1 Laboratory of Experimental Pediatric Surgery, Department of Surgical Sciences, Dentistry, Gynecology and Pediatrics, University of Verona, 37129 Verona, Italy
2 Neuroimmunology Unit, Institute of Experimental Neurology, IRCCS San Raffaele Scientific Institute, 20132 Milan, Italy
3 Neuropsychopharmacology Laboratory, Section Pharmacology, Department of Diagnostic and Public Health, University of Verona, 37129 Verona, Italy
Academic Editor: Rebecca Piccarducci
Abstract
Background: Mesenchymal stromal cells (MSC) from bone marrow have been
reported to undergo the initial phases of neural differentiation in response to
an increase of intracellular cAMP. We investigated the possibility that a similar
effect applies to chorion-derived MSC. Methods: The intracellular
concentration of cAMP was increased either by forskolin, to promote its
synthesis, or by inhibitors of its degradation. The consequent reduction in the
expression of mesenchymal markers was associated with the appearance of
neuron-like morphology in a subset of cells. The effect was measured and
characterized using biomarkers and an inhibitor of cAMP response element-binding
protein (CREB). Results: The dramatic morphological change induced by
all the treatments that promoted intracellular cAMP was transient and peaked on
the third day. After that, cells returned to the typical fibroblast-like
appearance within 24 hours. The distinctive morphology was associated to the
expression of neuregulin 1, doublecortin, neuron-specific class III
Keywords
- cAMP
- chorion mesenchymal stem cells
- placenta
- cAMP response element-binding protein
- regenerative therapy
- neural differentiation
Full term placenta represents an abundant source of mesenchymal stromal cells (MSCs) and could offer an ideal source of cell precursors, particularly for pediatric autologous cell therapy. In addition to their self-renewal ability, immunomodulatory properties and plasticity, chorion derived MSCs (C-MSCs) are neither teratogenic nor liable of ethical limitations [1, 2]. However, as compared to bone marrow MSCs (BM-MSCs) [3] and progenitor cells from other tissues, C-MSCs have been largely under-investigated for neural regenerative therapies.
Several authors described how an increase of the signaling molecule cAMP in the cytosol of MSCs derived from bone marrow [4, 5, 6, 7, 8, 9], Wharton’s jelly [10] amniotic fluid [11] produces a rapid morphological change and other features reminiscent of neuronal cells. Although the effect is transient [4] and does not lead to full commitment to neurons or glial cells phenotypes, it is likely to represent an early phase of the phenomenon [4]. Recently, IBMX-treated adipose derived MSCs were also shown to undergo morphological and phenotypic changes. Proteomic analysis supported an association to neuronal features deemed most promising in consideration of the resulting increase in b-FGF, a known neuronal growth factor for MSCs [12].
cAMP signaling is highly pleiotropic and is involved in the development of all three germ layers. cAMP formation is promoted by adenylyl cyclase, typically activated by stimuli acting via G-protein-coupled receptors. Its degradation to AMP is mediated by phosphodiesterases, crucial to control cAMP levels within the cell. cAMP activates several signaling pathways, most often mediated by the activation of protein kinase A (PKA) which in turn phosphorylates target proteins including other kinases, ion channels and transcriptional factors. Long-term effects are mediated by protein synthesis-dependent processes that involve the transcription factor cAMP response element-binding protein (CREB) [13]. cAMP is a universal second messenger and CREB role in neuronal development is well established. Being able to control this signaling pathway can be considered a crucial step in order to take full advantage of an untapped source of mesenchymal cells, like C-MSCs.
This study aims to reproduce and characterize the effect of cAMP on C-MSC. Three approaches known to increase intracellular cAMP produced a rearrangement of C-MSCs morphology that we prove to be reversible by time-lapse microscopy. The effect was prevented by a CREB-specific inhibitor and was modulated by potential regulators of neural development . These results support the potential for extending to C-MSCs the studies for the development of novel cell therapies for neural damages [14].
Forskolin, 3-isobutyl-1-methylxanthine (IBMX), and dibutyryl-cAMP (db-cAMP) were from Serva, Germany. Cell culture media and Pen Strep are from Life Technologies Europe BV. KG-501 was from Società Italiana Chimici, SIC, Italy. b-FGF was from Merck Darmstadt Germany. Sphingosine-1-phosphate (S1P) and arginine-vasopressin (AVP) were from Sigma Aldrich.
C-MSCs were enzymatically isolated from human term placenta and characterized as
previously described [15]. In the experiments shown, cells were passaged 6–10
times. For neuron-like cell induction, C-MSCs were plated in 96 well plates
(10
After three days of treatment with the induction medium, C-MSCs were fixed using
4% paraformaldehyde (PFA) + 2% sucrose for 15 minutes and washed three times
with phosphate buffer saline (PBS). Permeabilization and blocking was performed
using PBS containing 10% FCS and 0.1% TritonX-100 for 30 minutes at room
temperature. Coverslips were incubated overnight with primary antibodies raised
against doublecortin (1:200 cat. #4645, Cell Signaling Technology, Inc. Danvers,
USA), pro-neuregulin 1 (1:100 cat. CSB-PA09589A0Rb, Cusabio, Technology, Houston,
USA), neuron-specific class III
Neuron-like cells were clearly different from fibroblasts because they manifested a number of protrusions and smaller nuclei, as compared to the larger, flat fibroblast phenotype. The number of neuron-like cells was quantified by manual counting using the Plugin Cell Counter of the software ImageJ Fiji (version 2.1.0/1.53c, NIH, Bethesda, Maryland, USA).
The fluorescent signal was quantified in confocal images and analyzed using ImageJ Fiji software. The background was measured in non-populated areas and subtracted. The specific signal was calculated by measuring the mean fluorescence intensity of the whole cell surface for over 30 cells. The intensity was normalized to the average signal of a comparable number of untreated cells stained and analyzed in parallel.
C-MSCs were seeded in 96-well plates, after replacement of the growth medium
with an induction medium, cells were placed in an EVOS® Onstage
Incubator (Life Technologies) at 37 °C, 5% CO
Real-time PCR was performed on cells treated with forskolin (50
The statistical measurements values were presented as mean
The p value was represented as follow: * p
C-MSCs were isolated from human placentas at term and propagated in vitro. We characterized the mesenchymal properties of these preparations in terms of immunophenotype and potency in a previous paper [15].
Similarly to what was repeatedly documented for other MSCs typically derived from bone marrow [4, 5, 6, 7, 8], a subset of C-MSCs acquired a neuron-like morphology in response to a treatment aimed to raise the concentration of intracellular cAMP. The effect was unambiguous because in response to forskolin the large flat fibroblasts raised around the nucleus to form a central dense body surrounded by a branched cytoplasm (Fig. 1, Supplementary Videos 1,2).
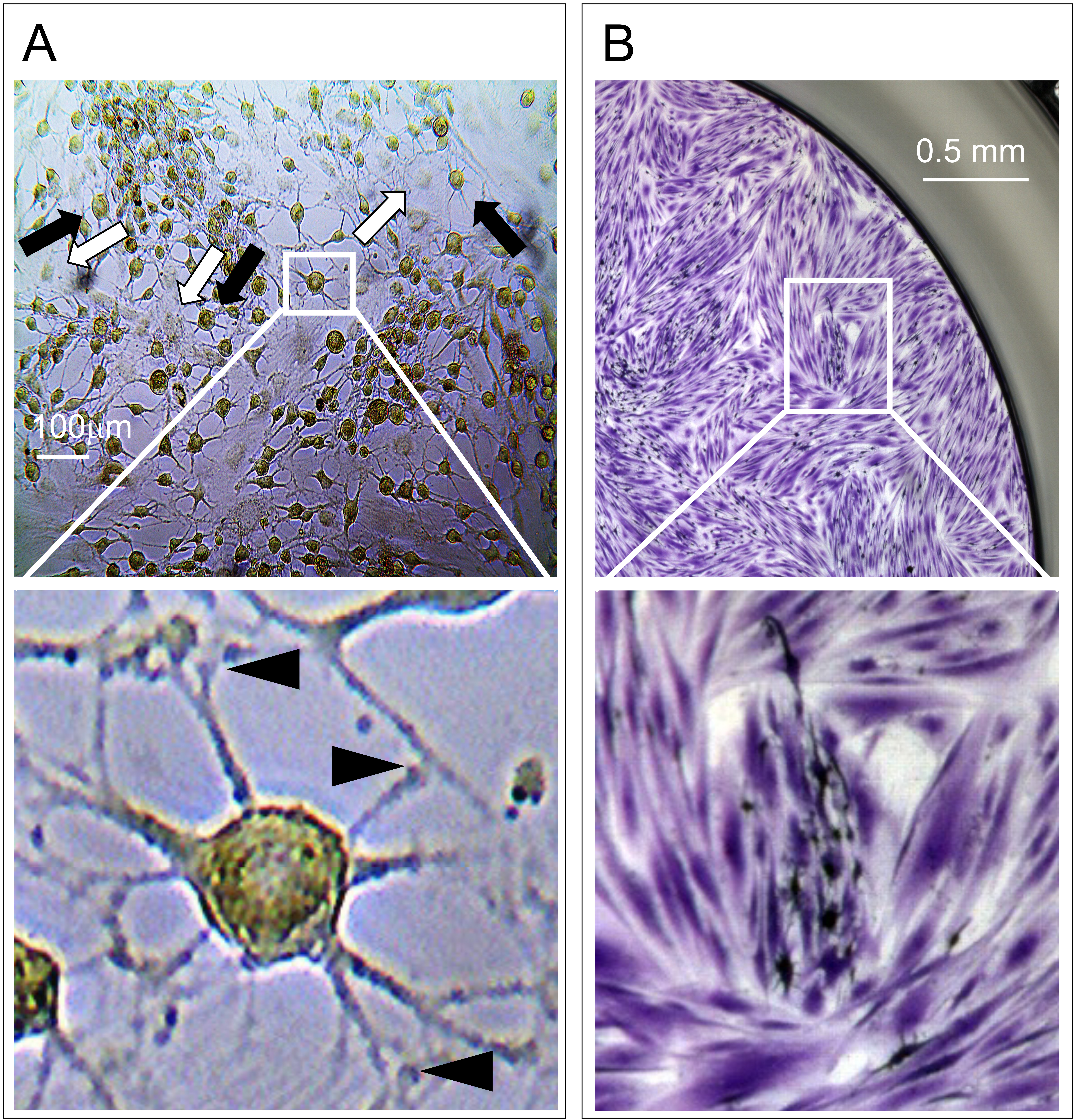 Fig. 1.
Fig. 1.Forskolin induces neuron-like morphology in
C-MSC. (A) Phase-contrast image of C-MSCs after 3 days of treatment
with 50
The robust stress fibers, typical of mesenchymal fibroblast-like cells, remained well organized in the majority of the cells that did not respond to the treatment. On the contrary, actin filaments were completely disorganized in the cells that assumed the neuron-like morphology (Fig. 2A).
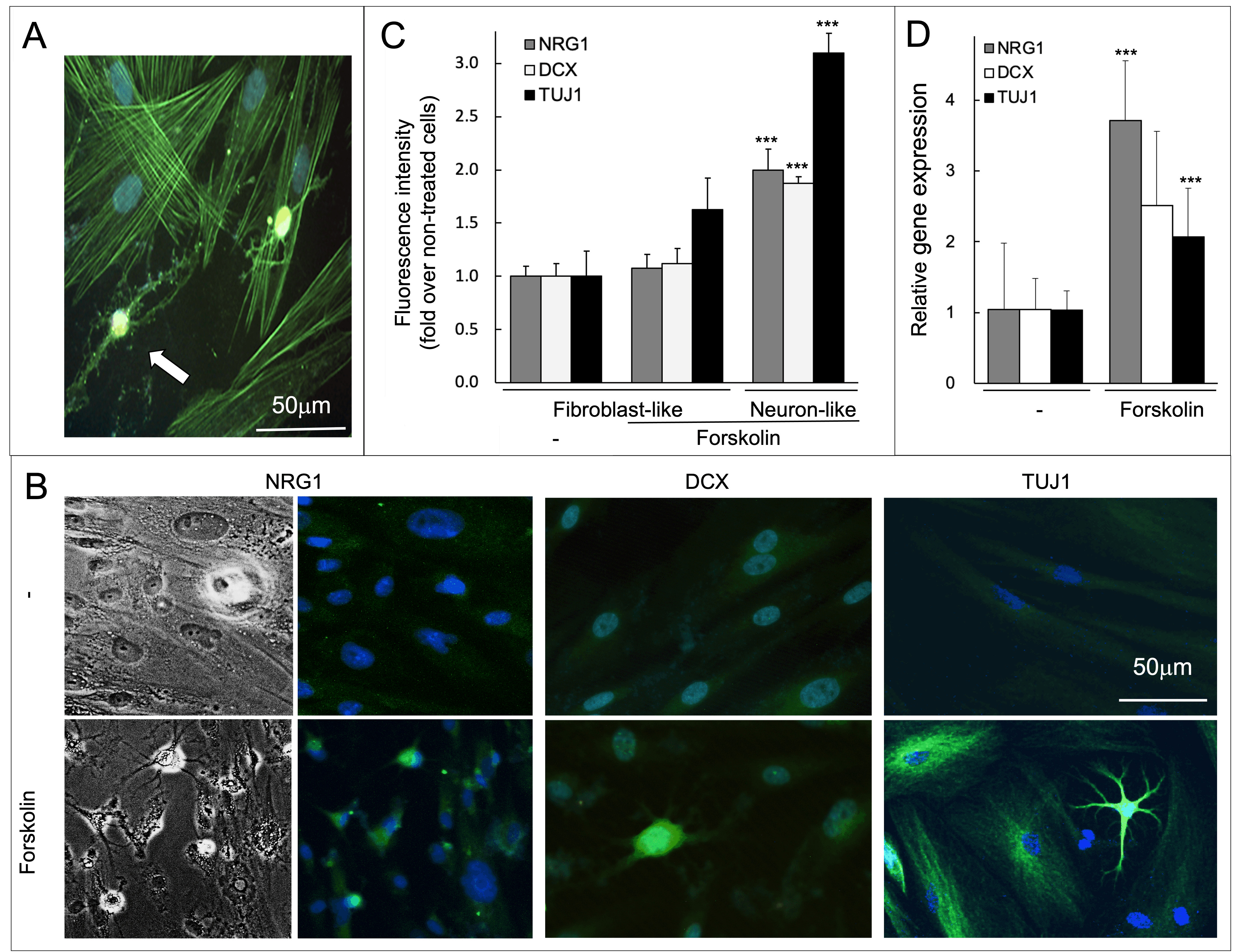 Fig. 2.
Fig. 2.Forskolin-induced rearrangement of the actin cytoskeleton and
the expression of neuronal markers. (A) C-MSCs after 3 days of treatment with
forskolin, phalloidin staining reveals the actin cytoskeleton in green,
Hoechst-stained nuclei are in blue. The white arrow indicates a neuron-like cell.
(B) Representative images of C-MSCs after 3 days of treatment with forskolin or
vehicle. The panels on the left show phase-contrast images. All the other images
present in panel (B) show cells stained for neuronal markers as indicated (green)
or the nuclei (blue). (C) Fluorescence intensity was quantified in cells stained
as in panel (B) and plotted as mean
The effect on cell morphology was associated with an increase in the expression
of three neuronal markers, doublecortin (DCX), neuregulin 1 (NRG1), and
neuron-specific class III
A component of FCS was suggested to inhibit the acquisition of a neuron-like morphology by BM-MSC [4]. Likewise, 10% FCS concentration in the growth medium of C-MSCs reduced the number of neuron-like cells as compared to 1% FCS (Fig. 3A).
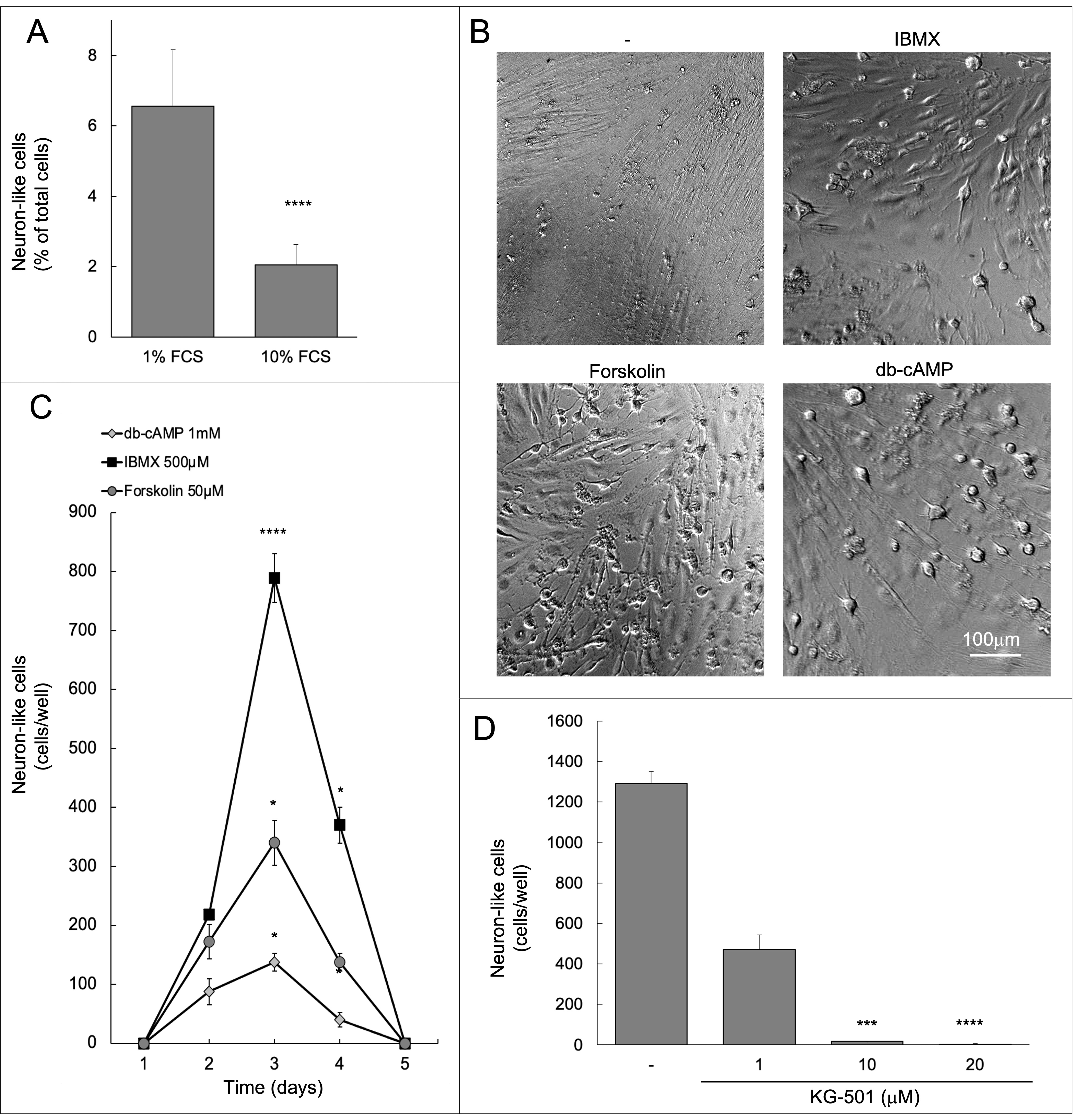 Fig. 3.
Fig. 3.Neuron-like morphology is transient and mediated by
transcription. (A) Neuron-like cells were counted 3 days after stimulation with
50
The same dramatic change of morphology observed in response to forskolin was reproduced by two additional strategies, IBMX and the cAMP analog db-cAMP, that increase the intracellular concentration of cAMP acting on phosphodiesterases [9]. The three approaches replicated experimental settings applied by other authors to mesenchymal cells derived from other tissues [2, 4, 5, 6, 7, 8, 12, 16] and produced an identical effect on cell morphology (Figs. 1,3B). Although the treatments demonstrated different efficacy, in all cases the peak was after three days (Fig. 3C). Eventually, neuron-like cells returned to their original morphology indistinguishable from all other fibroblast-like cells (Supplementary Videos 1–4), which excludes a toxic effect.
Further increasing the concentration did not show saturation of the effect, yet
the morphological differences became less obvious, possibly as a consequence of
toxic effects that over 100
cAMP-responsive elements binding protein (CREB) phosphorylation by PKA controls
neurogenesis by supporting newborn neurons and its role is conserved throughout
evolution [17]. In order to verify whether the appearance of neuron-like cells
involved CREB, the specific inhibitor KG-501 was simultaneously administered in
culture with forskolin. In the presence of 1
b-FGF is central to a highly orchestrated and complex sequence of cues required
for the acquisition of a neuronal phenotype [19] and has often been combined with
stimuli raising cAMP to promote MSCs neural differentiation [9, 20]. Combining 40
ng/mL b-FGF with 50
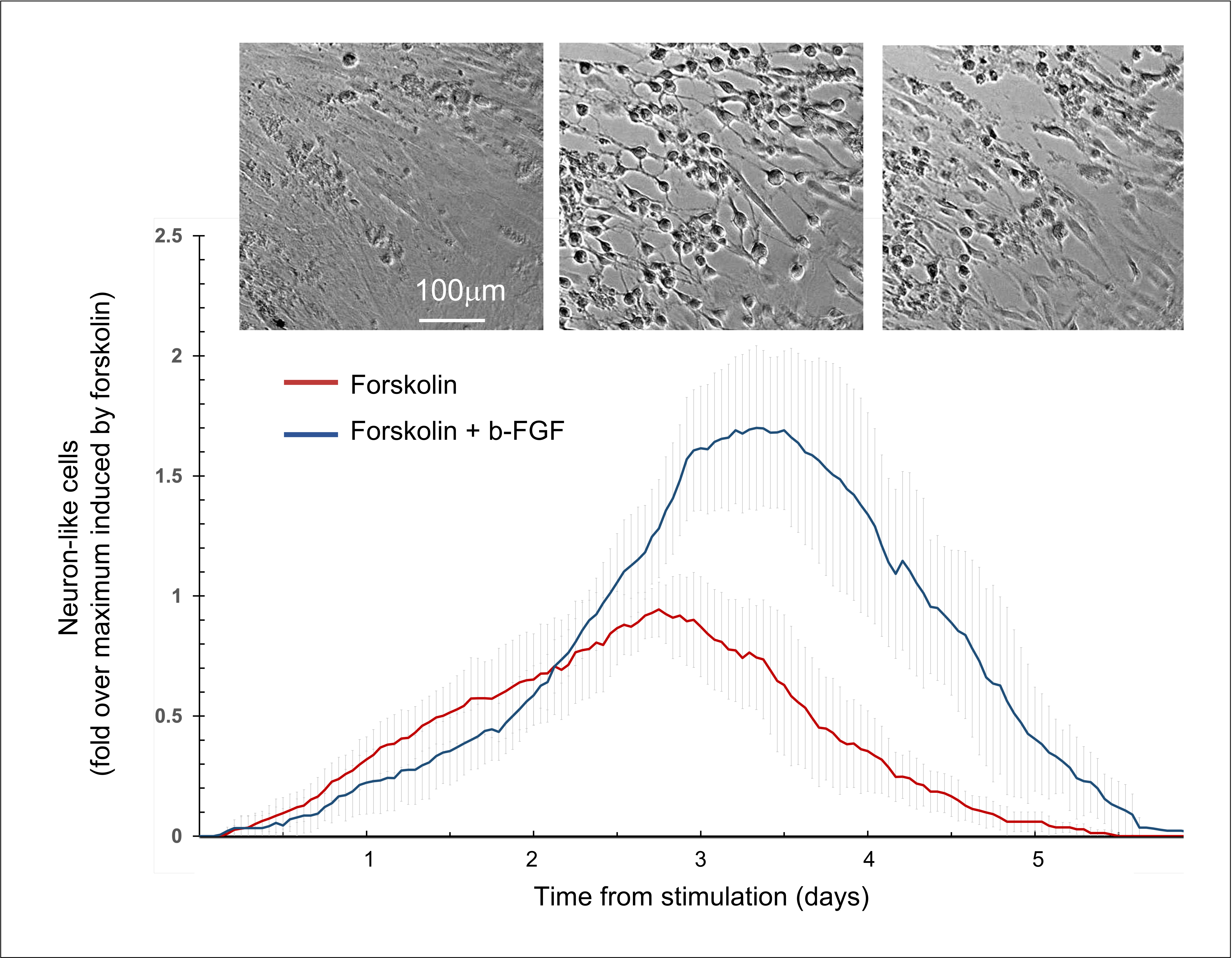 Fig. 4.
Fig. 4.b-FGF promotes neuron-like morphology. The appearance
of cells characterized by a neuron-like morphology was monitored in time lapse
documenting the time extent before the cell would return to a fibroblast-like
aspect. For each experiment (n = 3) data are normalized to the maximum number of
neuron-like cells achieved after stimulation with forskolin alone (mean
The expression levels of Sox2 and Oct4 are tightly balanced to support self-renewal and pluripotency. Forskolin treatment produced a dramatic decrease in both transcription factors by the third day (Fig. 5). Expression remained low when all cells recovered the fibroblast-like appearance and the decrease was not prevented by b-FGF.
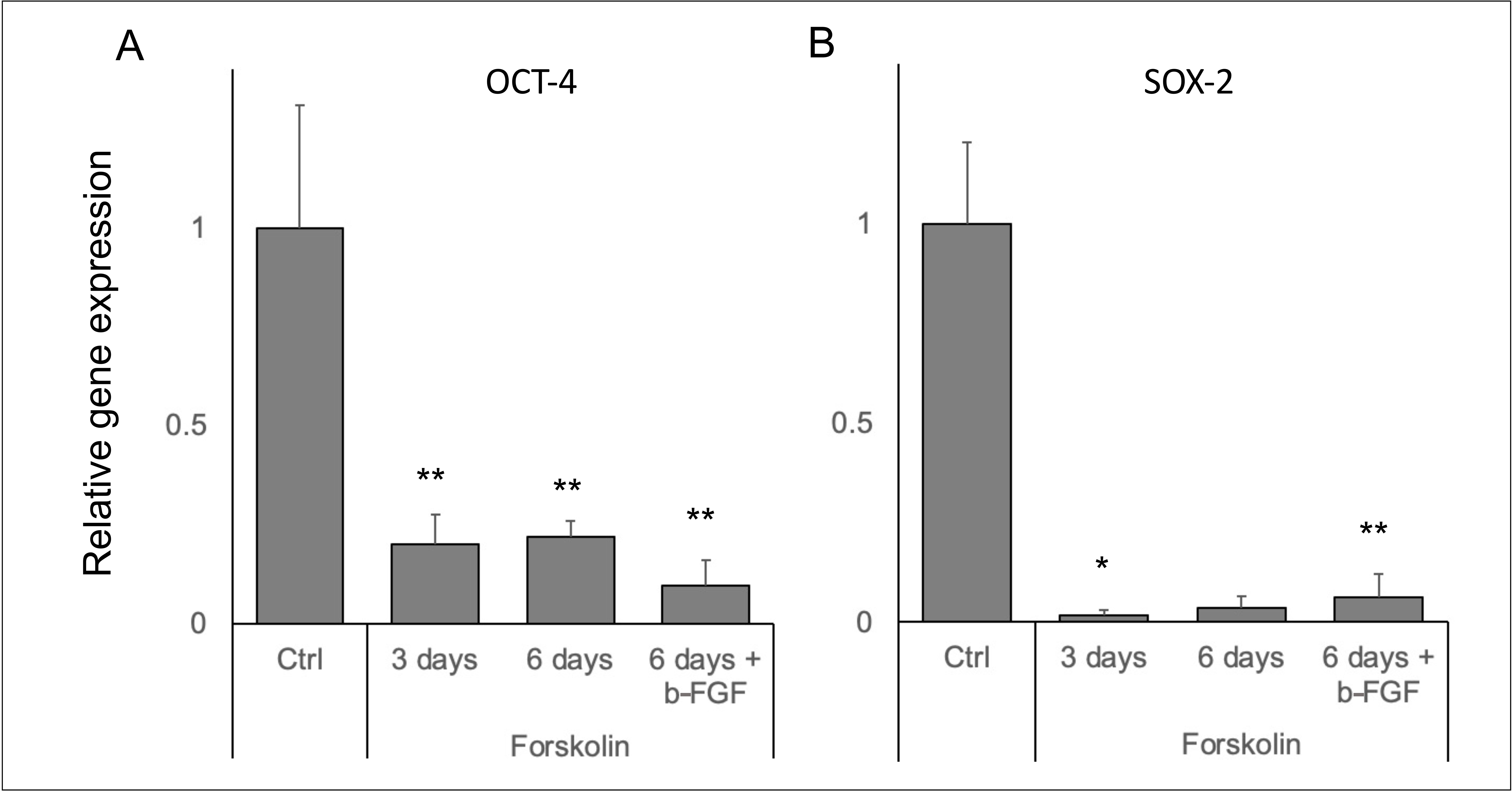 Fig. 5.
Fig. 5.Forskolin induced reduction in the expression of pluripotent markers. The expression of pluripotent markers was measured by real-time PCR in C-MSC treated as indicated. (A) OCT-4 mRNA expression. (B) SOX-2 mRNA expression. For each condition, data are normalized to the gene expression of untreated cells (n = 3, performed in duplicate, mean
S1P is a bioactive lysophospholipid with versatile biological effects, mainly
exerted through the binding of their specific receptors [21]. S1P is a powerful
stimulator of neurogenesis by binding to various G protein coupled receptors
(GPCRs) to activate or inhibit signalling pathways and by cross-talking with
other cytokine and growth factor receptors [22, 23]. Our lab previously
demonstrated that S1P activates ERK1/2 in C-MSCs in a dose-dependent manner.
ERK1/2 activation was transient in time and it was completely inhibited by
pertussis toxin, indicating that the pathway was fully Gi-dependent [15].
Combining 10
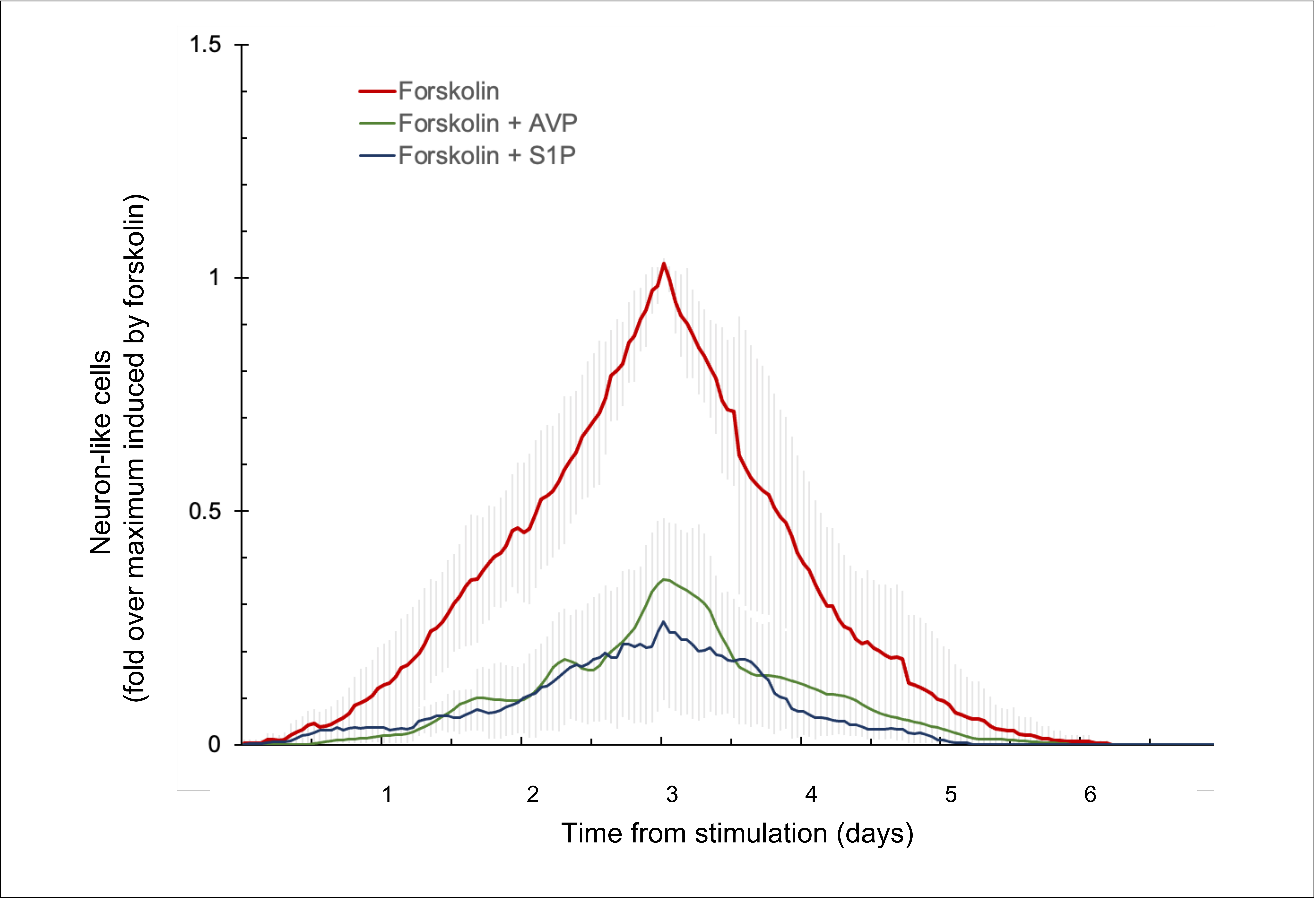 Fig. 6.
Fig. 6.S1P and AVP prevent neuron-like morphology. The appearance of
cells characterized by a neuron-like morphology was monitored in time lapse. For
each experiment (n = 6) data are normalized to the maximum number of of
neuron-like cells achieved after stimulation with forskolin alone (mean
AVP, a hormone synthesized from the AVP gene in hypothalamus neurons, regulates
various aspects of stem cells life. All three subtypes of AVP receptors are found
in mice adipose-derived MSCs (A-MSCs) whereas human A-MSCs expressed only AVPR1
subtype [24]. Activation of AVPR1 activates Gq-proteins and on turn PLC-
The most common source of human MSCs for “cell therapy” is the bone marrow. However, only 1 out of 10,000 of nucleated cells in the marrow is mesenchymal and an invasive procedure, particularly in newborns, is required to obtain reasonable yield of cells.
Placenta could provide a virtually unlimited supply of MSCs from a tissue that is normally discarded at birth. C-MSCs exploitation could lead to highly innovative treatments, particularly for autologous therapies in newborns, including neurological disorders. We demonstrated that the exposure of C-MSCs to different agents that increase cytosolic cAMP reproduces very closely the neuron-like phenotype that has been thoroughly investigated for BM-MSCs [3, 4, 5, 6, 7, 8].
Several authors reported the differentiation of MSCs into neural cell types and astrocytes, albeit the effective functional properties of the derived lineages remain highly debated.
The reliability of neural markers is also questionable considering the heterogeneity and relative abundance of transcripts and proteins expressed by MSCs [4]. Neural circuit development and regeneration is a highly complex process determined by a sequence of cues and interactions. In this sense, we hypothesize that our and others observations in a number of MSCs only represent a particular step triggered by cAMP, however, a detailed understanding of the cues that govern each step of neural differentiation is crucial in order to finally allow effective cellular therapies to treat damages of the neural tissue [6]. cAMP signalling is key to neural progenitor cells proliferation and differentiation, and consequently to nerve regeneration [26]. cAMP’s effects on morphological maturation are believed to depend on PKA and CREB activation. Once activated, CREB can bind to the promoter region of genes containing CRE. Whereas phosphorylated CREB is basically absent in stem cells, it is transiently up-regulated in neural progenitor cells and young neurons during the first few weeks of differentiation. During this time the level of CREB phosphorylation correlates with the total dendritic length and the morphological maturity of the young neurons [27].
Alone or in combination with other agents, such as b-FGF and BDNF, cAMP has been
reported to promote neuron-like morphology in BM-MSCs [3] with timeframes ranging
from hours to days [6]. The rapid effect of other agents that produced dramatic
morphological changes, i.e.,
Albeit our initial characterization of these cells using a set of mesenchymal markers described a homogeneous population of precursors [15], we found that the morphological effect only occurred in part of the cells. Noticeably, neuron-like cells were not distributed randomly but in clusters (Fig. 1 and Supplementary Videos). It is thus possible that our preparation included a subset of cells with a specific potential to respond to cAMP by acquiring the neuron-like morphology. Additional research is required to understand the reason why only a well-defined subset of cells undertook the process.
If C-MSCs respond to cAMP similarly to BM-MSCs, the molecular basis of the
phenomenon remains to be better characterized. KG-501 experiments suggest the
involvement of CREB which was shown to mediate the differentiation of neuronal
precursor cells in response to cAMP [16, 28, 29]. In addition, CREB obtained
growing attention as a key regulator of synaptic plasticity in the adult brain
[30]. Forskolin reduced the expression levels of SOX-2 and OCT-4 to an extent
that was almost complete suggesting that not only it occurred to “neuron-like
cells” but also to the large majority of the cells conserving the
fibroblast-like shape. The expression levels of SOX-2 and OCT-4 play a pivotal
role as lineage specifiers [31] including for neuronal commitment [32], and the
treatment with forskolin most likely compromised the possibility of C-MSCs to
undergo neuronal commitment. We suggest that a raise of cAMP in C-MSCs cytosol
activates a transcription program that remains incomplete and it is thus turned
off. As a consequence, cells do not die but revert to the fibroblast morphology
as shown by our videos. A trophic effect of b-FGF leading to neuron-like
phenotypic changes was previously reported not only for neural progenitors [33],
but also for bone marrow [3] and other mesenchymal cells [34] and for pancreatic
S1P inhibited the appearance of neuron-like cells. The effect could be explained
with the activation of Gi proteins and the consequent inhibition of adenylyl
cyclase responsible for the production of cAMP. In this respect, AVP should not
have produced the same effect since it signals through GPCRs that activate Gq/11
and Gs. Both, S1P and AVP, can promote PLC
The authors recognize some limitations to this study. Time-lapse recording allows only to observe morphology. This approach proved that neuron-like cells can return to the original fibroblast-like aspect and indistinguishable from all other cells. However, it did not detail the nature of neuron-like phenotype. Transcriptomic and proteomic analysis of MSCs derived from several origins and treated with IBMX suggested that a similar morphology alteration coincide with neurite substructure development [12]. A similar analysis could be instrumental to define more precisely the significance of this process also in MSC derived from placenta. Microdissection strategies or better markers will allow to selectively tag the small cell population and explore the specific nature and physiological significance of neuron-like cells in future RNAseq studies.
A full commitment to neural phenotype could be tested with electrophysiological analysis by patch-clamp of single cells to proof the excitability, if any, associated with this neuron-like phenotype [11].
We conclude that, as previously described for other MSCs, agents promoting an increase of cAMP produce a dramatic reorganization of the cytoskeleton of a subpopulation of C-MSCs. The development of protrusions towards other cells is prevented by a CREB inhibitor and it is fully reverted within 72 hours from the stimulation.
The possibility to take advantage of C-MSCs for therapeutic use appears even more exciting than the exploitation of MSCs from other sources because of the several advantages mentioned above. Further characterization of the specific subset of C-MSC with a neuron-like phenotype and a deeper understanding of the underlying mechanism could allow to guide C-MSCs towards full neural commitment and differentiation.
GI, FS and LG designed the research study. GI, GR, FS, AB and AD performed the research. GR, FS, AB, CB, AD, LO, MDC provided help and advice on this project. LO, and MDC provided materials. GI, GR, FS, AB, AD and LG wrote the manuscript. GI and LG supervised the project. LG contributed to funding acquisition. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
The study was conducted according to the guidelines of 243 the Declaration of Helsinki and approved by the Ethics Committee of the Azienda Ospedaliera 244 Universitaria Integrata di Verona, no. 0054.
This study was performed in the LURM (Laboratorio Universitario di Ricerca Medica) Research Center, University of Verona.
This research received no external funding.
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/j.fbl2708249.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.






