. In 2010 it was demonstrated first time that resveratrol induces the expression of SIRT1 protein in human cancer cells [4]. Resveratrol is a polyphenol found in grapes, wines, peanuts and various herbs and functions as phytoestrogen [5]. Since then, numerous studies have shown its beneficial effects through preventing or slowing down the progression of wide range of diseases like diabetes, ischemic injuries, cardiovascular disease, cancer and other age-related diseases [6, 7, 8, 9]. SIRT1 inactivation is implicated in COVID-19 severity and its lower level is a predictor of uncontrolled COVID-19 [10]. Its agonistic effect was described in relation to drugs like fenofibrate or statins [11, 12].
Metabolic syndrome is described as a disease where at least three of the next
symptoms are represented: abdominal obesity, high triglyceride and low high
density lipoprotein (HDL) level, high blood pressure, high fasting glucose level,
increased risk of blood clotting and tendency to develop inflammation. Severe
obesity is a metabolic disease associated with chronic inflammation. Furthermore,
chronic inflammation when expression of pro-inflammatory cytokines IL-1
| Numbered | Life style or treatment | Disease | Tissue type | SIRT1 level | Metabolic changes, clinical parameters after intervention OR disease & states | Signaling/ cellular mechanism | Study type | Cases no. | Reference | ||
| mRNA | protein | activity | |||||||||
| 1 | CPAP | healthy OSAS vs. healthy | blood | NA | increased* | increased* | increased*NOx, decreased*AHI&ODI&AI, improved* sleeping architecture&efficiency | NFkB, AP-1/decreased oxidative stress, | Controlled clinical trial | 35 | [35] |
| 2 | aging | healhty | blood | NA | increased* | NA | decreased*LDL, increased*HDL, decreased*eNOS | PON-1/oxidative stress, SNP effect | Randomized controlled trial | 120+115+103 | [39] |
| 3 | CR (short) | healhty | blood, skeletal muscle | NA | decreased | NA | decreased*weight, BMI, insulin, leptin, triglycerides, T3 and increased* growth hormon, FFA, ketone bodies, FT4, SHGB, cortisol, ALP, creatinine | Akt ,AS160, S6kinase, 4E-BP1, FOXO1; insulin/mTOR; ERK; CRE/energy-nutrient sensing pathways | Original article | 12 | [27] |
| 4 | CR (long) | obease and lean women | subcutaneous adipose tissue | increased* | NA | NA | decreased*weight, serum insulin, leptin and increased* FFA | NA | Original article | 9/12 | [26] |
| 5 | CR (long) | healthy overweight | PBMC | NA | increased* | increased* | decreased*weight, BMI, %fat, visceral fat area, mean blood pressure, HOMA-R, IRI, FFA, IL-6 and increased* VO2 max | p-SIRT1, p-AMPK | Original article | 4 | [25] |
| 6 | CR /E (long) | healthy overweight | skeletal muscle | increased* | NA | NA | decreased* EE, T3, DNA-damage, insulin and increased* insulin-sensitivity | PPARGC1A, TFAM, eNOS, PARL, mtDNA/mitochondrial function | Randomized controlled trial | 36 | [31] |
| 7 | CR/E (long) | obese | white adipose tissue | increased* | NA | NA | decreased* PARP activity and increased* NAD+ biosynthesis, NAMPT | PARP activity/NAD+ biosynthesis, NAMPT | Controlled clinical trial | 19 | [32] |
| 8 | CBT | overweight/obese | plasma | NA | NA | NA | dietary intake preferences, ghrelin concentration | SIRT1 and CLOCK SNP effects | Original article | 1465 | [41] |
| 9 | diet | healthy | plasma, PBMC | increased* | NA | NA | decreased* AGEs, 8-iso-prostanes, VCAM-1, TNFa, RAGE and increased* SIRT1, PPARg | PPARg, SIRT1/oxidative stress, inflammation | Randomized controlled trial | 67+18 | [37] |
| 10 | diet | healthy | serum, PBMC | NA | NA | NA | decreased* LDL-C and increased* HDL-C | SIRT1 haplotype2 | Original article | 707+723 | [29] |
| 11 | diet | healthy | blood | NA | NA | NA | decreased* airway abstruction and increased* FEV1 | SIRT1 SNPs | Original article | 3224/ 1152+1390 | [36] |
| 12 | E (aerob) | healthy | skeletal muscle | increased* | NA | NA | increased* PGC1 |
mitochondrial biogenesis | Clinical trial | 40 | [30] |
| 13 | E (aerob) | healthy | blood | increased* | NA | NA | decreased* IFNγ, IP-10, IL-1 |
TLR4 pro-inflammatory; IFNγ, PPARγ, PGC-1 |
Clinical trial | 30 | [38] |
| 14 | E (anaerob) | healthy | skeletal muscle | NA | increased* | NA | increased* p-AMPK, SIRT1 | p-AMPK | Randomized controlled trial | 15 | [33] |
| 15 | E (aerob) | healthy | skeletal muscle | no change | NA | NA | decreased* total body and intraabdominla fat-mass, glycated haemoglobin and increased* oxygen consumption, insulin sensitivity, total body fat-free mass | no activation of mitochondrial biogenesis | Randomized controlled trial | 11+11+11 | [34] |
| 16 | depressed emotional state | emotional disorders | PBMC | decreased* | NA | NA | no change in cortisol | NA | Original article | 28/59+44 | [40] |
| 17 | LAGB | sever obesity | adipose tissue, liver | increased* | increased* | NA | decreased* BMI, fasting glucose, insulin, HOMA, ALT, GGT, CRP, leukocyte count and increased* SIRT1,3,6 | liver inflammation, fibrosis, steatosis, adipocytokine expression | Original article | 29 | [28] |
| AGEs, advanced glycation end-products; AHI, apnea-hypopnea index; AI, arousal index; ALP, alkaline phosphatase; BMI, body mass index; CBT, cognitive behaviour therapy; CPAP,continous positive arway pressure; CR, calorie restriction; E,exercise; F3, triiodothyronine; FFA, free fatty acid; FT4, free thyroxine; LAGB, laparoscopic adjustable gastric banding; NOx, nitic oxide derivatives; ODI, oxygen desaturation index; OSAS, obstructive sleep apnea syndrome; PBMC, peripherial blood mononuclear cells; SNP, single nucleotide polymorphysm; WL, weight loss; *, significant change. | |||||||||||
With this review, we aimed to demonstrate that well designed interventions can increase SIRT1 expression and, consequently, improve the metabolic and energy profile in diseases where SIRT1 levels are attenuated including COVID-19. Moreover, we aimed to discuss whether these interventions could be part of preventive and complementary therapies in the medical practice.
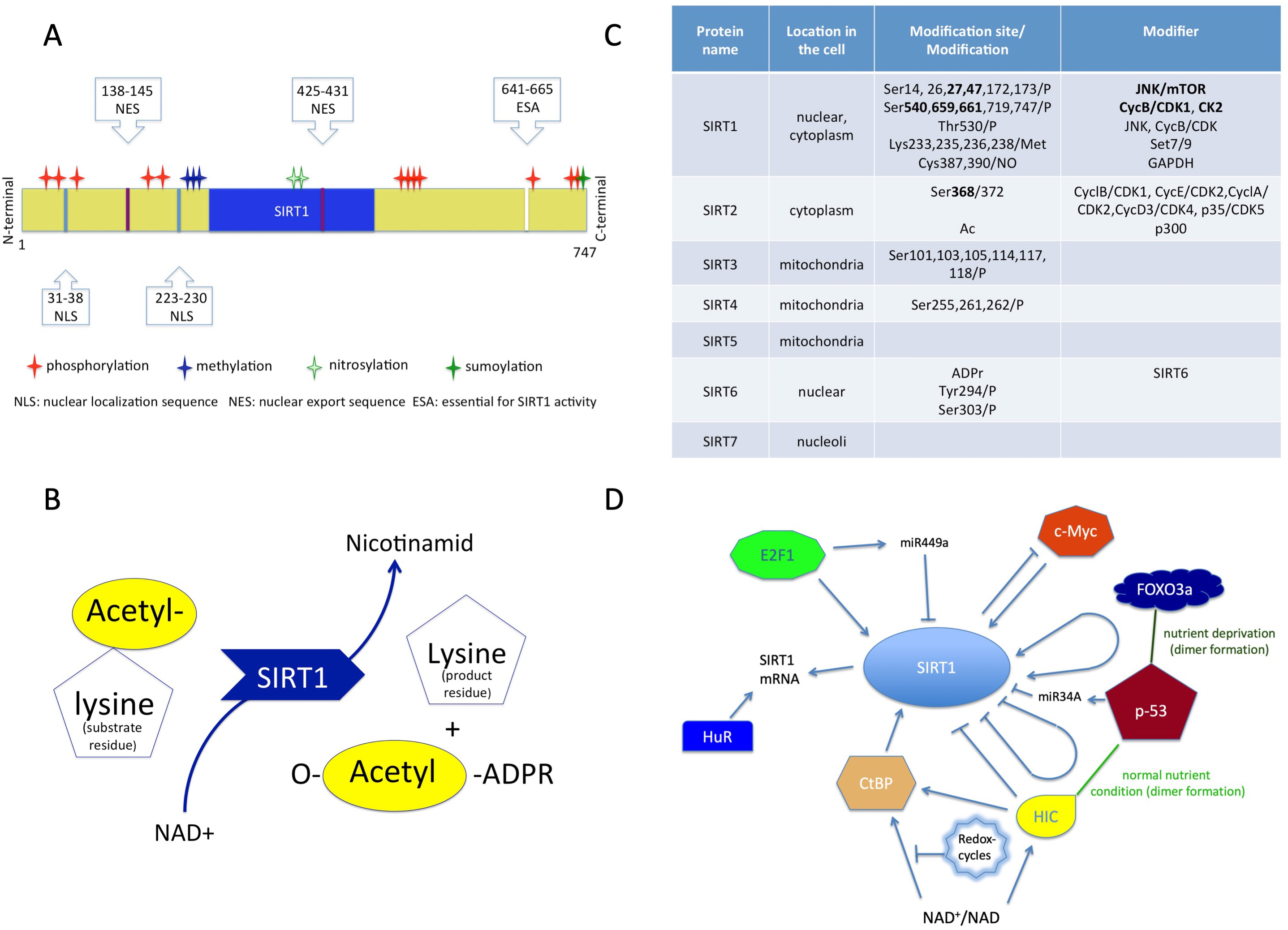
SIRT1 is a class III human histone deacetylase enzyme (HDAC) and as the member of sirtuin family, plays crucial role in orchestrating homeostasis [44, 45]. As an epigenetic modifier, this protein regulates chromatin structure and transcription processes as well. The sirtuin group is structurally and functionally different from the other three HDAC groups, so the members of this group cannot be modulated by classic HDAC inhibitors [45, 46, 47]. SIRT1 is a monomer protein consisting of a highly conserved catalytic domain (blue region in Fig. 1A), two nuclear localization sites (NLS) and two nuclear export sites (NES) (Fig. 1A) [48, 49].
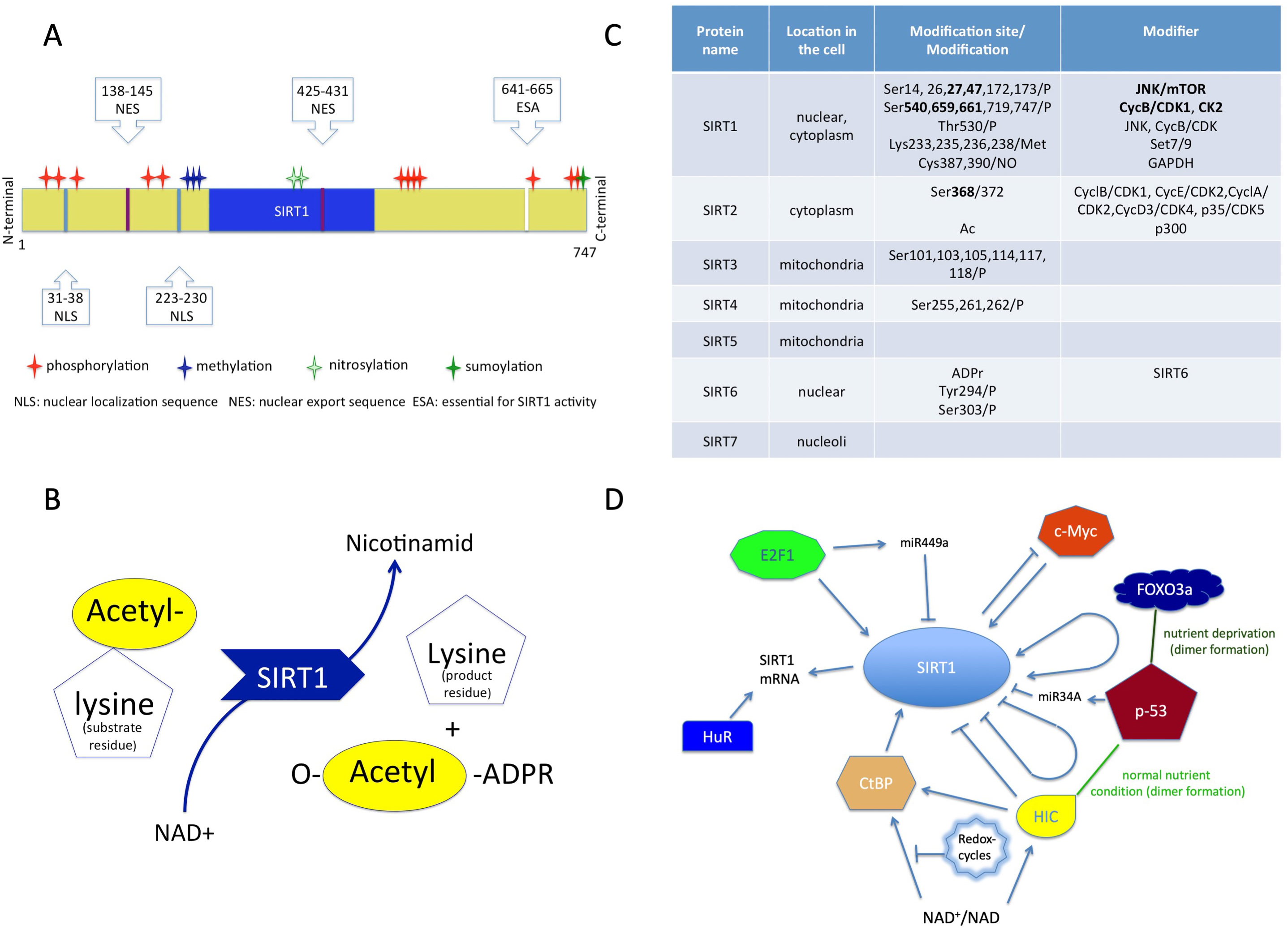 Fig. 1.
Fig. 1.Structure and function of sirtuins. (A) Structure of SIRT1 protein consisting of a conserved (blue) region, two nuclear localization sites (NLS), two nuclear export sites (NES) with the modifications. (B) The transacetylation process. (C) The localization of the sirtuins in the cell, and their modifiers with modifications. (D) Molecules which affect SIRT1 expression. NAD+, Nicotinamid-adenin-dinucleotid; ADPR, Adenozin-diphosphate-ribose.
The catalytic pocket engages both the NAD+ co-factor and the acetyl-lysine substrates [46, 48]. The enzymatic reaction is transacetylation (Fig. 1B) [46, 50].
The activity of SIRT1 protein can be modulated by the availability of NAD+ and also by posttranslational modifications (Fig. 1C). Phosphorylation at the terminal sites orchestrates substrate binding, whereas desumoylation reduces and nytrosilation impairs its catalytic activity [47].
The modulation of SIRT1 activity can occur through protein-protein interactions as well [50]. The active regulator of SIRT1 (AROS) enhances SIRT1 activity by binding to its NH2-terminal [47]. The protein called deleted in breast cancer 1 (DBC1) abrogates SIRT1 activity through binding to the catalytic domain. Additionally, SUMO1/Sentrin specific peptidase 1 (SENP1) has also been identified in the reduction of SIRT1 activity [47, 51].
The gene expression of SIRT1 is regulated by cellular redox-changes and environmental factors (e.g., nutrients).
The protein expression level of SIRT1 can be modulated by both transcriptional and translational pathways by E2F1 and p53 (Fig. 1D) [52]. The RNA-binding protein HuR increases the stability of SIRT1 mRNA [52, 53]. Additionally, the tumor suppressor miR-34a downregulates the expression of SIRT1 and attenuates the resistance to chemotherapy agent 5-fluoro-uracil [54]. The miR499a inhibits SIRT1 expression and miR199a attenuates SIRT1 activity [52].
Non-histone proteins deacetylated by SIRT1 are closely related to pathways
involved in metabolic adaptation to fasting [55]. During this adaptation process
specific gene transcriptions are induced through activation of transcription
factor FOXO3 by SIRT1 [56]. SIRT1 has tissue-specific functions in metabolic and
energy homeostasis, and plays a significant role in maintaining serum glucose
levels during starvation [56]. To achieve the latter, SIRT1 deacetylates and
activates PPAR
SIRT1 can activate AMPK through deacetylating and activating its upstream
activator, liver-kinase-B1 (LKB1). Similarly, AMPK can induce SIRT1 expression by
increasing NAD+ level [68]. Moreover, AMPK can activate PGC-1
Clinical trials and human studies investigating calorie restriction (CR) (Table 1) described that adipose tissues in lean people showed 120% higher SIRT1 level
compared to obese patients at the baseline [26]. Long-term CR significantly
increased the SIRT1 mRNA expression levels (130% higher) compared to baseline
levels in obese women [26], and significantly increased the activation levels of
SIRT1 and AMPK proteins in peripheral blood monocytes (PBMCs) as well [25].
However, in short-term CR, neither SIRT1 level nor activation of AMPK pathway was
changed, although activation of insulin/mTOR pathway was significantly reduced
[27]. On the contrary, both short and long-term CR significantly decreased body
weight, BMI, insulin level (with unchanged level of insulin receptor
CR also has a beneficial effect on the circulatory system based on studies
showing that SIRT1 and endothelial nitric oxide synthase (eNOS) positively
regulate each other upon CR when eNOS induces SIRT1 expression, while SIRT1
deacetylates eNOS and promotes its activity. Vascular endothelium produces nitric
oxide (NO) through eNOS and supports general endothelial health; as NO is
important in relaxing muscle and lowering blood pressure. Additionally, CR
improves myocardial ischaemic tolerance by facilitating SIRT1 entry into the
nucleus by eNOS, and upregulation of SIRT1 protects against cardiac hypertrophy
resulting from PPAR
Hypothalamus is the main systemic coordinator of mammalian physiology including regulation of diurnal activities like feeding, body temperature, energy expenditure and other metabolic functions. CR increases SIRT1 levels in the dorsomedial and lateral hypothalamus triggering behavioral changes to higher physical activity and increased body temperature [55]. SIRT1 upregulates the core transcription factors, BMAL1 and CLOCK, and directly regulates their cyclic repressors as well by leading to degradation. Because AMPK is also able to induce degradation in cyclic repressors which suppress many nuclear receptors thus it is also able to modify nuclear receptor-dependent gene expression programs [69].
Metabolic parameters negatively correlated by SIRT in a clinical trial using laparoscopic adjustable gastric banding (LAGB) to ensure CR. Significantly decreased BMI, fasting glucose, insulin, HOMA, liver enzymes such as ALT, GGT, inflammatory CRP and leukocyte counts after 6 months of weight loss compared to the baseline. The mRNA levels of SIRT1, 3 and 6 significantly increased in the adipose tissue. In the liver, SIRT1 and 3 protein levels increased as well. Expression of all three mRNA significantly and negatively correlated with BMI and serum glucose, and SIRT1 mRNA expression showed significantly negative correlation with liver portal fibrosis, steatosis, GGT levels, adipo-cytokines and receptor expressions in both tissue types (Table 1) [28].
SIRT1 regulates lipid and glucose metabolism, thus consequently lipid profile, where dyslipidaemia is a cardiovascular risk with high LDL-C (low-density lipoprotein-C) and low HDL-C (high-density lipoprotein-C) levels. The genetic variant haplotype 2 of SIRT1 significantly decreased LDL-C and increased HDL-C levels in both sexes but only with low n-6/n-3 polyunsaturated fatty acid (PUFA) intake [29].
Clinical trials investigating the effect of exercise alone or with CR have found
similar result to CR interventions (Table 1). In more details, acute and chronic
stimuli due to one- and three-days cycling, respectively, increased mitochondrial
biogenesis through significantly increased PGC-1
The sequential course of inflammation is directed by reprogrammed metabolism and
bioenergy [73]. SIRT1 is involved in this process, and switching glycolysis to
fatty acid oxidation in monocytes during adaptation to acute inflammation [76].
Reactive oxygen species and ATP generated by anabolic glycolysis are required for
rapid defense against invading microorganisms, while lipolysis/fatty acid
oxidation is necessary to regeneration and repair, and it leads to immune
repression. Failure in this homeostasis results in many chronic and acute
inflammatory diseases such as obesity, diabetes, metabolic syndrome,
atherosclerosis, Alzheimer’s disease or sepsis [73]. The repression of anti-aging
gene SIRT1, which is involved in epigenetic processes that alter the immune
system, was implicated to diabetes and multiple organ dysfunction syndrome [77].
During acute systemic inflammation response, which could be induced by COVID-19
as well, cardiovascular and microvascular functions are impaired leading to
multiple organ failure [73]. On the contrary, at the molecular level, the shift
in NAD+ availability increases nuclear NAD+ level and activates SIRT1, which then
induces facultative heterochromatin formation for gene silencing in promoter
regions of proinflammatory genes such as TNF-
Obstructive sleep apnoea syndrome (OSAS) is described as poor sleeping
quality with decreased blood oxygen levels due to repeated
airway obstruction. This increases blood pressure, hypoxia/re-oxygenation events,
activates the pro-inflammatory NF
It has been known for a long time that PPAR
The association between low back pain and oxidative stress has already been
reported [83, 84]. However, a recent clinical study also found that exercise
intervention was effective in reducing nonspecific low back pain due to
significantly decreased TLR-4 related pro-inflammatory signals (TLR-4 mRNA,
IFN
Age-related diseases, such as cardiovascular disease, Alzheimer’s and
Parkinson’s diseases or cancers, are supposed to be developed by a low degree of
systemic inflammation with advanced age [73, 85, 86]. Oxidative stress is
significantly increased by aging and is closely related to mitochondrial
dysfunction, which promotes the production of reactive oxygen species [87]. SIRT1
and AMPK, however, regulate mitochondrial biogenesis and clearance of defected
mitochondria (mitochondria fission) through PGC-1
Stress level and emotional state are also important risk factors in development of metabolic disorders and cancer [93, 94]. Patients with mood disorders such as major depressive disorder and bipolar disorder have been studied in both depressed and remission states. The mRNA levels of SIRT1, 2 and 6 were significantly decreased in peripheral blood monocytes in mood disorders and depressed states compared to healthy controls. In contrast, in remission state these were comparable to those seen in healthy subjects. This result suggests that SIRT1, 2 and 6 are state-dependent and might be associated with pathogenesis and pathophysiology of mood-disorders. However, serum cortisol level did not change dramatically during remission and there was no significant correlation with SIRT levels. It suggests that chronic stress factor was still present (Table 1) [40].
Cognitive behavioral therapy has been applied to support weight loss in another study. Carriers of double minor allele of SIRT1 (rs1467568; AG or AA) and CLOCK (rs1801260, TC or CC) showed significantly higher resistance to weight loss, lower weekly weight loss at higher plasma ghrelin concentration, evening preference and lower adherence to Mediterranean diet compared to homozygotes for both major alleles (GG and TT, respectively) (Table 1) [41].
Calorie restriction, adequate physical activity and emotional state increase the level of SIRT1 in the body, resulting in beneficial changes in levels of inflammatory and stress markers, thus promoting balanced epigenetic regulation and increasing healthy life expectancy (Table 1) [88, 95].
SIRT1 regulates the availability of several target genes and transcription factor genes involved in epigenetic regulation [96], and many of which are also involved in aging and age-related diseases [52].
Autophagy is a process in which organelles and macromolecules are directed into lysosomes for degradation. This process is used by cells for normal turnover or production of nutrients in response to energy shortage [69]. AMPK is also involved in this process by activating pro-autophagic complexes, directly triggering the autophagy cascade, initiating autophagosome formation or inhibiting mTOR, an autophagy suppressor molecule [97, 98]. P53 can also inhibit mTOR and activate AMPK thus inducing autophagy. Although in most cases p53 is positively regulated by AMPK [68], it can also inhibit autophagy [99].
P53 is a tumor suppressor gene and is also able to arrest the cell cycle by DNA repair and apoptosis [68]. It controls the metabolic switch between glycolysis and oxidative phosphorylation (Wartburg effect) and favors TCA cycle while limiting glycolytic flux [100]. P53 can regulate SIRT1 expression depending on the nutrient status of the cells [52, 56]. In starving cells, p53 complexed with FOXO3 induces SIRT1 expression, while under normal conditions HIC1, an epigenetically regulated repressor, mediates the suppression of SIRT1 gene expression [52].
However, SIRT1 can also regulate p53 through deacetylation thus inhibiting DNA damage and stress induced cellular senescence [88]. SIRT1 prevents nuclear translocation of p53 and blocks transcription-dependent induction of apoptosis, while promotes its cytosolic accumulation and transport to mitochondria, suggesting transcription-independent induction of apoptosis. Although firstly SIRT1 was considered as a tumor promoter, recent studies have suggested it to be a tumor suppressor by facilitating mitochondria-dependent apoptosis [101]. Moreover, in chronic stress condition SIRT1 can inhibit corticosterone-induced autophagy and enhances apoptosis [102].
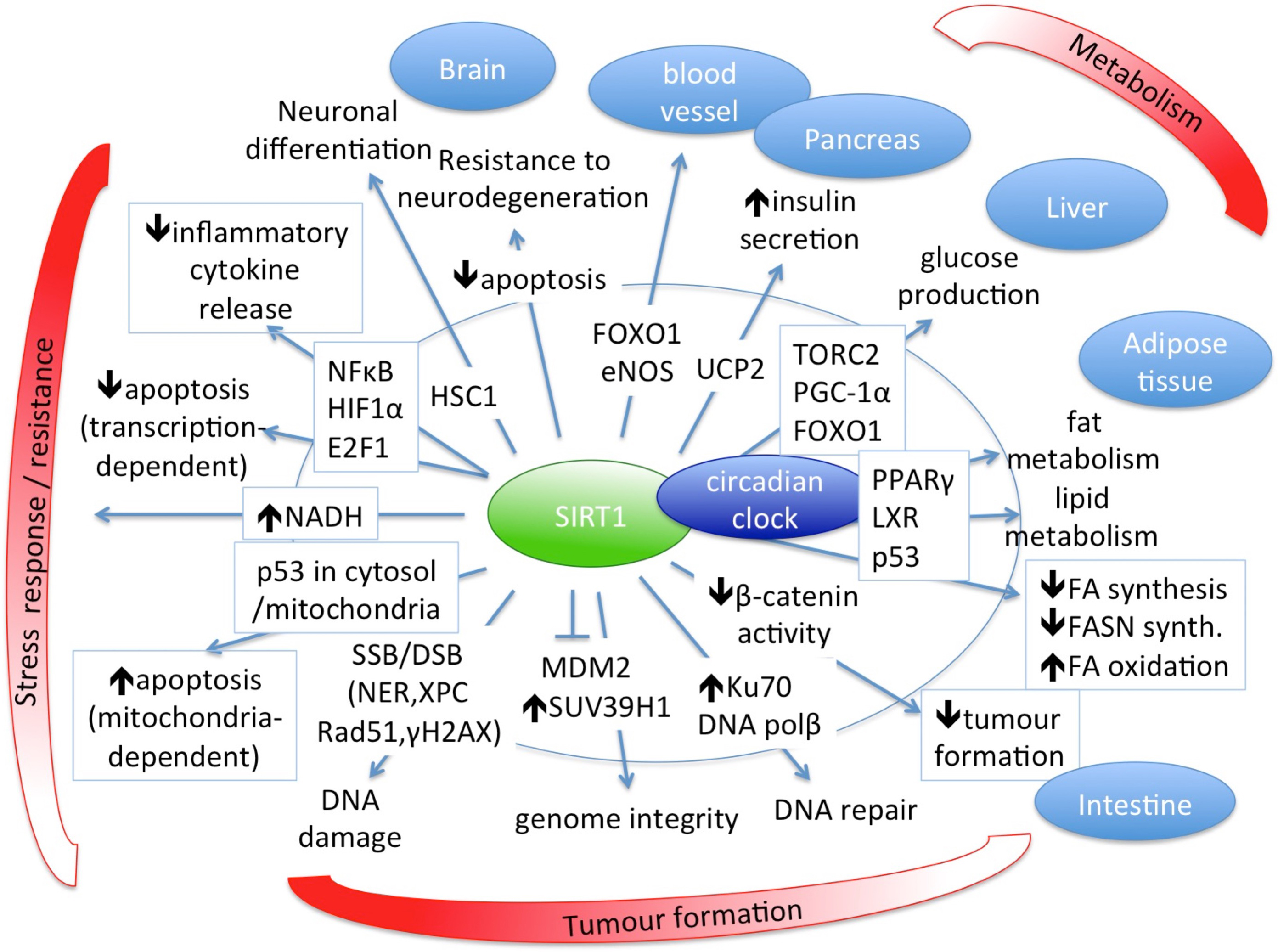
The main role of SIRT1 in biological processes and its regulatory targets for normal healthy homeostasis are summarized in Fig. 2 [27, 31, 48, 52, 56, 101, 103, 104, 105].
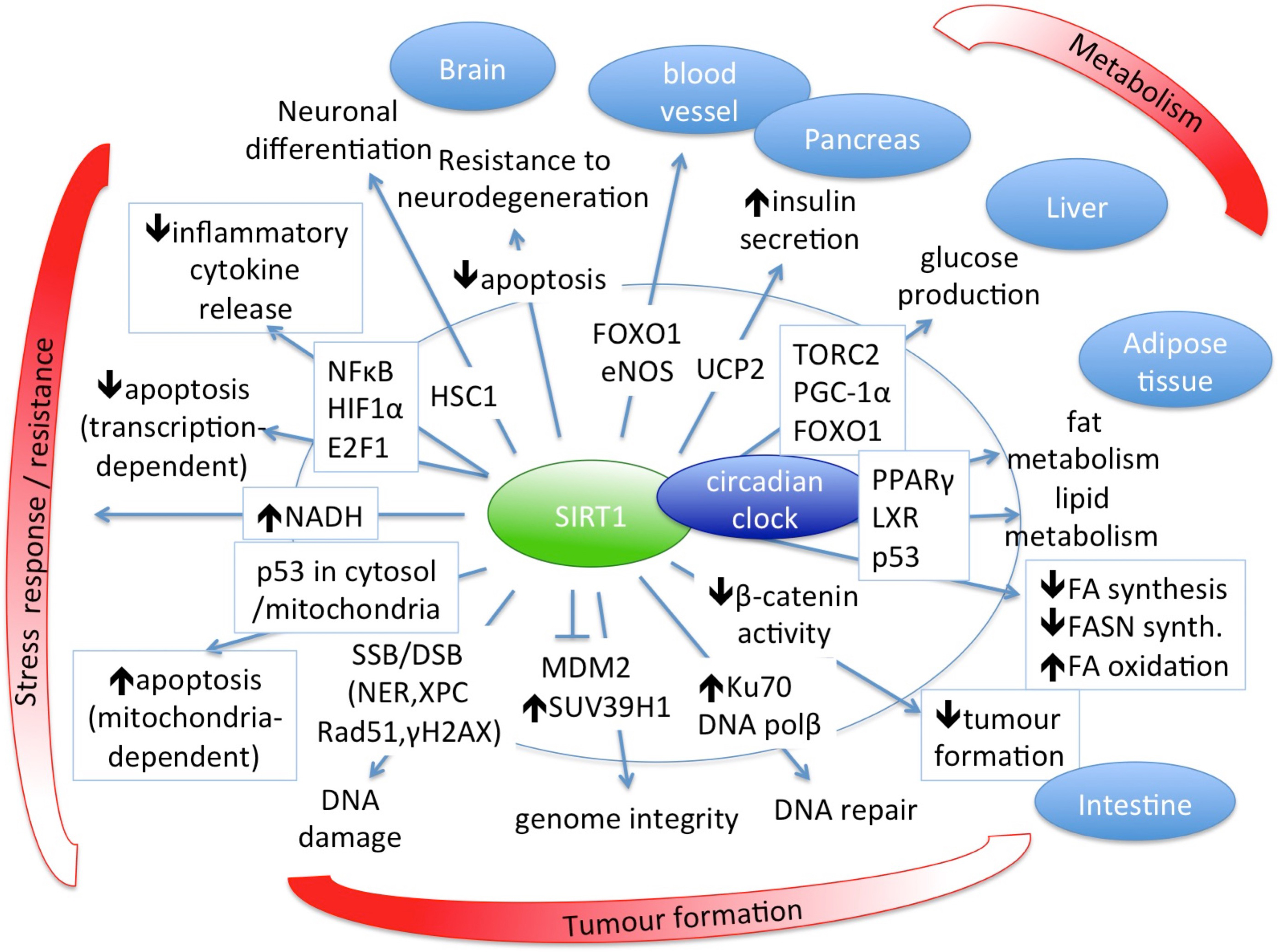 Fig. 2.
Fig. 2.SIRT1 has central role in several biological processes and is an important regulator of normal healthy homeostasis.
Research on metabolic disorders and cancers has increased interest in lifestyle factors such as diet, physical activity, stress level, habits and behaviors as these significantly influence human physiology during the actual duration of their effects. These factors, whether they exist continuously or for a short time but regularly, take their effects into account over a long period of time. Thus, because they act through specific epigenetic modifications [44, 103], they can modify homeostasis, resulting in further genetic alterations [106, 107]. It has been established that lifestyle factors may have a greater impact on colorectal cancer risk than genetics, based on a Spanish cohort study [108].
Although the clear positive effect of SIRT1 and AMPK is still controversial in the development of cancer, several studies have found a positive correlation between SIRT1 expression and longer overall survival (OS) in cancer patients [109, 110].
Colorectal cancer. Several studies have found significant correlation between higher expression or overexpression of SIRT1 and TNM stages (classification of malignancies, T - tumor, N - Node, M - metastases), depth of invasion, lymph node metastasis, increased stem cell markers and decreased suppressors of metastasis [111, 112, 113, 114, 115]. Similarly, in serrated lesions, which are alternative routes for colorectal cancer formation, high expression of SIRT1 and c-Myc was strongly associated with higher grades of malignancy along with the presence of KRAS or BRAF mutations [116]. In two prospective studies, overexpression of SIRT1 was more common in microsatellite instable-high (MSI-high), CpG island methylator phenotype (CIMP-high) and CIMP-high/MSI-high tumors, but only in BRAF-mutated tumors that were positively and significantly associated with CIMP-high/MSI-high tumors. SIRT1 was also associated with overexpression of FASN and hypermethylation of several promoters and CpG islands [117]. In contrast, another study reported that lymph node metastasis was negatively correlated with SIRT1 [118]. Beneficial effect of resveratrol through apoptosis induced in colon cancer cells and sensitization of chemoresistant colon cancer cells to various drugs has been demonstrated in another study as well [119]. Moreover, it has been suggested that exercise-induced alterations in the systemic milieu can influence important regulatory mechanisms in the tumor microenvironment such as angiogenesis, immune regulation and metabolism, and lead to cumulative antitumorigenic effect (Table 2, Ref. [17, 111, 112, 113, 114, 115, 116, 117, 118, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135]) [136].
| Numbered | Cancer type/SIRT1 induccer | Disease | Tissue type | SIRT1 level | Metabolic changes, clinical parameters after intervention OR disease & states | Signaling/ cellular mechanism | Study type | Cases no. | Reference | ||
| mRNA | protein | activity | |||||||||
| 18 | CRC | cancer | colorectal cancer tissue (FFPE- IHC) | NA | increased* | NA | depth of invasion, lymph node metastase, TNM stage/poor prognosis | NA | Systematic review and meta analysis | 50-497/7 studies | [111] |
| 19 | CRC | cancer | colorectal adenocarcinoma tissue (FFPE- IHC) | NA | increased* | NA | metastasis (0.02), poor prognosis (p |
CD133+, stemness | Original article | 102 | [112] |
| 20 | CRC | cancer | colorectal cancer tissue (FFPE- IHC) | NA | increased* | NA | Fra-1, OS | Fra-1 | Original article | 90 | [113] |
| 21 | CRC | cancer | colorectal cancer and liver metastasis (microarray) | NA | increased* | NA | TNM stage, metastasis, OS | miR199b-CREB-KISS1/progression | Original article | 60 (16 meta) | [114] |
| 22 | CRC | cancer | normal colon, colon adenoma, colon carcinoma | NA | increased* | NA | diagnosis, prognosis | NANOG, NCF2, ELF, TGF |
Original article | 167 | [115] |
| 23 | CRC | cancer | colorectal cancer tissue (FFPE- IHC) | NA | increased*/decreased* | NA | differentiation, stage/lymph node metastasis | differentiation, progression | Original article | 40 | [118] |
| 24 | CRC | cancer | serrated lesions of colorectum (FFPE-IHC) | NA | increased* | NA | NA | c-Myc/KRAS, BRAF mutation | Original article | 121 | [116] |
| 25 | CRC | cancer | colorectal cancer tissue (FFPE- IHC) | NA | increased* | NA | tumour grade (0.003), mucinous component (0.04), CIMP-high (0.002), MSI-high (p |
BRAF, MSI, CIMP, FASN/methylation of CACNA1G, IGF2, MLH1, NEUROG1, RUNX3, SOCS1, MINT31 and p14 | Original article | 485 | [117] |
| 26 | B | cancer | breast cancer tissue (FFPE- IHC) | NA | increased* | NA | TNM, DFS, OS | NA | Meta-analysis | 28-200/6 studies | [126] |
| 27 | B | cancer | breast cancer tissue (FFPE- IHC) | NA | decreased* | NA | DFS, OS, | Notch1/Snail | Original article | 150 | [127] |
| 28 | B | cancer | breast cancer (microarray) | decreased* | NA | NA | metastasis | EMT | Meta-analysis | 1181/12 studies | [128] |
| 29 | B | cancer | breast cancer tissue (FFPE- IHC) | NA | decreased * (HRBC, H2BC) increased* (TNBC) | NA | LNM, LVM, DFS, OS, HRBC/H2BC/TNBC | NA | Original article | 427 | [129] |
| 30 | B | cancer | breast cancer tissue (FFPE- IHC) | decreased* | NA | NA | grade3, IDC | NA | Original article | 64 | [130] |
| 31 | B | cancer | breast cancer tissue (FFPE- IHC) | NA | increased* | NA | LNM, DFS, TNBC | NA | Original article | 344 | [131] |
| 32 | B | cancer | breast cancer tissue (FFPE- IHC) | NA | increased* | NA | LNM, DFS | E-cadherin, Vimentin, Snail/ EMT | Original article | 319 | [132] |
| 33 | B | cancer | MS | [17] | |||||||
| 34 | L | cancer | NSCLC tissue (FFPE- IHC) | NA | increased* | NA | TNM, LNM, Ki67 | HIF1/ hypoxia, oxidative stress | Original article | 125 | [120] |
| 35 | L | cancer | NSCLC tissue (FFPE- IHC) | NA | increased* | NA | DFS, OS | NA | Original article | 105 | [121] |
| 36 | L | cancer | NSCLC tissue (FFPE- IHC) | NA | increased* | NA | TNM, LNM, DFS, OS, | cortactin | Original article | 163 | [122] |
| 37 | L | cancer | NSCLC tissue (FFPE- IHC) | NA | increased* | NA | TNM, DFS, OS, chemotherapy resistance | chemotherapy resistance | Original article | 295 | [123] |
| 38 | L | cancer | NSCLC tissue (FFPE- IHC, PCR) | increased* | increased* | NA | OS, prognosis | HIC-SIRT1-p53 loop, acetyl-p53 | Original article | 97 | [124] |
| 39 | L | cancer | sputum | NA | NA | NA | OS, risk of SCC | SNP, haplotype | nested case-control study | 17000 (267+383 selected) | [125] |
| 40 | P | cancer | PIN, PCa tissues | decreased* | NA | NA | H2A.Z | H2A.Z, mTOR, c-Myc | Original article | 57 | [133] |
| 41 | P | cancer | prostate cancer tissue | increased* | NA | NA | Ki67 | Ki67, methylation | Original article | 47 | [134] |
| 42 | P | cancer | prostate cancer tissue | NA | increased* | NA | Gleason grade | Gleason grade | Original article | 41 | [135] |
| ADC, adenocarcinoma; DFS, disease free survival; FASN, fatty acid synthase; H2BC, Her2 positive breast cancer; HRBC, hormon receptor-positive breast cancer; LNM, lymph node metasta metastasis; LVI, lymhovascular invasion; NSCLC, non-small cell lung cancer; OS, overal survival; PCa, prostate carcinoma; PIN, prostatic intraepithelial neoplasia; SCC, squamous cell carcinoma; TNBC, triple negative breast cancer; *, significant change. | |||||||||||
Lung cancer. SIRT1 showed significantly higher expression in lung tumors than in normal tissues and was positively associated with Ki67 index, TNM stage and lymph node invasion. It is suggested as an independent prognostic factor for shorter disease free survival (DFS) and OS, and for higher chance to chemotherapy resistance [120, 121, 122, 123]. In squamous cell carcinomas (SCCs), high levels of SIRT1 effectively deacetylated and inactivated p53. In SIRT1 positive non-small cell lung cancer (NSCLC) cases, low HIC1, a suppressor of SIRT1, was associated with poor prognosis [124]. However, a case-control study investigating SNPs suggested that SIRT1 is a tumor suppressor in radon-induced cancer in miners (Table 2) [125].
Breast cancer. In a meta-analysis of non-stratified studies, SIRT1 expression was significantly and positively correlated with high TNM stages, decreased DFS and a higher rate of lymph node metastases (LNM) [126]. However, other studies found opposite correlations where increased SIRT1 level reduced EMT markers [127, 128]. In stratified evaluations, lower expression of SIRT1 correlated with the presence of LNM in hormone receptor-positive breast cancer (HRBC), HER2 overexpressed/amplified and ER/PR negative breast cancer (H2BC), and in invasive ductal carcinoma (IDC). Similarly, in another study, lower SIRT1 expression correlated with poorer DFS only in HRBC group [129, 130]. Increased level of SIRT1 was associated with increased LNM only in TNBC groups [129, 131, 132]. Additionally, SIRT1 expression was significantly lower in TNBC than in hormone positive HER2 negative breast cancers. Moreover, low SIRT1 expression was associated with worse OS in the latter group, while in TNBC, high SIRT1 level was associated with a poor prognosis [110]. However, metabolic syndrome is associated with an increased risk of breast cancer, poor prognosis and mortality, and is also associated with a decreased SIRT1 level (Table 2) [17].
Prostate cancer. mRNA expression of SIRT1 was significantly downregulated in prostatic intraepithelial neoplasia (PIN) and prostate carcinoma (PCa) [133]. In contrast, other studies have reported that SIRT1 mRNA levels were significantly increased in prostate cancer tissues, positively correlated with Ki67 mRNA expression [134], and were associated with the Gleason grade 3 (Table 2) [135].
Resveratrol, a natural compound, has been used to mimic calorie restriction
[137] because it is known to significantly increase forced vital capacity (FVC)
function of the lung [36] and respiratory quotient (RQ) as well, while
significantly decreasing sleeping metabolic rate, systolic blood pressure, mean
arterial pressure and many other clinical and systemic parameters [137].
Resveratrol treatment significantly decreased intrahepatic lipid content but
increased that in muscle cells. Resveratrol significantly increased AMPK
phosphorylation, PGC-1
| Numbered | Cancer type/SIRT1 induccer | Disease | Tissue type | SIRT1 level | Metabolic changes, clinical parameters after intervention OR disease & states | Signaling/ cellular mechanism | Study type | Cases no. | Reference | ||
| mRNA | protein | activity | |||||||||
| 43 | resveratrol | healthy, obese | skeletal muscle | NA | increased* | NA | decreased* ALT, insulin, HOMA, triglycerides, leptin, intrahepatic lipid, leukocyte number, TNF |
p-AMPK, PGC-1 |
Randomized, double-blind study | 11 | [137] |
| 44 | SRT21204 | healthy | plasma | NA | NA | NA | decreased* IL-6, IL-8, CRP and increased* F1+2, TATc | LPS /inflammation | Randomized, double-blind placebo controlled trial | 24 | [138] |
| 45 | SRT21204 | healthy old | serum | NA | NA | NA | decreased* serum cholesterol, triglycerids, half-life of recovery after exercise (ADP,PCr) and increased* HDL:LDL ratio | mitochondrial oxidative phosphorylation | Phase I. clinical trial | 24 | [139] |
| 46 | SRT21204 | healthy, cigarette smokers | serum | NA | NA | NA | decreased* cholesterol, LDL, triglycerids, | no effect on blood flow rate, coagulation factros, macrophage activation | Randomized, double-blind placebo controlled, crossover trial | 24 | [140] |
| 47 | Selisistat | healthy | blood | NA | NA | NA | NA | gene expression differences | Randomized, double-blind placebo controlled, crossover trial | 22+66 | [141] |
| F1+2, prothrombin fragment; MOG, malate/octanoyl-carnitine/glutamate substrate; MOGS, MOG/succinate substrate; TATc, thrombin-antithrombin complex; *, significant change. | |||||||||||
In parallel with exercise, the small molecule SIRT1 activator, SIRT2104, significantly attenuated plasma cytokines IL-6, IL-8 and CRP in lipopolysaccharide-injected human patients but increased the levels of prothrombin fragment and thrombin-antithrombin complex. There was no effect on the level of von Willebrand Factor, fibrinolytic response, number of leukocytes and gene expression profile [138]. Other studies have recorded significantly decreased recovery period after exercise as well as significantly decreased serum lipid levels after SIRT2104 treatment (Table 3) [139, 140].
The SIRT1 inhibitor Selisistat (SEN0014196) has a specific transcriptional signature in blood cells, involving transmembrane transport, cholesterol/lipid/steroid homeostasis and redox processes genes (Table 3) [141].
Experimental knowledge of the beneficial effects of proper diet and exercise has been known since ancient times and can be used effectively, however, the molecular mechanism behind these has only recently been revealed. Our primary aim was to explore lifestyle specific changes of SIRT1, a key epigenetic regulator, which influences the development of metabolic syndrome and related cancer, and describe the molecular aspects of these mechanisms.
In general, we found that optimal levels of lifestyle factors correlated to increased levels of SIRT1 improving metabolic and inflammatory parameters; some of them increased mitochondrial function and increased the flexibility of alternation between different energy sources. Briefly, SIRT1 are activated and/or induced by AMPK in energy/nutrient-sensing pathways by glucose deprivation or exercises in many tissues. This is the result of increased AMP level which is the sign of low cellular energy [142]. Moderate and high-intensity exercise increases the AMP/ATP ratio [143, 144], while low-intensity exercise only if it prolongs to exhaustion [145]. SIRT1 activation then leads to increased fat mobilization and lipid oxidation in both skeletal muscle and differentiated adipocytes [57, 146, 147]. During exercise, increased mitochondrial respiration results in elevated NAD+/NADH.H+ ratio which also induces SIRT1 levels, so this feedback loop maintains the activity of the proper metabolic pathways to meet energy demand. However, beside the activation loops, there are inhibition circles as well in this regulation which impede biosynthetic pathways including fatty acids, and thus diminish fat-induced inflammation levels [148]. Improved exercise tolerance has other benefits such as decreased ROS production, oxidative stress and subsequent DNA–damage [25]. Despite the beneficial effects of a long-term CR with elevated SIRT1 levels, initially low baseline levels of SIRT1 in adipose tissue can significantly reduce the effectiveness of CR [32]. Similarly, the minor alleles of SIRT1 and CLOCK in the carriers also increased resistance to weight loss, increased ghrelin levels and lower adherence to the Mediterranean diet [41]. These results suggest that SIRT1 level may serve as a predictive marker of metabolic imbalances or effectiveness of a complementary therapy for weight loss, high blood pressure, cardiovascular disease, T2DM or cancers. However, a well-defined range of healthy and unhealthy levels of SIRT1 in different tissues, like adipocytes, skeletal muscle cells or PBMCs, should be determined which requires further studies for its appropriate use in health services.
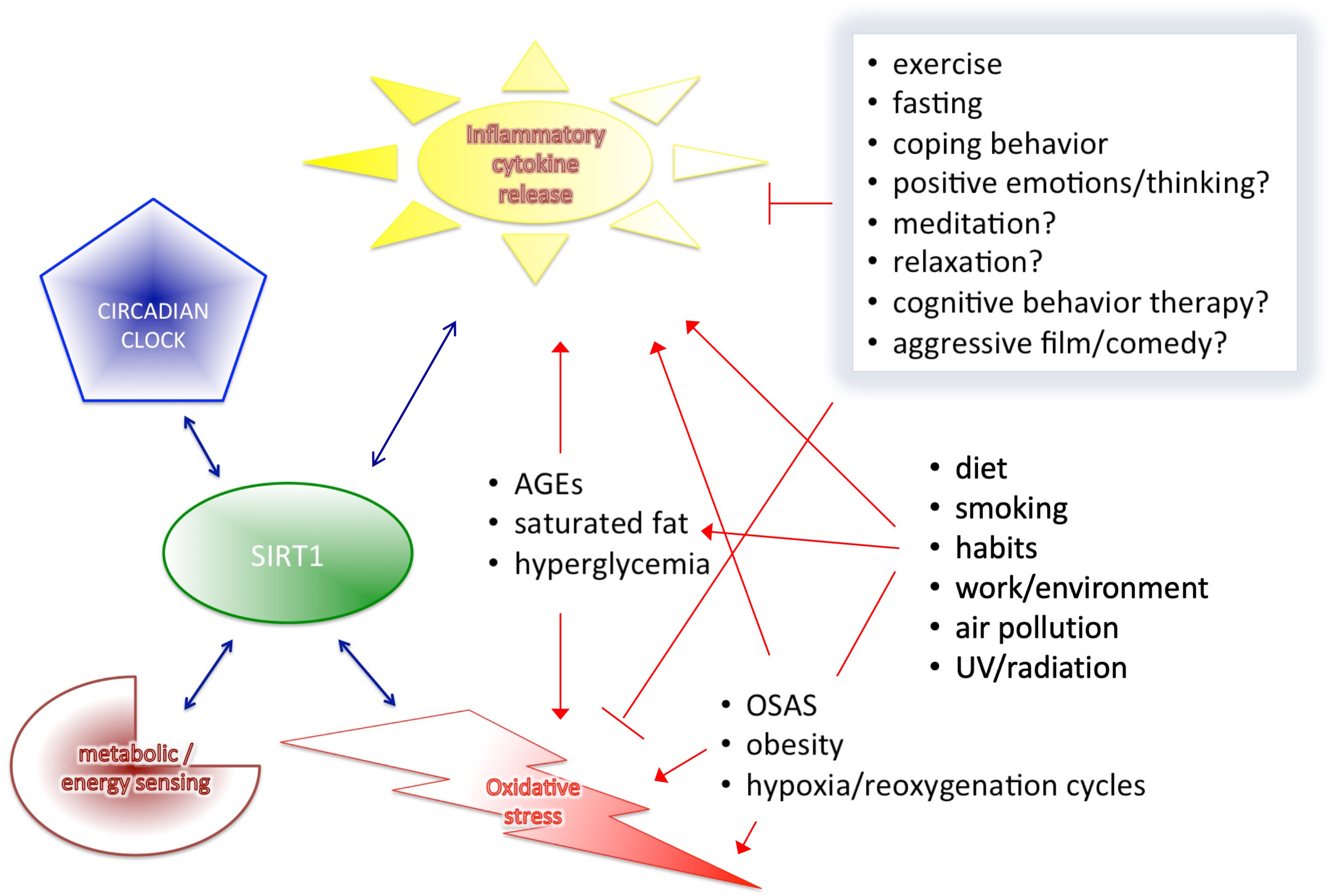
Based on the results of the cited human clinical trials and human studies, we described that SIRT1 interplays with major components of health-regulatory processes, such as metabolic/energy sensing system, pro-inflammatory cytokine release, oxidative stress control, however, the circadian rhythms itself cannot discussed as a separate entity from all others (Fig. 3). Circadian rhythm is the basis of all diurnal behaviors, like eating or activity level in general, and all energy and metabolic processes required for these functions. Therefore, it is necessary to outline this essential component and its connections to all the above mentioned mechanism related to SIRT1 expression. The proper alignment of the circadian clock with the environmental “time generators” is necessary for normal, healthy metabolic regulation [149]. Therefore, desynchrony resulting from a generally imbalanced lifestyle can cause similarly elevated leptin, glucose and insulin levels, as well as increased blood pressure leading to increased risk of obesity, T2DM, hyperlipidaemia, high blood pressure and cardiovascular disease.
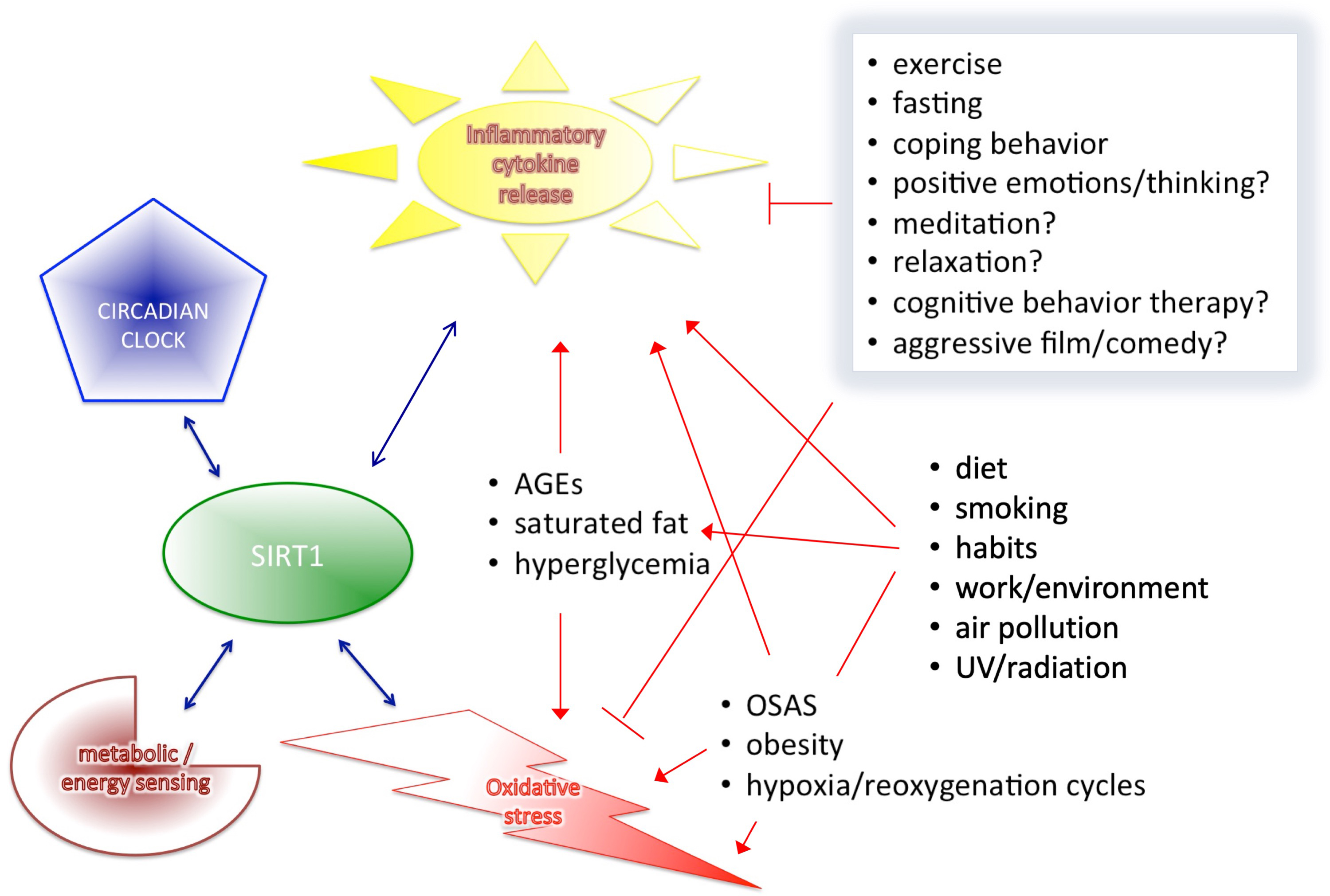 Fig. 3.
Fig. 3.SIRT1 interplays with the four major components of health-regulation.
As reported, circadian rhythm alterations are frequent in metabolic syndrome,
cancers and mood disorders [149, 150]. Food availability is predominant for
peripheral clocks in the peripheral tissues, and is able to resynchronize them
independently from the central clock. Thus, not surprising that a number of
metabolic pathway regulator transcription factors (such as Rev-erb-alpha,
ROR-alpha, PPAR) display tissue specific circadian expression in peripheral
tissues (liver, white and brown adipose tissue, skeletal muscle). It has been
identified that smoking is also able to disrupt circadian rhythm, decrease SIRT1
expression and is strongly associated with metabolic imbalance and inflammation
as well [151, 152]. The CLOCK/NAD
Defense mechanisms related to inflammation are also important regulators of
homeostasis (Fig. 3). They include SIRT1, AGER and pattern recognition receptors
(PRR), like TLRs, and stop infections and other non-desired mechanisms in the
human system [37, 82]. TLR is originally activated by bacterial lipids leading to
subsequent release of IFN
As we described earlier, oxidative stress is involved not only in immune system
function but in aging, telomere attrition, DNA-damage, apoptosis and
angiogenesis, and increases the level of MMP9, which is also known in cancer
development, however, SIRT1 is able to block MMP-9 expression and regulates all
these functions as well [158, 159, 160, 161]. Interestingly, a recent paper suggests that
Treg cells, which are also the focus of recent cancer research, sustain and
amplify tumor suppressor capacity in the microenvironment through oxidative
stress [162]. However, it is promising that repeated exercise may reprogram these
important regulatory mechanisms induced by oxidative stress in distant tissue
microenvironments, i.e., in tumors or other tissues that are not directly
involved in the training response, resulting in a cumulative antitumorigenic
effect [136]. In addition, we found that SIRT1 inducers exert a similar effect as
CR and exercises without side effects during the duration of the studies, which
may be an alternative if the other two are inappropriate for some reason
[137, 138, 139, 140, 141]. In tumors, SIRT1 expression varied by tumor types and subgroups.
However, as we have found, the main drawback of all cancer papers is that mostly
only the tumor samples and the surrounding normal tissues were examined.
Unfortunately, these data do not allow us to compare these results with
epidemiology papers that measured wider and systemic effect of SIRT1, rather than
local tissue changes in cancer studies. Therefore, these data can give
information for diagnostic, progression or local treatment purposes but do not
explore the background and causal factors of tumor development. Higher levels of
SIRT1 could reflect a necessity of higher defense in the body to eliminate a more
aggressive tumor. However, data suggest that DNA damaging agents probably would
not be sufficient in tumors overexpressing SIRT1 because SIRT1-regulated pathways
bypass this by induction of survival [52]. Additionally, non-cancer related data
detailed above suggest that higher SIRT1 expression is able to reduce MMP-9
either by downregulation of NF
In summary, in our review we focused on SIRT1 and related pathways as a molecular aspect of different lifestyles. Our findings support that interventions to prevent metabolic disorders can similarly prevent the development of cancer, as it has known relation to chronic inflammation and metabolic syndromes. With this review, we aimed to promote health science-related research and highlight the importance of beneficial lifestyle factors as effective tools for prevention and complementary treatment to reduce the development of diseases.
ABCA1, ATP-binding cassette transporter A1; ADPR, Adenozin-diphosphate-ribose;
AGEs, glycation end products; AGER1, AGE receptor 1; AROS, active regulator of
SIRT1; CIMP-high, CpG island methylator phenotype; CLOCK, circadian locomotor
output cycles kaput; COVID-19, coronavirus disease; CR, caloric restriction;
DBC1, deleted in breast cancer 1; DFS, diseases free survival; EE, energy
expenditure; EMT, epithelial to mesenchymal transition; eNOS, endothelial nitric
oxide synthase; FXR, farnesoid X receptor; FEV1, forced expiratory volume in 1s;
FOXO1, FOXO3, Forkhead box O 1 or 3; FVC, forced vital capacity; H2BC, HER2
overexpressed and/or amplified and ER and/or PR negative breast cancer; HDAC,
histone deacetylase enzyme; HDL(-C), high-density lipoprotein(-C); HOMA-R,
homeostasis model assessment for insulin resistance; HRBC, hormone
receptor-positive breast cancer; IR, insulin resistane; IRI, insulin resistance
index; IDC, invasive ductal carcinoma; LAGB, laparoscopic adjustable gastric
banding; LDL(-C), low-density lipoprotein(-C); LNM, lymph node metastases;
LXR
ZN—design, data collection, visualization, writing manuscript, paper review, final approval; EK—paper review; IT—paper review.
Not applicable.
We would like to thank to Katherine McAllister, Robbie Carson and Sarah Osman at Queen’s University Belfast for their kind support at the beginning of the manuscript preparation.
This work was supported by the predoctoral research fellowship of the National Research, Development and Innovation Office of Hungary obtained for Eva Kiss (ÚNKP-21-4-I-SE-19).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.