1 School of Pharmacy, Zunyi Medical University, 563000 Zunyi, Guizhou, China
2 Department of Pharmacy, Zunyi Medical and Pharmaceutical College, 563000 Zunyi, Guizhou, China
3 Department of Pharmacy, The Third Affiliated Hospital of Zunyi Medical University, 563000 Zunyi, Guizhou, China
†These authors contributed equally.
Academic Editor: Luigi De Masi
Abstract
Background: Lactate dehydrogenase (LDH) is one of the important enzyme
systems for glycolysis and gluconeogenesis. It can catalyze the reduction and
oxidation reaction between propionic acid and L-lactic acid, which is usually
overexpressed in cancer cells. Therefore, inhibiting the activity of LDH is a
promising way for the treatment of cancer. In this study, an effective method
based on ligand fishing and ultra performance liquid chromatography-mass spectrum
(UPLC-MS) was established to screen and identify active ingredients from
Selaginella doederleinii with potential inhibitory activity for LDH.
Methods: Firstly, LDH was immobilized on the magnetic nanoparticles
(MNPs), three immobilization parameters including LDH concentration,
immobilization time and pH were optimized by single factor and response surface
methodology for maximum (max) immobilization yield. Then, a mixed model of
galloflavin and chlorogenic acid (inhibitors and non-inhibitors of LDH) was used
to verify the specificity of immobilized LDH ligand fishing, and the conditions
of ligand fishing were further optimized. Finally, combined with UPLC-MS,
immobilized LDH was used to simultaneously screen and identify potential LDH
inhibitors from the ethyl acetate extract of Selaginella doederleinii.
Results: The prepared fishing material was comprehensively characterized
by scanning electron microscopy (SEM), transmission electron microscope (TEM),
X-ray diffraction (XRD) and fourier transform infrared spectrometer (FT-IR). The
optimal immobilization conditions were obtained as LDH concentration of 0.7
mg/mL, pH value of 4.5, and immobilization time of 3.5 h. Under these conditions,
the max immobilization yield was (3.79
Keywords
- lactate dehydrogenase (LDH) inhibitor
- functionalized magnetic nanoparticles
- Selaginella doederleini
- biflavonoids
- ligand fishing
According to data released by the Global Cancer Observatory of the International Agency for Research on Cancer, a subsidiary of the World Health Organization, the number of new cancer patients worldwide in 2020 was 19.3 million, and it is expected to increase by 30.2 million in 2040 [1]. Among all cancers, female breast cancer has surpassed lung cancer as the most common one [2]. Information on the Our World in Data website shows that an estimated 9.6 million people died of various forms of cancer in 2017. With the rising morbidity and mortality, cancer is a serious threat to human health and has become a major global public health problem [3]. Therefore, it is imperative that the search for effective cancer drugs is accelerated.
The Warburg effect is a common feature of cancer cells, which, unlike most normal cells, tends to “ferment” glucose into lactic acid even under aerobic conditions [4]. Some scholars believe that the energy generated by excessive glycolysis supports the growth of cancer cells [5], and that the production of a large amount of lactic acid causes extracellular acidification, which leads to the invasion and metastasis of cancer cells [6]. Thus, in part, it is possible to deprive cancer cells of the energy they need to survive and hinder their invasion and metastasis by inhibiting lactate dehydrogenase (LDH) during glycolysis process [7]. LDH is the rate-limiting enzyme that catalyzes the conversion of pyruvate to lactate during glycolysis and plays a crucial role in the Warburg effect [8]. LDHA and LDHB are two isoenzymes of LDH [9]. By inhibiting the activity of LDHA, the proliferation and migration of prostate cancer cells could be inhibited and reduced [10], and this phenomenon was also observed in ovarian cancer cells [11], hepatocellular cancer cells [12], and pancreatic cancer cells [13]. Taken together, LDHA inhibitors are one of the key drugs of interest for the treatment of cancer.
At present, several screening methods for enzyme inhibitors have been
established, including computer virtual screening technology [14], spectrum
effect binding [15], ligand fishing technology [16], as well as others. Because
ligand fishing technology has the advantages of high efficiency, good stability,
and strong specificity, it has been widely used in screening inhibitors [17]. For
example, lipase was immobilized on magnetic nanoparticles (MNPs), and bamboo
leaves were screened for lipase inhibitors. Three were subsequently found and
identified as isoorientin, orientin, and isovitexin with inhibition rates of
56.93
Selaginella doederleinii belongs to the Selaginella family, which is a traditional Chinese herbal medicine usually used in folk medicine as an antitumor herb [21]. Carbohydrates, alkaloids, and flavonoids are its main chemical components [22]. Pharmacological research of Selaginella doederleinii mainly revolves around its antitumor effects. For example, the ethyl acetate extract of Selaginella doederleinii was used to investigate its anti-colorectal cancer effects, and the results showed that it inhibited the proliferation of HT-29 and HCT-116 cell lines by inducing autophagic death and apoptosis through PI3K-Akt-mTOR and AMPK alpha signaling pathways [23]. HPLC was used to analyze the ethyl acetate extract of Selaginella doederleinii, which mainly contained eight kinds of biflavonoids, and the extract inhibited the growth of A549 lung cancer cells through the mitochondrial apoptosis pathway, decreasing Ki67 expression and microvascular density [24]. Thus, the ethyl acetate extract contained the main active antitumor components from this plant, but the active ingredient remained unidentified.
The discovery of active natural products from widely existing medicinal resources in nature has been an important way to develop innovative drugs [25]. Therefore, in this study, LDHA was immobilized on amino-modified MNPs, the synthesis process was optimized, and the synthesized materials were characterized. Subsequently, ethyl acetate extracts from Selaginella doederleinii were screened for inhibitors of LDHA using the ligand fishing technique. Finally, potential LDHA inhibitors were identified by UPLC/MS.
LDH (subunit: LDHA, 100 U/mg) and (3-aminopropyl)triethoxysilane were
acquired from Aladdin Biochemical Technology Co. (Shanghai,
China). Fe
An Agilent 1260 equipped with diode array detector was purchased from Agilent (California, USA). ACQUITY UPLC H-Class with Quaternary Solvent Manager was from Waters (Massachusetts, USA). The 759S UV-Vis spectrophotometer equipped with a unique monochromator optical path and photoelectric converter was purchased from JINGHUA Technology Instrument Co. (Shanghai, China). The vortex meter with an amplitude of 4.5 mm (circular oscillation) was purchased from JOANLAB Experimental Instruments Co. (Zhejiang, China). A Thermo Scientific™ Multiskan™ FC microplate reader with a detection wavelength range of 340–850 nm was purchased from Thermo Fisher Scientific (Massachusetts, USA).
The Selaginella doederleinii (Guizhou, China) powder that had passed through a 60-mesh sieve was extracted twice with 75% ethanol for 2 h under reflux. The two extracts were combined and evaporated with the excess solvent until there was no alcohol smell. Finally, it was extracted three times with ethyl acetate and dried under reduced pressure and stored at 4 ℃ [26].
LDH-functionalized MNPs were prepared according to the literature with minor
modifications [27]. Fifteen volumes of 50% ethanol were added to the mixture of
Fe
One mL of 5% GA was added to an Eppendorf tube containing 3 mg of
MNPs-NH
Scanning electron microscopy (SEM), transmission electron microscopy (TEM),
X-ray diffraction (XRD), and fourier transform infrared (FTIR) spectroscopy were
used to characterize Fe
A unit of LDH activity was defined as the amount of enzyme that catalyzes 1
(Where A and Am are the decrease and max decrease in absorbance under each set of conditions, respectively.)
The Coomassie brilliant blue method was used to measure the yield of immobilized
LDH [29]. One hundred
(Where [E] and [M] represent the difference in the amount of LDH in the supernatant before and after immobilization and the mass of MNPs, respectively.)
Three parameters including LDH concentration, pH of the LDH solution, and
immobilization time were investigated to obtain the optimal conditions for LDH
immobilization on Fe
Based on the experimental results in the above Subsection 2.6.1, a three-factor, three-level response surface analysis experiment was designed with the relative enzyme activity (Y) as the response value. The parameters were as follows: concentrations were 0.3, 0.6, and 0.9 mg/mL, pHs were 3, 4, and 5, and incubation times were 2, 3, and 4 h. Seventeen experiments were conducted based on the above design, as shown in Table 1.
| Factors | Coded levels | ||
| −1 | 0 | 1 | |
| LDH concentration (mg/mL) | 0.3 | 0.6 | 0.9 |
| immobilization pH | 3 | 4 | 5 |
| Immobilization time (h) | 2 | 3 | 4 |
To clarify the relationship between the three parameters in Section 2.6.1 and
the LDH immobilization yield, 100
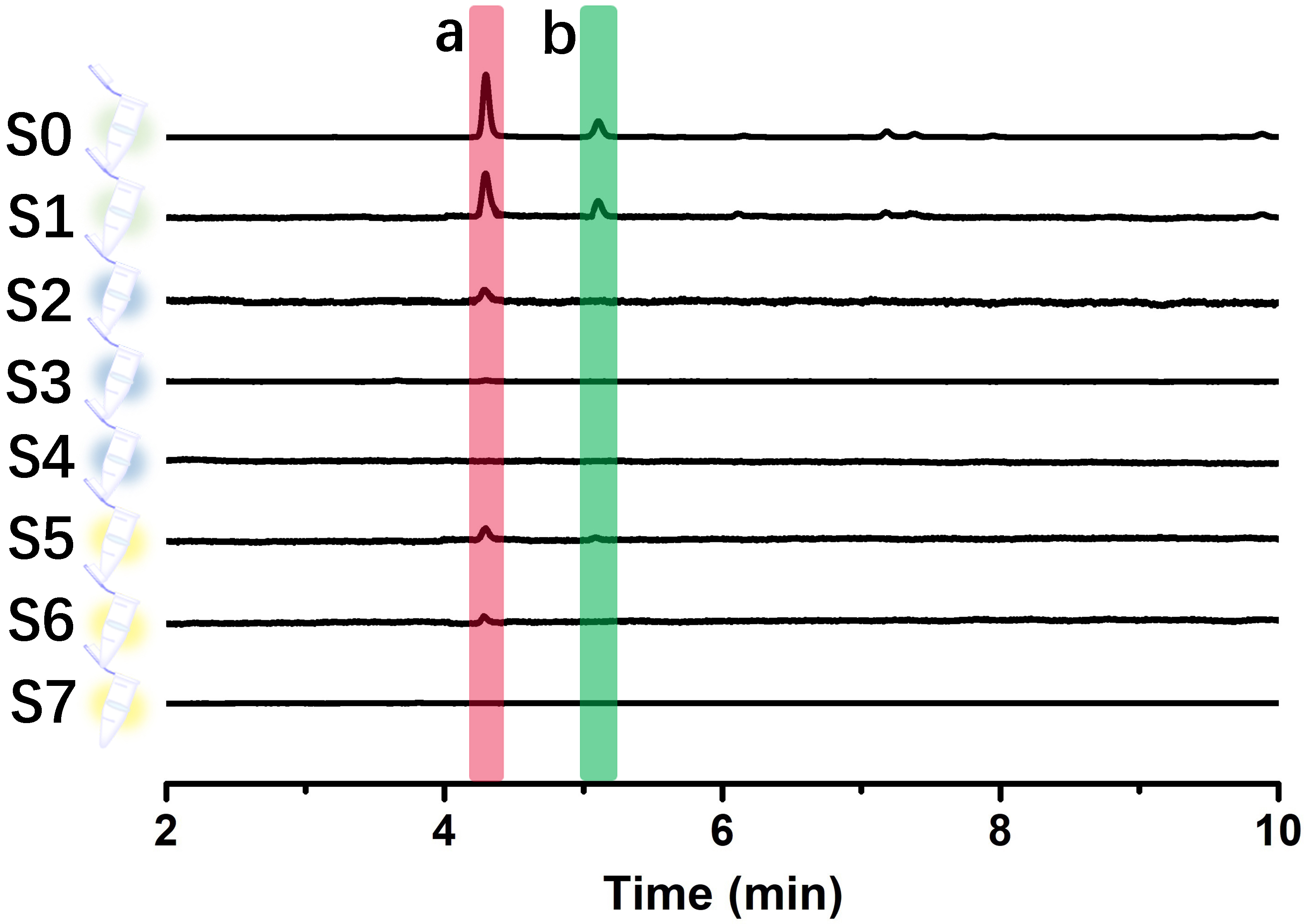
Immobilized LDH was added to a mixture containing galloflavin and chlorogenic acid (standard LDH inhibitor and non-inhibitor) for specific detection. The incubation time (10–60 min) and elution time (10–60 min) of ligand with immobilized LDH and the concentration of methanol eluent (20%–100%) were optimized. The procedure was as follows: 3 mg of immobilized LDH was added to 1 mL of a mixture of galloflavin and chlorogenic acid (S0) and incubated for 30 min. After magnetic strip separation of immobilized LDH, the supernatant was collected (S1). Next, the immobilized LDH was washed three times with 1 mL of buffer, and the washing solutions were collected (S2–S4). Then, 1 mL of methanol was added to the immobilized LDH and eluted 3 times, and the elution solution was collected each time (S5–S7). Finally, S1–S7 were analyzed using HPLC.
On the basis of the optimal ligand fishing conditions obtained in Section 2.8, 3 mg of immobilized LDH was added to 1 mL of the Selaginella doederleinii methanol solution (S0) and incubated for 30 min. The supernatant (S1) was then collected from the immobilized LDH. The supernatants (S2–S4) were then collected by washing three times with 1 mL buffer. Then 1 mL methanol was used to elute three times, and the eluates were collected (S5–S7). UPLC/MS was used to analyze all samples (S0–S7).
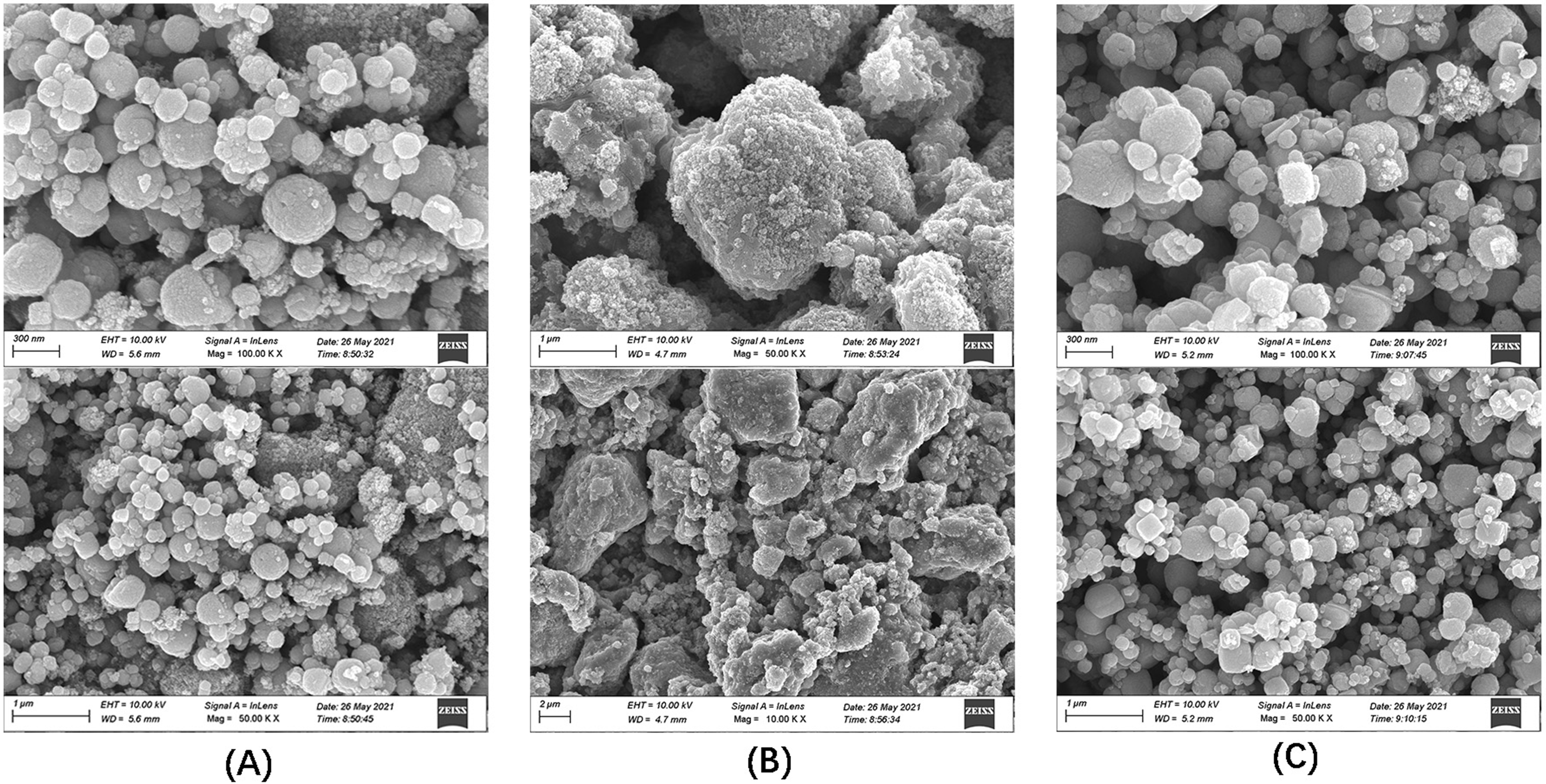
SEM is an important technique to use to observe the microscopic morphology of
materials because of its great advantages in resolution, depth of field, and
microstructure analysis [30]. The SEM images of Fe
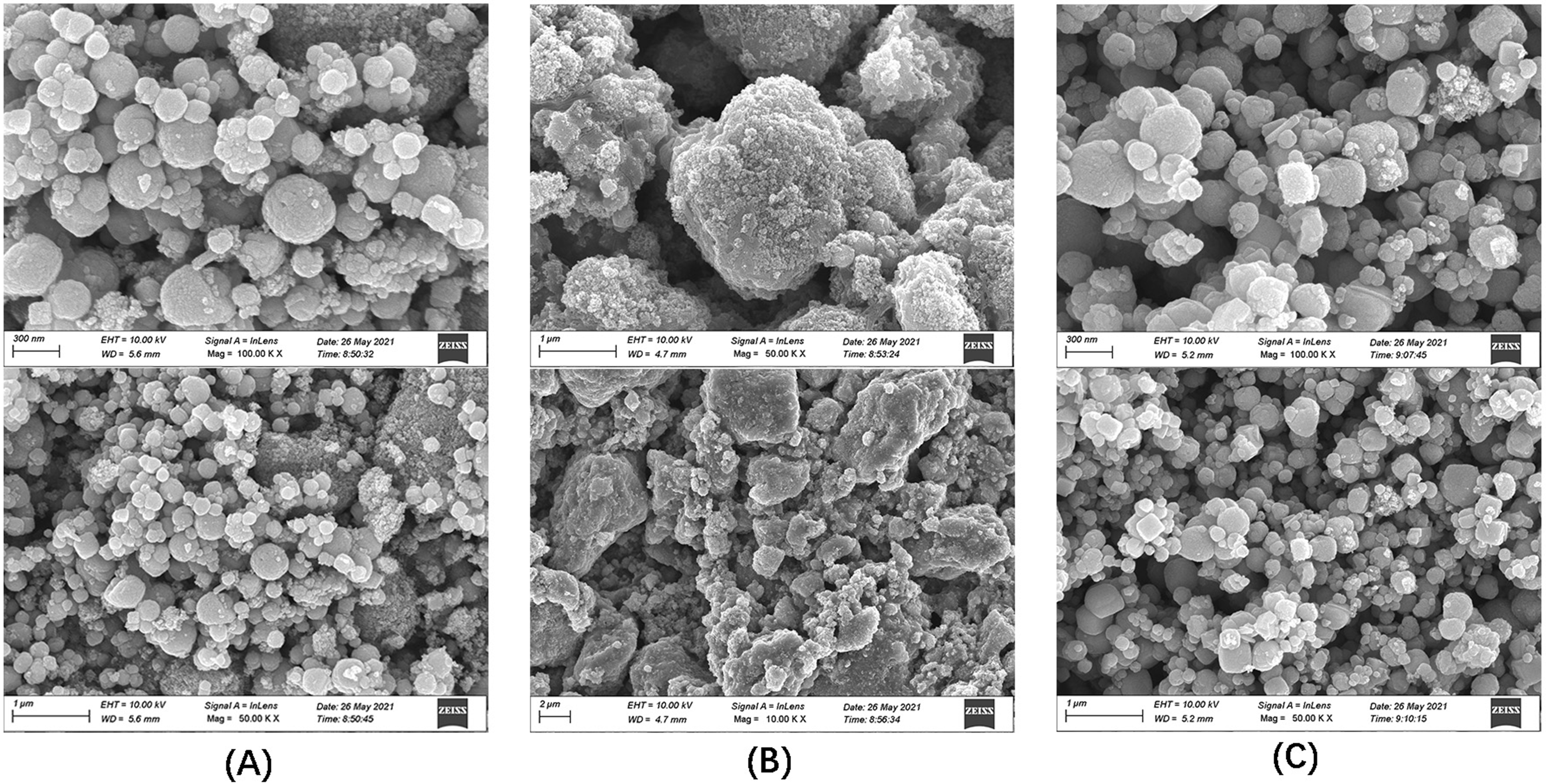 Fig. 1.
Fig. 1.SEM images of the three materials. (A) Fe
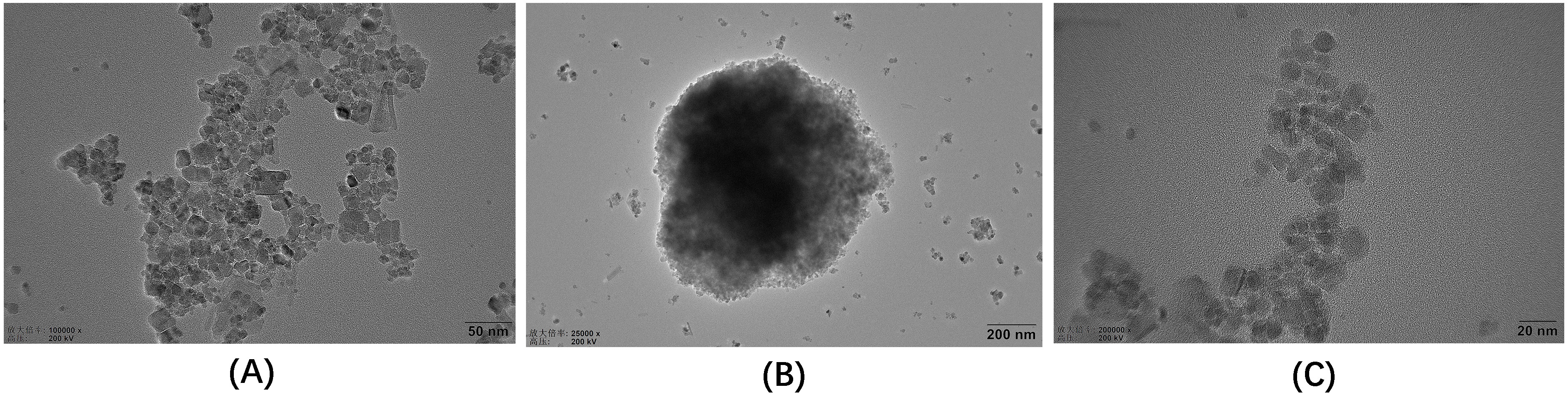
TEM uses an electron beam as a light source and an electromagnetic field as a
lens to project the accelerated and concentrated electron beam onto a very thin
sample. Because of the different density and thickness of the sample, images with
different brightness and darkness are formed. This method is usually used for
ultrastructural observation [31]. TEM images of Fe
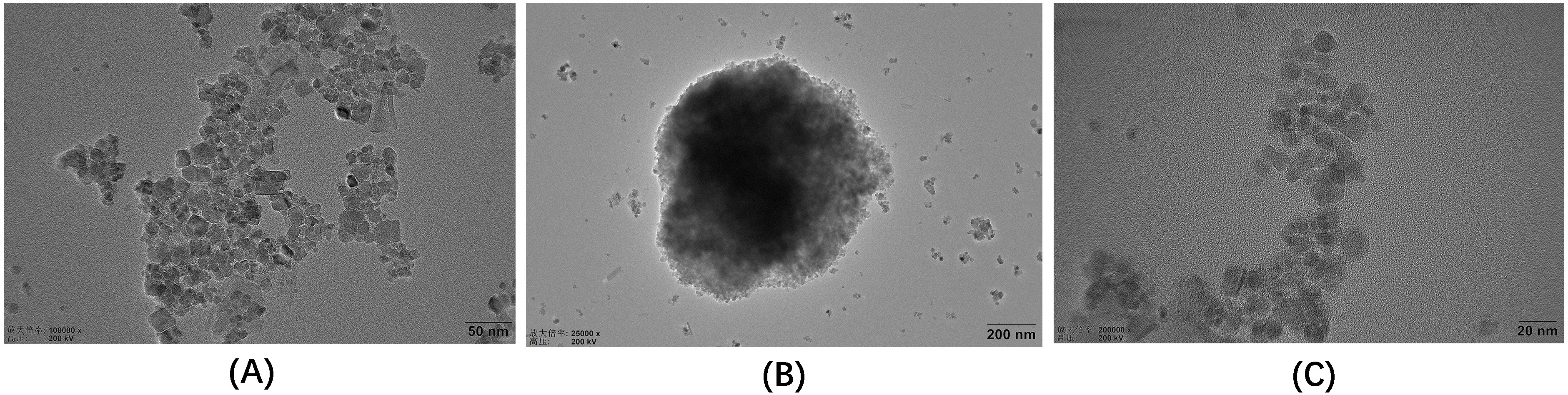 Fig. 2.
Fig. 2.TEM images of the three materials. (A) Fe
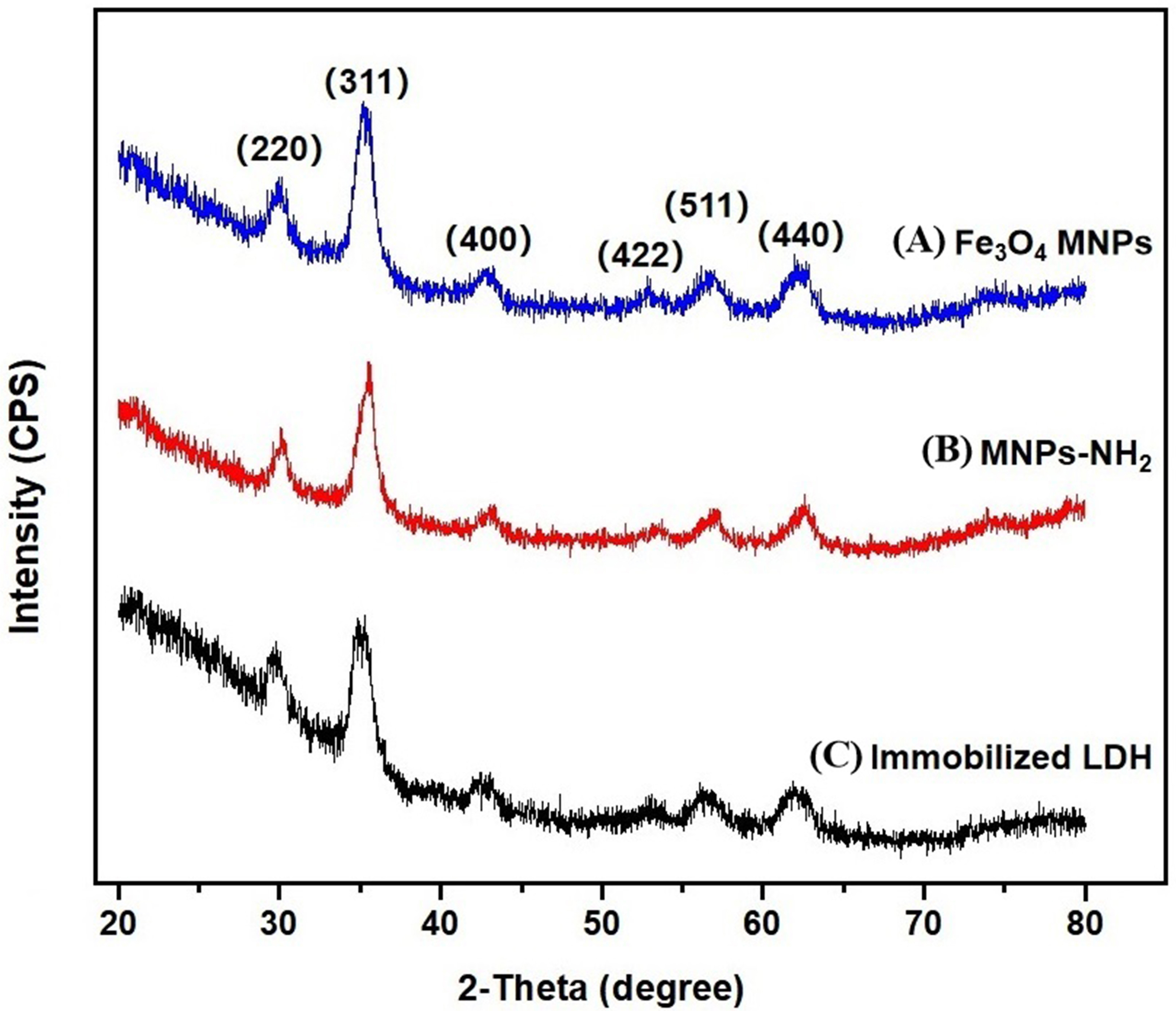
XRD is the most powerful approach applied to identify the structure of crystals
and has been successfully adopted for the development of new materials [32]. The
Fe
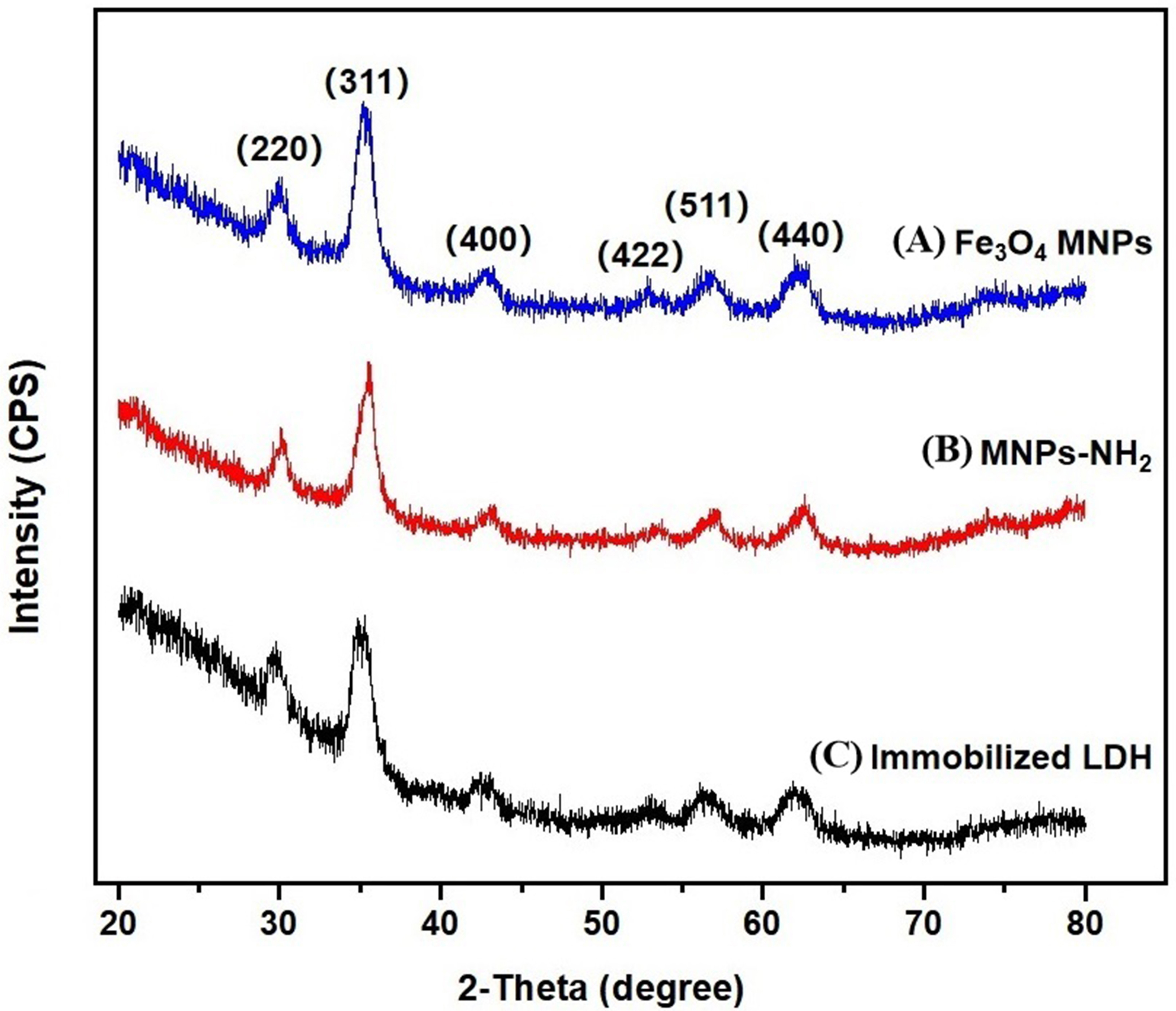 Fig. 3.
Fig. 3.XRD patternof the three materials. (A) Fe
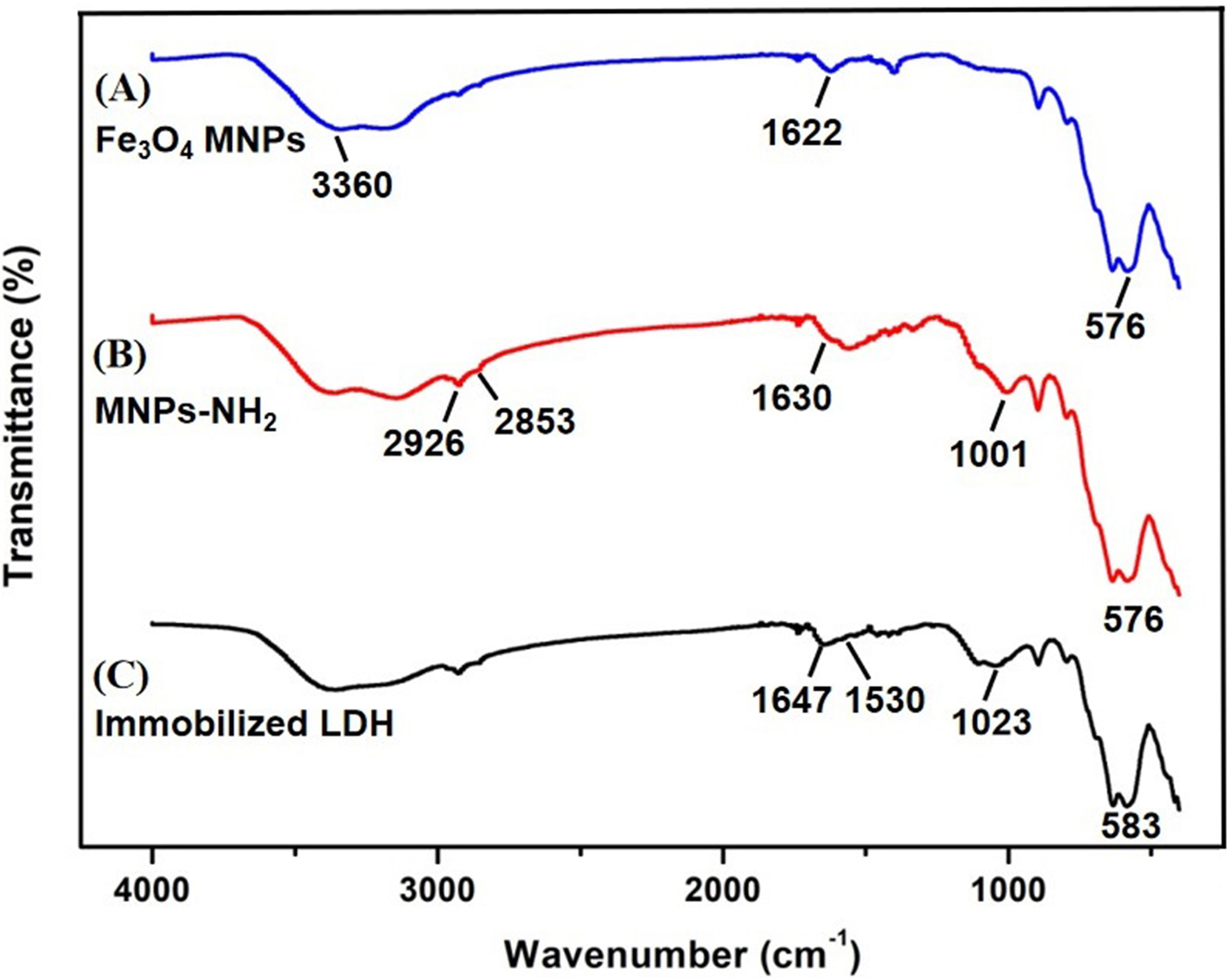
FT-IR spectroscopy has the advantage of providing high resolution and decrease
measurement time, so it is normally used for group structure analysis of
materials and qualitative and quantitative analysis of materials [34]. This could
be observed in the FT-IR images of Fe
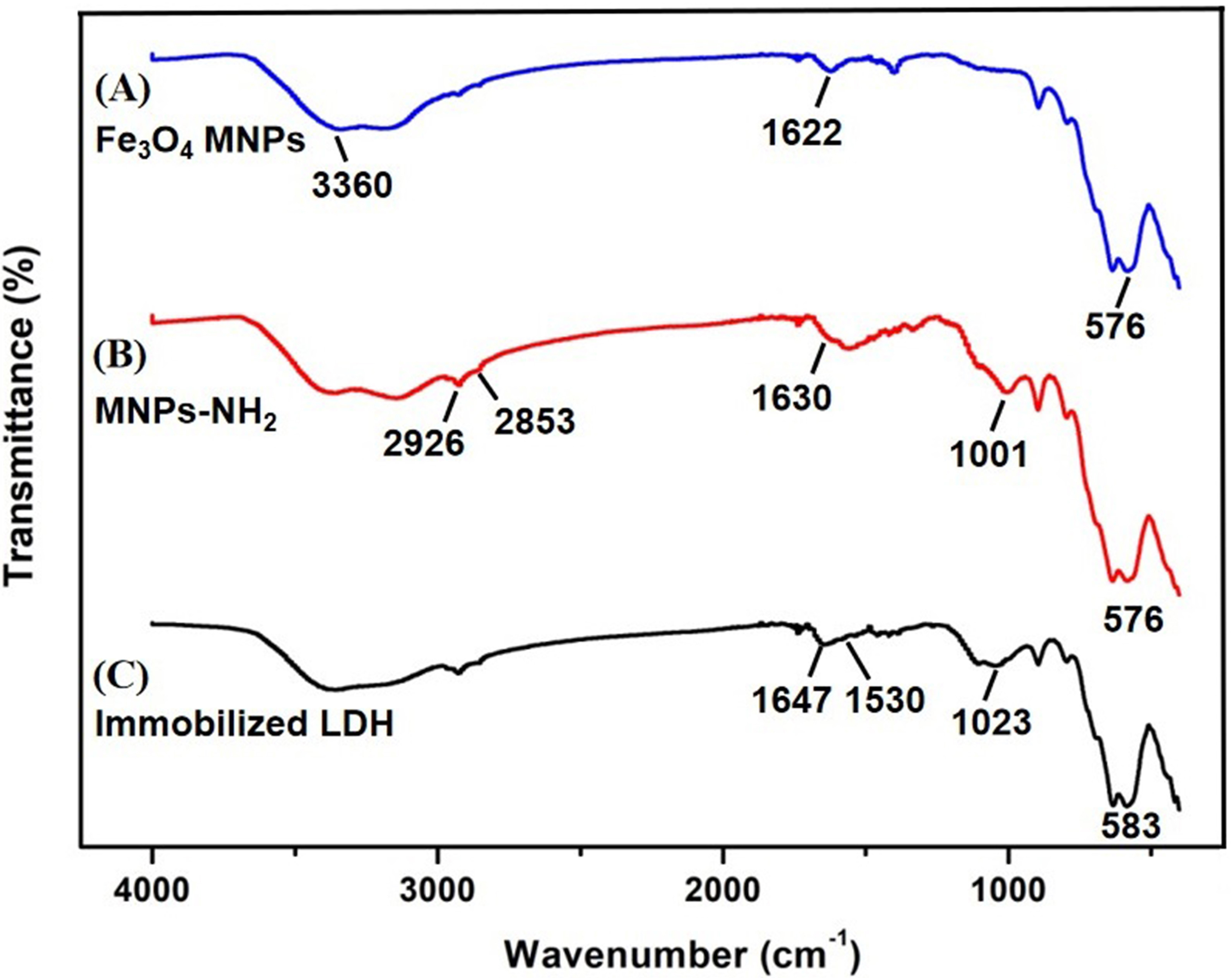 Fig. 4.
Fig. 4.FT-IR spectra of the three materials. (A) Fe
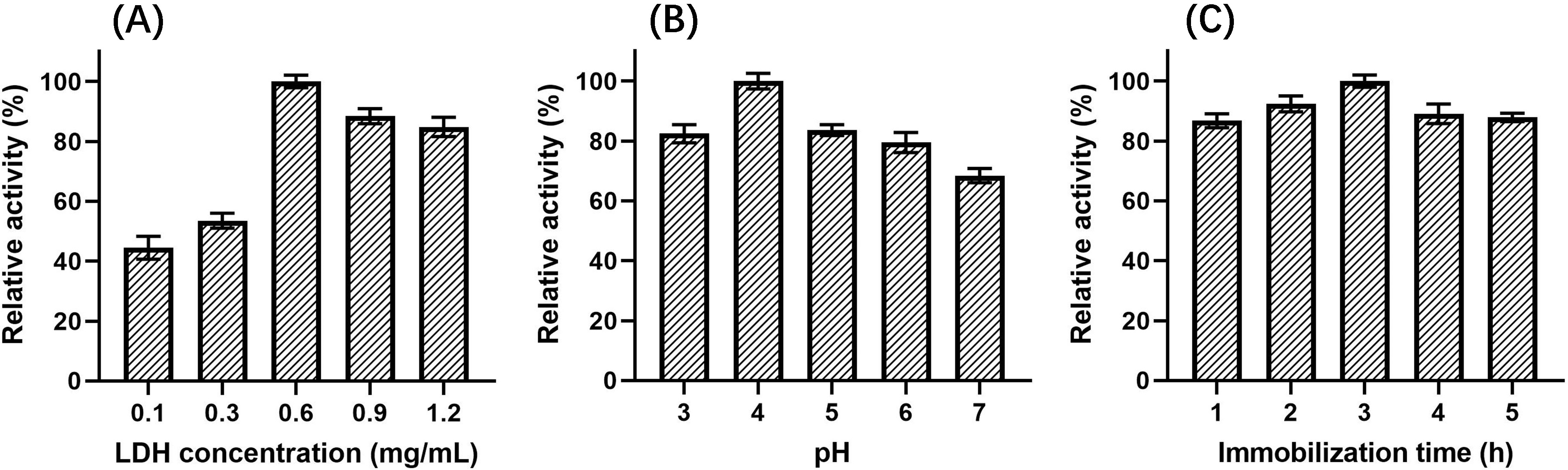
LDH concentration, pH, and immobilization time in the LDH immobilization process were optimized using single-factor experiments and response surface methodology.
The relationship between LDH concentrations, ranging from 0.1 mg/mL to 1.2 mg/mL
(pH 5, immobilization time 2 h), and the relative activity of immobilized LDH was
investigated, as shown in Fig. 5A. The relative activity increased dramatically
and reached a maximum as the enzyme concentration increased from 0.1–0.6 mg/mL.
When the concentration continued to rise to 1.2 mg/mL, the relative activity
decreased slowly. It showed that the activity of immobilized LDH increased with
the increase in the number of LDH immobilized on the surface of Fe
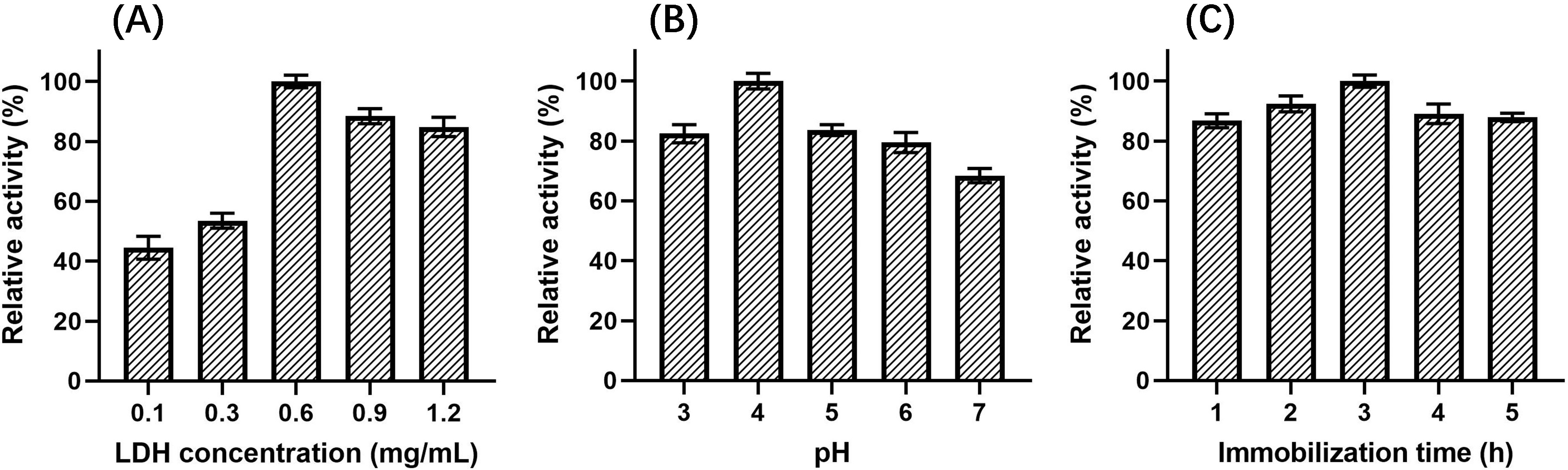 Fig. 5.
Fig. 5.Effect on relative activity of immobilized LDH. (A) LDH concentration. (B) pH. (C) Immobilization time.
The effect of immobilization pH, ranging from 3 to 7 (LDH concentration of 0.6 mg/mL, immobilization time 2 h), on the relative activity of immobilized LDH was also investigated. As shown in Fig. 5B, when the pH changed from 3 to 4, the relative activity of immobilized LDH increased and reached a maximum. However, when the pH increased to 7, the activity of immobilized LDH gradually decreased, which maight be caused by the increase of the electrostatic repulsion between LDH and the magnetic nanomaterials.
Different immobilization times were also investigated, ranging from 1 h to 5 h (LDH concentration of 0.6 mg/mL, pH 5), as shown in Fig. 5C. The relative activity increased gradually with time from 1–3 h. When the immobilization time was 3 h, the relative activity reached the maximum, but when the immobilization time was extended from 3 to 5 h, the relative activity decreased slightly. Thus, this illustrated that extension of time beyond 3 h was not conducive to the immobilization of LDH.
Compared with the enzyme immobilization conditions in other studies, our
conditions were optimized, especially the immobilization time was greatly
shortened to 3 h. For example, when immobilized beta-secretase was used to screen
for potential inhibitors in Dendrobii Caulis, the enzyme required 24 h
to immobilize on magnetic beads [35]. Immobilization of neuraminidase on the
surface of magnetic beads required an overnight incubation in 500
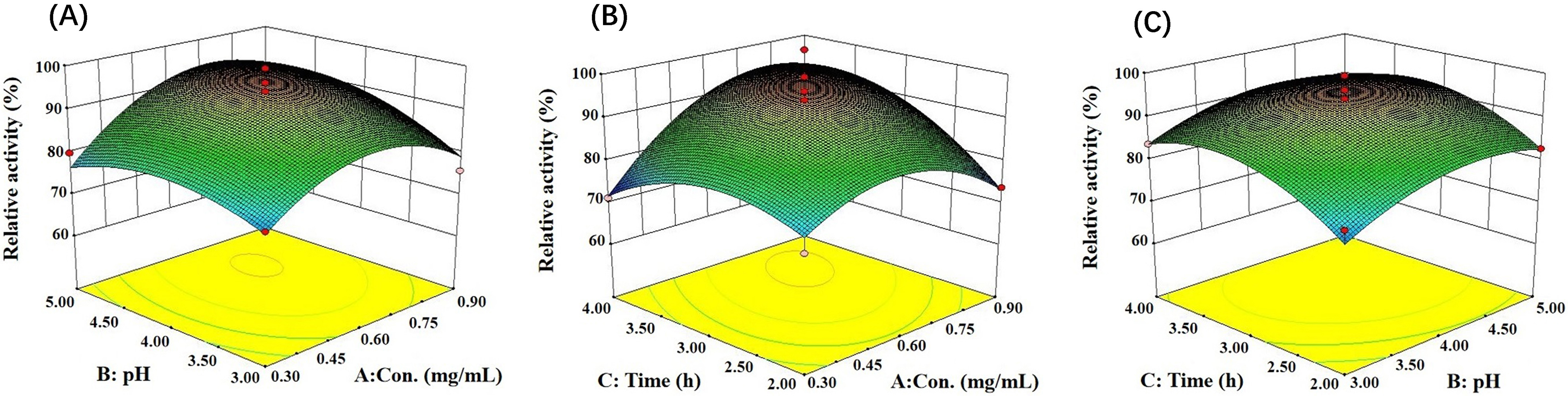
On the basis of the results of the single factor experiments, a response surface
test was performed, and Design Expert software (version 8.0, Stat-Ease Inc.,
Minneapolis, MN, USA) was used to analyze the results. The relative activity of
LDH reached the maximum when the pH was 4.43, the LDH concentration was 0.75
mg/mL, and the immobilization time was 3.49 h (Fig. 6). Simultaneously, the
analysis of variance showed that F = 7.27, p
(Where Y represents the relative activity, A, B, C are the LDH concentration, pH and time, respectively.)
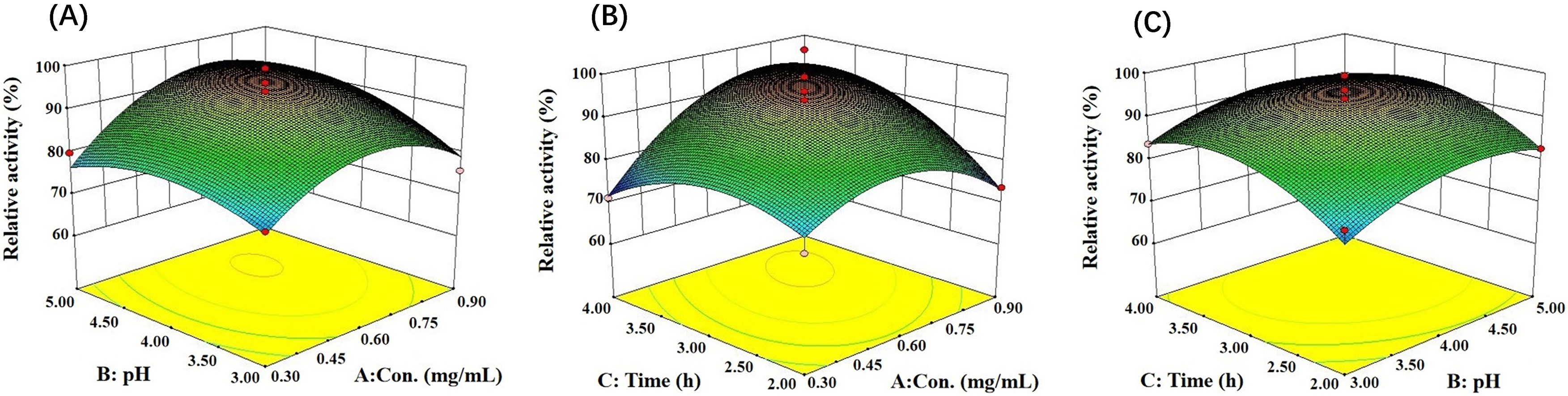 Fig. 6.
Fig. 6.Response surfaces and contour plots show the interaction of reaction conditions on immobilized LDH activity. (A) Concentration and pH. (B) Concentration and time. (C) Time and pH.
First, the Coomassie brilliant blue method was used to determine the standard
curve of LDH content, which was Y = 0.0438X + 0.0129 (R
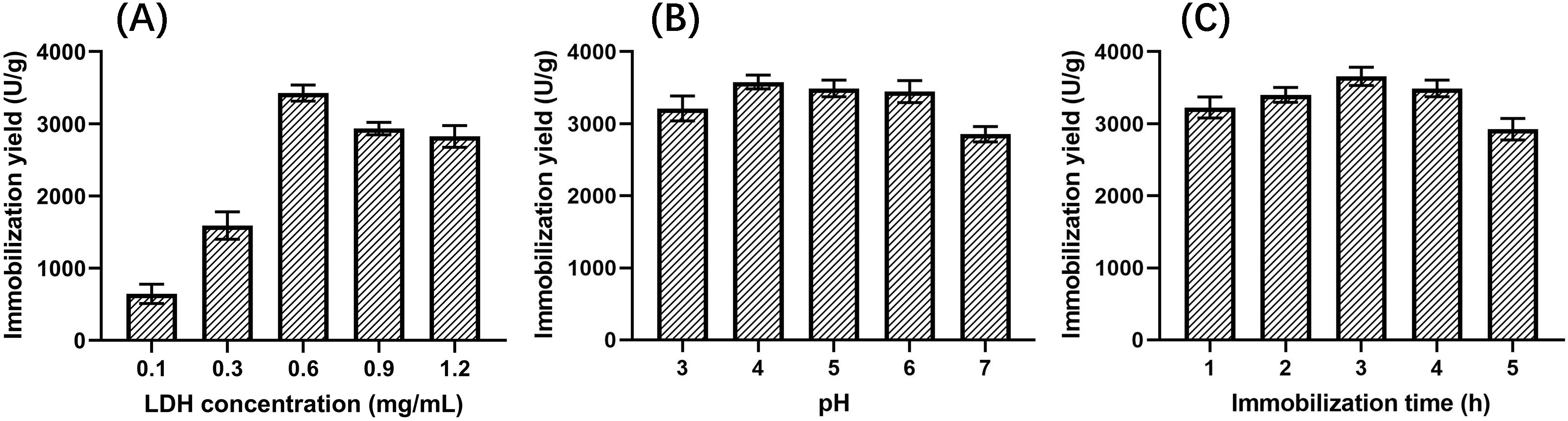
Three factors, including LDH concentration, pH, and immobilization time, were
investigated to explore the relationship with the LDH immobilization yield. As
could be seen in Fig. 7, the yield increased first and then decreased with the
increase in LDH concentration, and when the concentration was 0.6 mg/mL, the
yield reached a maximum value of (3.43
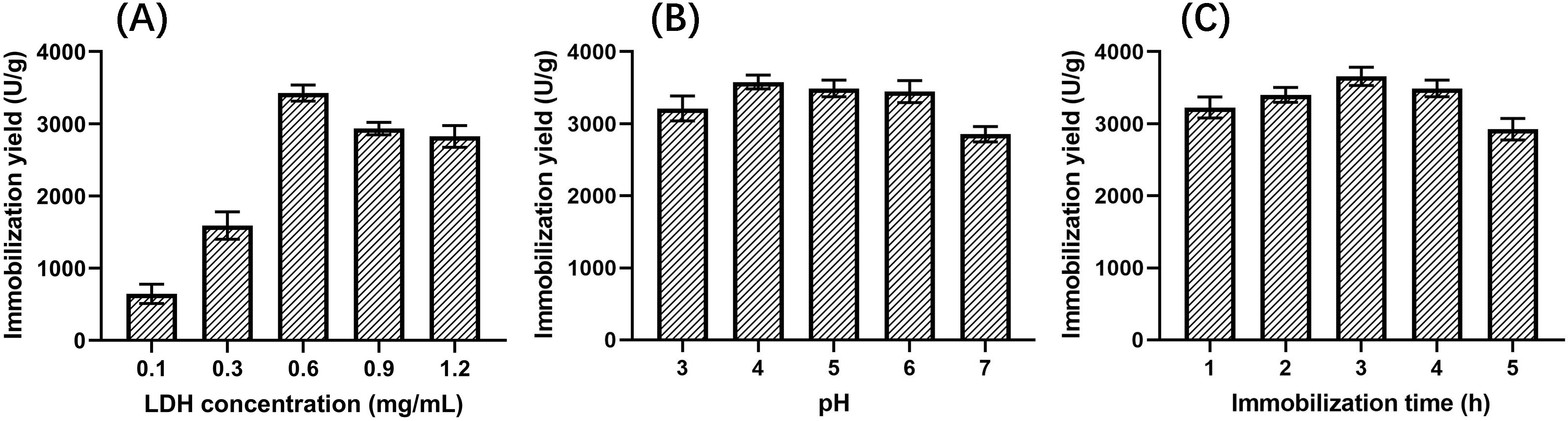 Fig. 7.
Fig. 7.Effects on the yield of immobilized LDH. (A) LDH concentration. (B) pH. (C) Immobilization time.
The optimal conditions for activity of immobilized LDH were determined in
single-factor experiments and the response surface test described in Section 3.2.
These conditions that were used for screening were an LDH concentration of 0.7
mg/mL, a pH value of 4.5, and an immobilization time of 3.5 h. Under these
conditions, the immobilized yield of LDH was (3.79
The peroxygenase was immobilized on monoaminoethyl-N-aminoethyl activated
agarose beads, and the immobilized yield of the enzyme was 7.5
Since chlorogenic acid could not bind to LDH, it was selected as a negative control, while galloflavin was selected as a positive control [40]. Therefore, a model mixture (containing galloflavin and chlorogenic acid) was used to test the specificity of immobilized LDH. This mixture model was then also used to optimize the experimental conditions for ligand fishing of immobilized LDH.
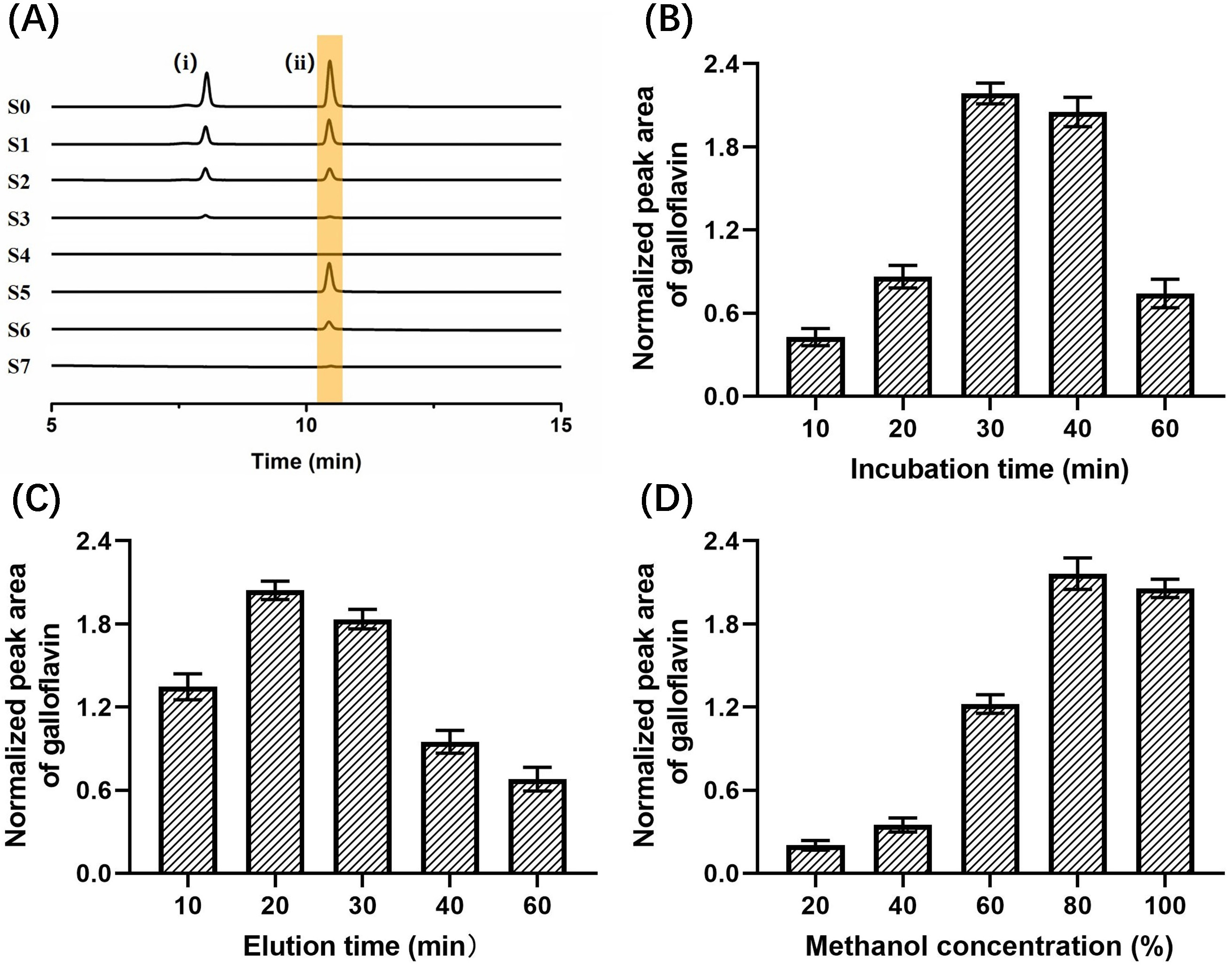
The specificity experimental results of immobilized LDH are shown in Fig. 8A. S2–S4 were the washing solutions of the buffer solution, and no substance was detected in S4, indicating that the components not bound to the immobilized LDH could be completely washed away after washing three times. S5–S7 were methanol eluates, in which only the positive control drug galloflavin was detected, while the negative control drug chlorogenic acid was not detected, proving that the immobilized LDH ligand was specific for ligand fishing.
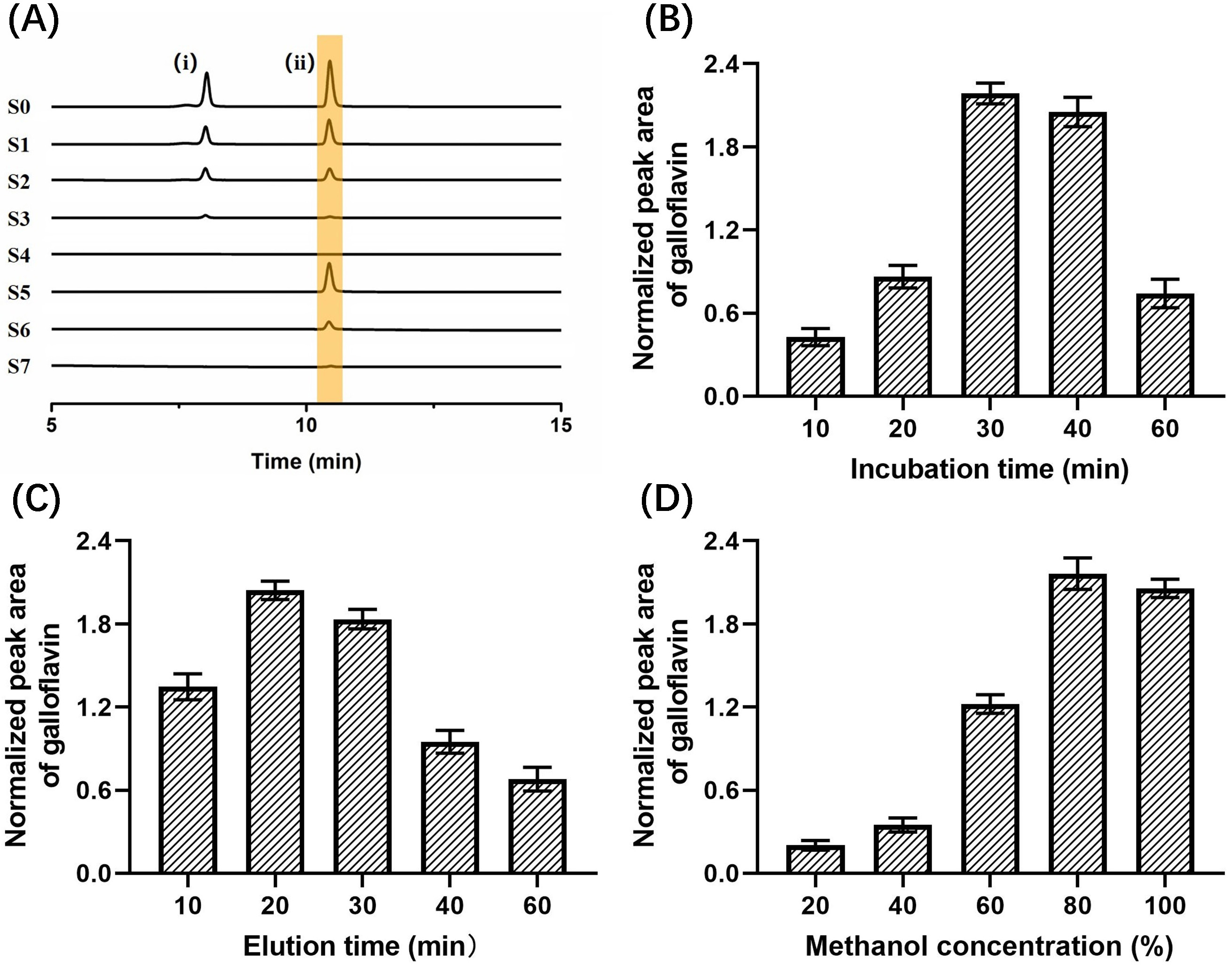 Fig. 8.
Fig. 8.Effects on the binding efficiency of galloflavin. (A) Base peak chromatograms acquired from different solutions (S0–S7) during the developed LDH-MNPs-based ligand fishing procedure from a model mixture containing chlorogenic acid (i), galloflavin (ii). (B) Incubation times. (C) Elution time. (D) Methanol concentration.
Then, the optimal incubation time between 10–60 min was selected as shown in Fig. 8B. When the incubation time was extended from 10 min to 30 min, the peak area of galloflavin increased significantly and reached the maximum. The peak area decreased slightly at the 40 min incubation time. However, as the incubation time was further increased to 60 min, the peak area decreased substantially. This result indicated that the active site of LDH reached saturation after 30 min of incubation.
As shown in Fig. 8C, the optimal resolution time between 10–60 min was 20 min. The peak area of galloflavin showed a trend of increasing first and then decreasing. When the elution time exceeded 20 min, the peak area gradually decreased. This phenomenon that was observed over time could have occurred because galloflavin was encapsulated by agglomerated and precipitated enzyme molecules for too long, resulting in a decrease in the peak area.
The optimal methanol concentration was obtained by investigating different methanol concentrations (20%, 40%, 60%, 80%, and 100%). When the methanol concentration increased from 20%–80%, the peak area gradually increased and reached the maximum value. However, the peak area decreased at 100% (Fig. 8D). It proved that 80% methanol was favorable for the elution of galloflavin.
In addition, the specificity of immobilized enzymes has also been investigated
in other published studies. For example, cyclooxygenase-2 (COX-2) immobilized on
nickel ion (Ni
The utility of immobilized LDH was further confirmed by screening potential inhibitors of LDH in Selaginella doederleinii ethyl acetate extracts using the ligand fishing method. The experimental results are shown in Fig. 9. Under optimal conditions, two compounds were screened in the final elution step. Subsequently, these two compounds were confirmed as amentoflavone and robustaflavone using the UPLC/quadrupole time-of-flight (Q-TOF)-MS identification method and comparison with MS data and related standards (As shown in Table 2).
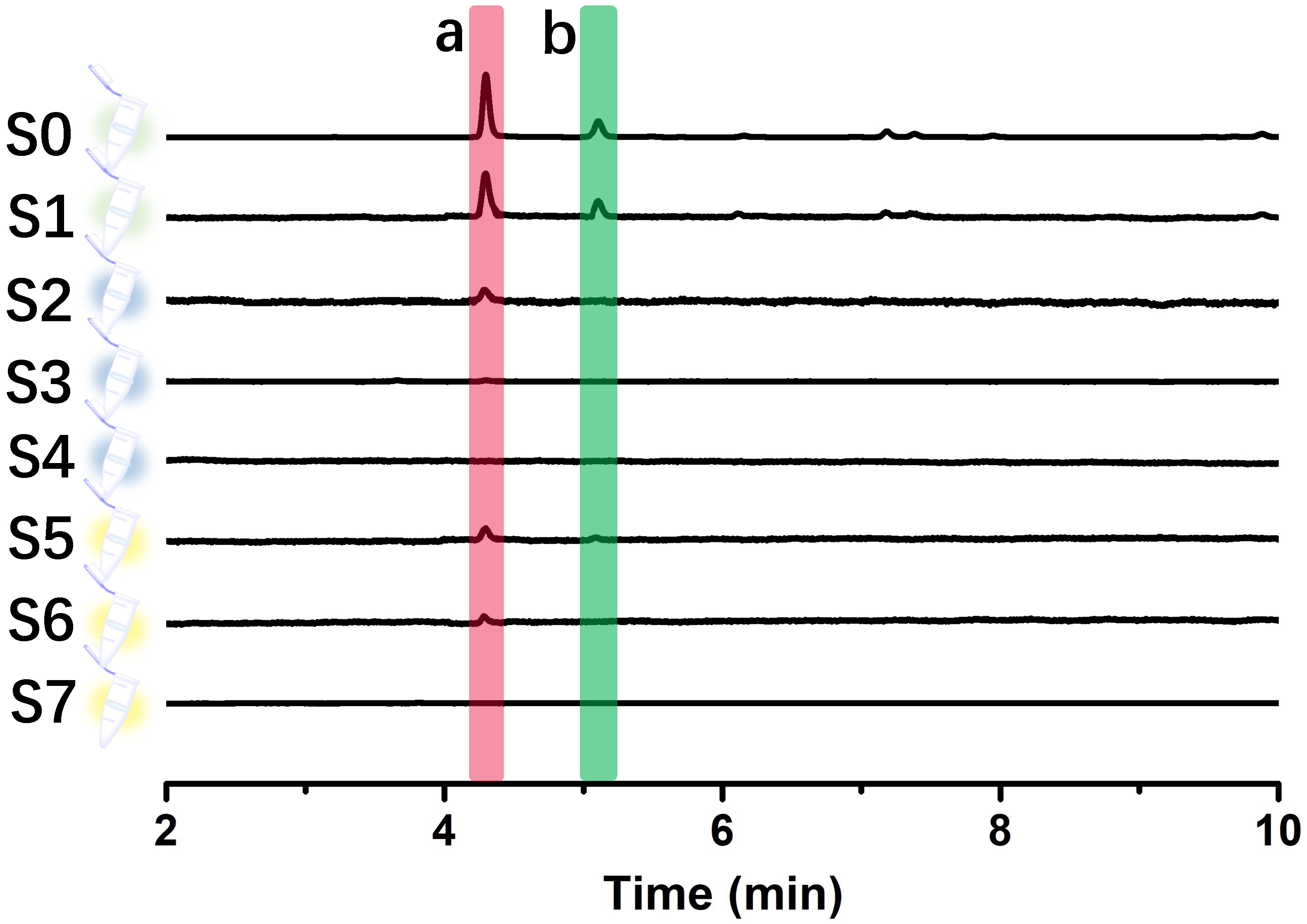 Fig. 9.
Fig. 9.UPLC chromatograms of different solutions (S0–S7) were obtained from the ethyl acetate extract of Selaginella doederleinii by an immobilized LDH-based ligand fishing approach.
| No. | t |
Analyte | Chemical structure | Neutral | Determined | Product ions (m/z) | Delta (ppm) | Formula |
| Mass (Da) | [M-H] | |||||||
| 1 | 4.27 | Amentoflavone | 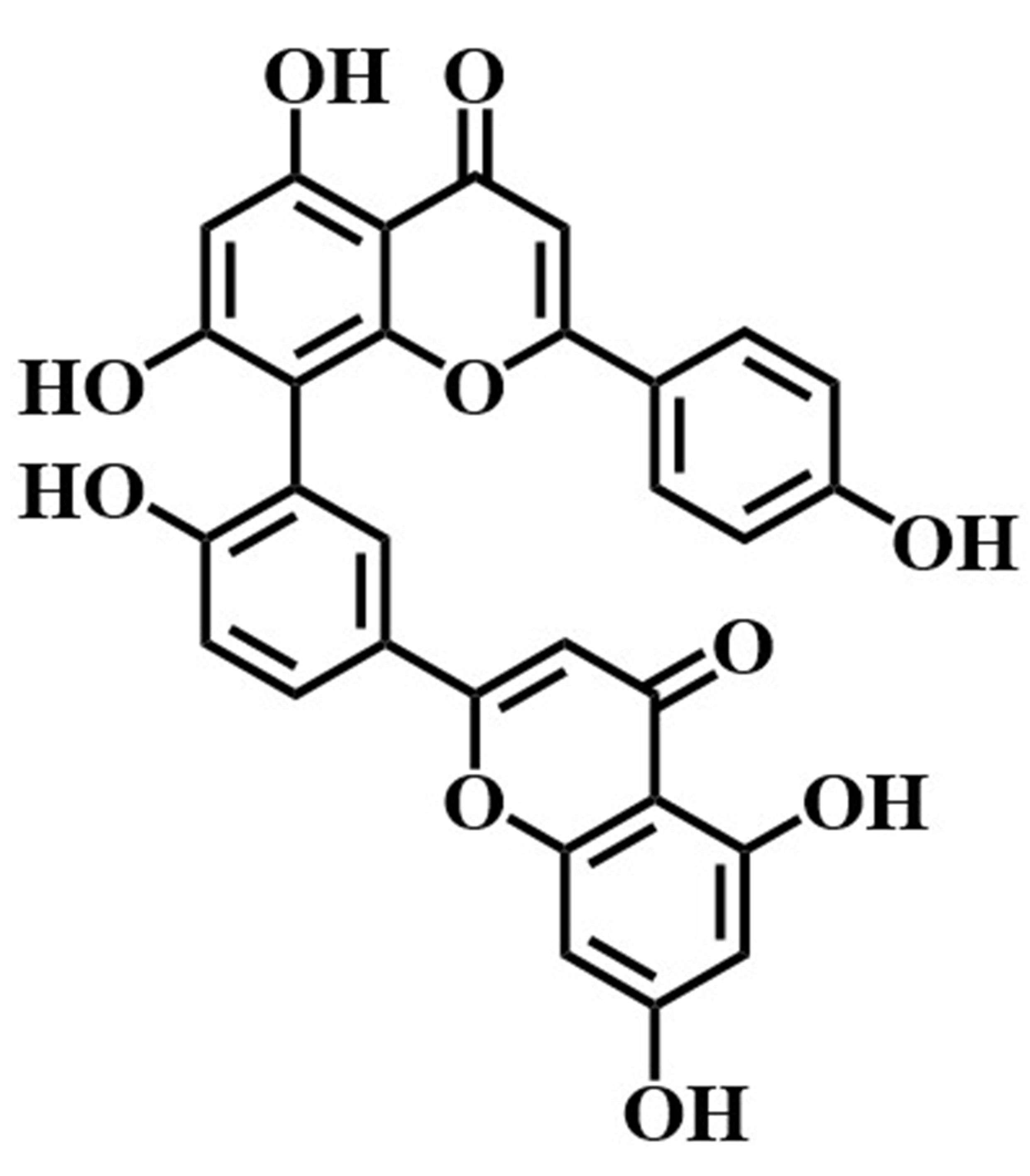 |
538.0658 | 537.0639 | 419.1025,401.0351, | –3.7 | C |
| 375.0682,153.1011, | ||||||||
| 121.1264 | ||||||||
| 2 | 5.09 | Robustaflavone | 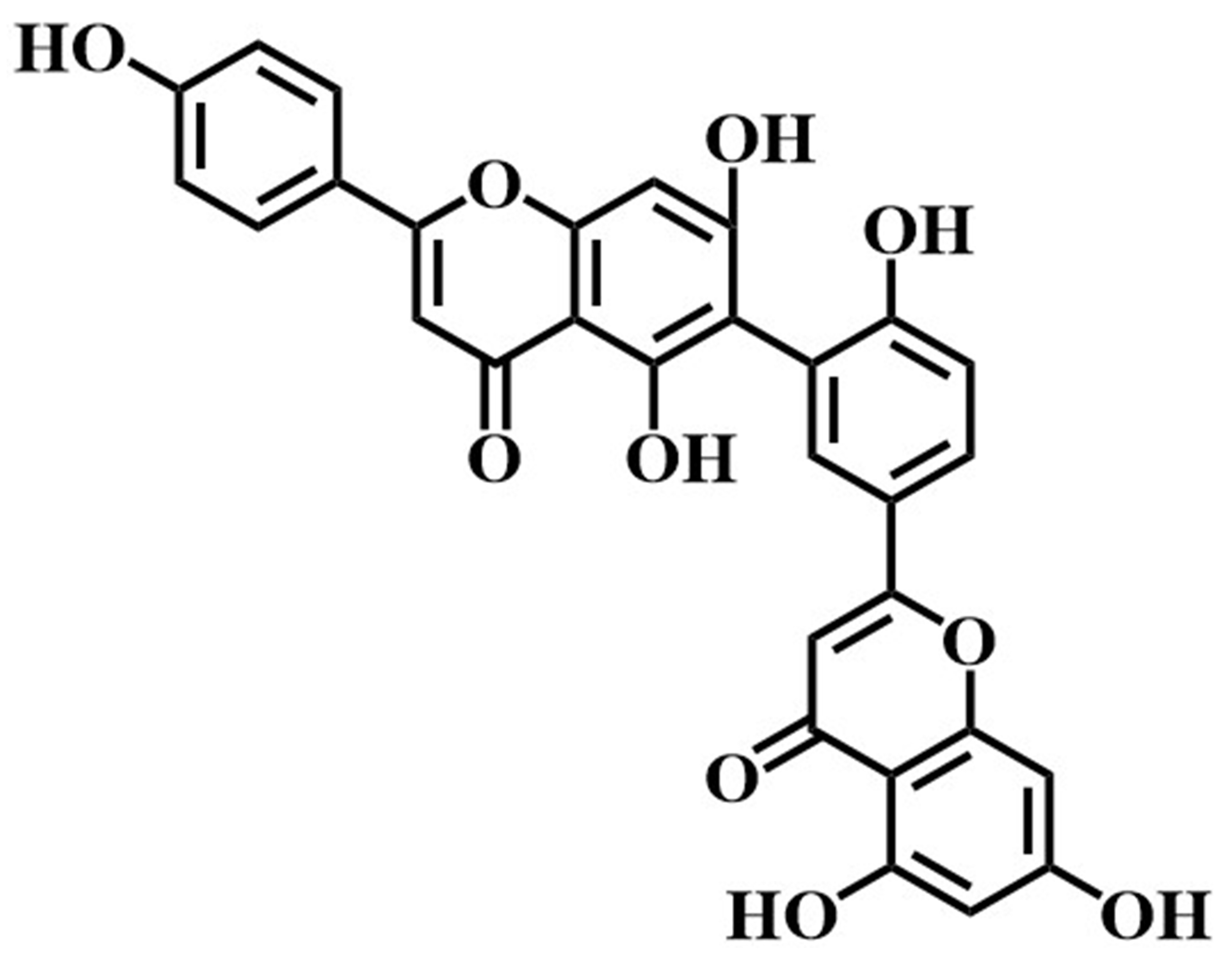 |
538.0846 | 537.0837 | 411.0897,385.0461, | –2.0 | C |
| 268.1167 |
Amentoflavone could effectively inhibit the content of LDH, exert antioxidant
effects and reduce hepatotoxicity. According to Li ’s report [44], 5 biflavonoids
were isolated from Hedyotis diffusa. Among them, amentoflavone could
significantly reduce the LDH content by the experiment of hydrogen
peroxide-induced hepatocyte injury. Especially, the LDH content decreased from
580 U/L to 286 U/L when amentoflavone was 250
Up to now, many literatures have reported that amentoflavone not only has
anti-tumor effects, but also has low toxicity to human normal cells. For example,
amentoflavone had a certain inhibitory effect on the proliferation of human
malignant melanoma A-375 cells with an IC
Compared with traditional screening methods, including chemical composition isolation and activity tracking [52], serum pharmacochemistry and network pharmacology [53], spectrum-effect relationship analysis and molecular docking [54], ligand fishing technology [55] could directly and accurately fishing out the active ingredients from complex natural products, simplifying the experimental steps and consuming less sample volume, further highlighting the specificity and efficiency of the technology.
Natural medicines have become the main source of modern new drug research and development because of their rich resources, diverse components, and wide range of biological activities. However, the traditional screening process is time-consuming and inefficient, which hinders the development of traditional Chinese medicines. Now, the ligand fishing technology with the advantages of fast screening speed, simple operation, and good specificity makes it suitable for screening potential active ingredients from multi-component systems, which has attracted more and more researchers’ attention.
Therefore, we have shown that by immobilizing LDH on the surface of amino-modified magnetic nanomaterials, potential LDH inhibitors from traditional Chinese medicines could be targeted and screened. First, we optimized the immobilization conditions for LDH using single-factor experiments and response surface methodology to obtain the maximum enzymatic activity and calculated the immobilization yield. Next, we used known LDH ligands and non-ligands to detect the specificity of immobilized LDH to further optimize the ligand fishing conditions. Finally, the immobilized LDH was applied for the first time to the screening of LDH ligands in ethyl acetate extracts of Selaginella doederleinii and combined with UPLC/Q-TOF-MS; we successfully identified two potential LDH inhibitors. These results show that ligand fishing is an effective method to screen natural products.
GW, SL, FZ and HL designed the research study. FZ and HL performed the research. CL, KF and YJ provided help and advice on research protocols, data interpretation and discussion. MW, SX, LZ and JY analyzed the data. FZ wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
This research was funded by the 2018 National Science Foundation of China 81860697, Zunyi Talent Team Project (2020) No.10(E-361), Guizhou Province Science and Technology Plan Project [2020]4Y086.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.