1 Chemisches und Veterinäruntersuchungsamt Karlsruhe, 76187 Karlsruhe, Germany
2 Abteilung Lebensmittelchemie, Technische Universität Kaiserslautern, 67663 Kaiserslautern, Germany
Academic Editor: Marcello Iriti
Abstract
Background: In the past 60 years, Cannabis sativa L. has been
an object of increasing interest because of the psychotropic effects of some of
its constituents. These effects mainly arise from the cannabinoid
Keywords
- cannabis
- cannabidiol
- $\Delta$9-tetrahydrocannabinol
- CBD
- $\Delta$9-THC
- e-cigarette
- e-liquid
- inhalation
- risk assessment
- benchmark dose
- toxicology
During recent years, cannabinoids such as cannabidiol (CBD) and
While
Different modes of action (MOAs) for CBD have been elucidated. CBD interacts
with multiple cellular pathways. For example, through interaction with cytochrome
P450 metabolic pathways, CBD can increase effects of
CBD is further known to increase beneficial effects of THC observed in studies
co-administering CBD and
Due to strict legislative regulations in most countries and its analgesic
effects,
In the last decade, the use of electronic cigarettes (e-cigarettes) has experienced a significant increase in many countries as an attractive alternative to smoking. E-cigarettes or vaporizers are deemed a reduced risk alternative to conventional cigarettes and have found a wide application as a substitute delivery system. The devices operate by heating so-called e-liquids until vaporization. The aerosol is inhaled by the consumer. E-liquids contain dissolved aroma compounds in an organic matrix such as glycerol and propylene glycol [16]. Recent cases of e-cigarette associated lung injury (EVALI) raised concerns about the safety of e-cigarettes. In 2020, 2807 cases of EVALI have been reported, resulting in the deaths of 68 consumers in the United States [17]. In other countries e-cigarette associated cases of hospitalization and even deaths have been reported [18]. Some mechanisms leading to EVALI have been investigated in animal models, such as oxidative stress and inflammatory reactions in lung epithelia resulting in a damaging of alveolar tissues and cells [19], which is assumed to be the result of the accumulation of vitamin E acetate in alveolar tissue.
The beneficial effect of CBD concerning bone health is subject to current research. CBD preparations were found to reduce bone loss and promote bone healing in rats [20, 21]. However, it needs to be elucidated, whether exposure to CBD via inhalation of CBD liquids has the same effect on humans. This is relevant concerning the discussed osteotoxic effect of nicotine and flavoring agents in e-liquids on bone integrity [22].
E-liquid preparations containing the non-psychotropic CBD are commercially
available. In the European Union (EU), e-liquids containing CBD are not necessarily
legal for sale because CBD may be a compound not in compliance with Art. 7 No. 6a
of directive 2014/40/EU, which prohibits the placing on the market of tobacco
products, which contain additives that create the impression that a tobacco
product has a health benefit [23]. Due to narcotic laws in most countries,
e-liquids are also not allowed to exceed certain thresholds of
This study aimed to conduct a benchmark dose modeling for CBD and
The first step in this study was to obtain suitable data from the scientific literature. Only in vivo data from studies in different animal models and human data were considered appropriate. The accepted animal models were mice, rats, and rhesus monkeys. The data acquisition was carried out using the Google Scholar search engine, the PubMed database of the National Library of Medicine, and the Cochrane Library, as well as additional online resources such as databases provided by publishers, including Nature and Science.
The major search terms and keywords used are listed below. To complement the data acquisition, combinations and variations of search terms and keywords were used as well. Slashes separating keywords in the list below indicate, that the separated keywords were used individually or in combination with each other.
Search terms and keywords:
• toxicity of CBD/
• acute/chronic toxicity of CBD/
• acute/chronic toxicity of CBD/
• CBD/
• vaporized CBD/
• oral CBD/
• intravenous (i.v.) CBD/
• bioavailability of oral and inhaled CBD/
• randomized controlled trials for CBD/
• combinations of the search terms and keywords mentioned above with author names
A further research strategy, which has been found useful, was to directly search for publications found in references of studies and reviews.
To be included, the literature data had to meet several criteria. A study
considered for inclusion in this research had to have administered at least 3
different doses and a control group receiving vehicle. Dose spacing was not
considered relevant. Furthermore, applied doses had to be administered in mg/kg
of body weight. Studies reported to have administered only fixed doses of CBD or
The reported results were expected to be presented as a mean effect with a standard deviation. Alternatively, the standard error of a dose group could be converted into a standard deviation by multiplying the value of the error with the square root of the number of animals in the respective dose group.
The test substances that were used had to be as pure as possible. The results of
formulations with combinations of CBD with
Data, which were only available plotted in diagrams, were included if data points for mean effect values and standard deviations were distinguishable from other plotted values. For numerical retrieval of plotted data, a dedicated plugin for ImageJ image processing software Version 1.8.0 (National Institutes of Health, Bethesda, MD, USA) was applied [26].
In toxicological risk assessment, the benchmark dose (BMD) approach is a more advanced statistical method than the established No Observed Adverse Effect Level (NOAEL) approach [27]. The BMD approach was first introduced by Crump [28] as an alternative method to NOAEL.
The benchmark dose (BMD) is defined by the United States Environmental Protection Agency (EPA) as the exposure level that corresponds to a percentage increase (usually 5 or 10%) in the probability of an adverse event (response) compared to a control scenario with no exposure [27]. This change in response to an exposure is called a benchmark response (BMR). Therefore, a BMR of 5% would be defined as a 5% increase in the number of subjects with an incidence of an adverse event. Benchmark models provide not only a BMD but also a confidence interval, which contains the value of the respective BMD. The limits of the confidence intervals obtained are called the benchmark dose lower limit (BMDL) and the benchmark dose upper limit (BMDU). In toxicological risk assessment the BMDL is used for estimating a reference value for consumer safety.
The BMD and its respective confidence interval, with the BMDL being the Point of Departure (POD), are calculated by fitting multiple statistical models. The benchmark dose software (BMDS), which has been developed by the EPA [29], performs automated fitting of selected models to dose-response data retrieved from toxicological studies.
The output delivered by the software contains the results of the models, namely, the BMD, the BMDL and the BMDU, with a recommendation for the most suitable model. The most suitable model is determined by comparing the Akaike information criteria generated in the output.
Before a BMD analysis could be carried out by using the BMDS, settings needed to be adjusted in the main workspace window. The data type obtained from publications included in this assessment was found to be continuous. For the benchmark response, the default setting for continuous data of one standard deviation (1SD) was selected.
To obtain RfD values for inhalation in humans, a concept has been designed for converting animal BMDLs into RfD values. This was an important step because most BMDLs considered reliable were obtained from analyzing dose-response data from animal studies in other routes besides inhalation (i.e., oral or i.v.). The conversion is carried out in several steps.
Initially, route-specific RfDs in humans have been calculated from animal BMDL for the same route by using an uncertainty factor (UF) of 10 to account for interspecies variability and an additional UF of 10 to account for intraspecies variability.
This equation yields an RfD for exposure in humans by the same administration
route as used in the animal model. The next step calculates a theoretical RfD for
the same dose-dependent response after an intravenous (i.v.) administration in
humans (RfD
This step is necessary because it allows one to calculate the final estimated
RfD for inhalation in humans (RfD
This approach allows for the estimation of an RfD for inhalation in humans from
BMDLs derived from animal models for different administration routes and can also
be used to convert applied doses, documented in the literature, to doses for
inhalation, which would lead to the same observed adverse effect. This
facilitated a comparison of the estimated RfDs with the RfDs in the literature.
To perform this approach for extrapolation, the values of the respective
bioavailability for each route need to be known. The respective bioavailability
values for human exposure to CBD and
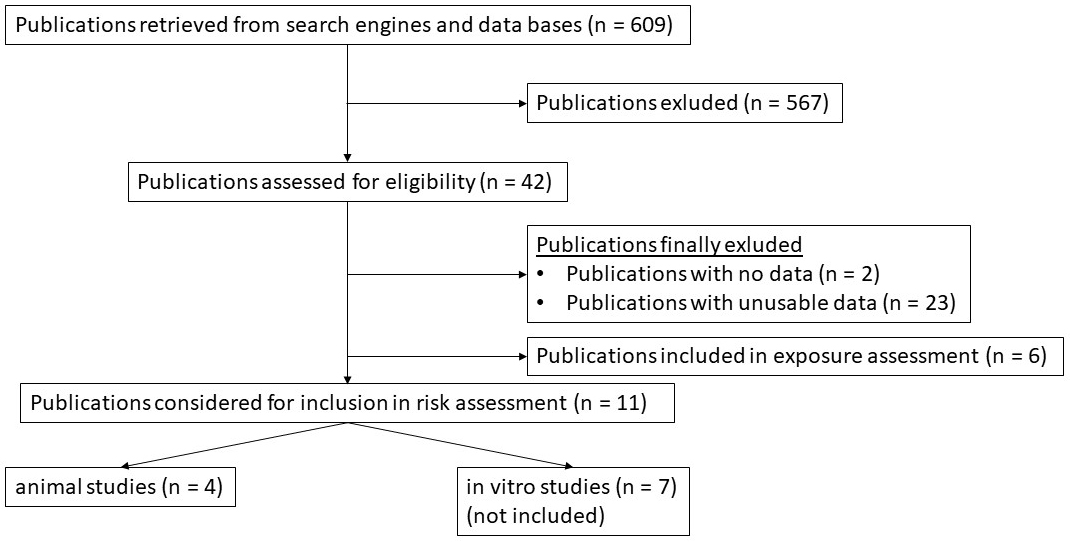
Before conducting this risk assessment, a literature review was performed to obtain usable dose-response data (Fig. 1). Data that met the inclusion criteria were included for a BMD analysis using the benchmark dose software (BMDS) provided by the EPA [29]. A total of 609 publications were screened for usable dose-response data, of which 567 publications were excluded. Of the remaining 42 studies, 2 studies did not contain dose-response data and 23 studies had no usable dose-response data. Six studies were included for exposure assessment and have been used to develop the dose conversion model, which has been introduced before. Among 11 studies that were considered to be used for a risk assessment using BMD modeling, 4 animal studies contained sufficient dose-response data for a BMD analysis.
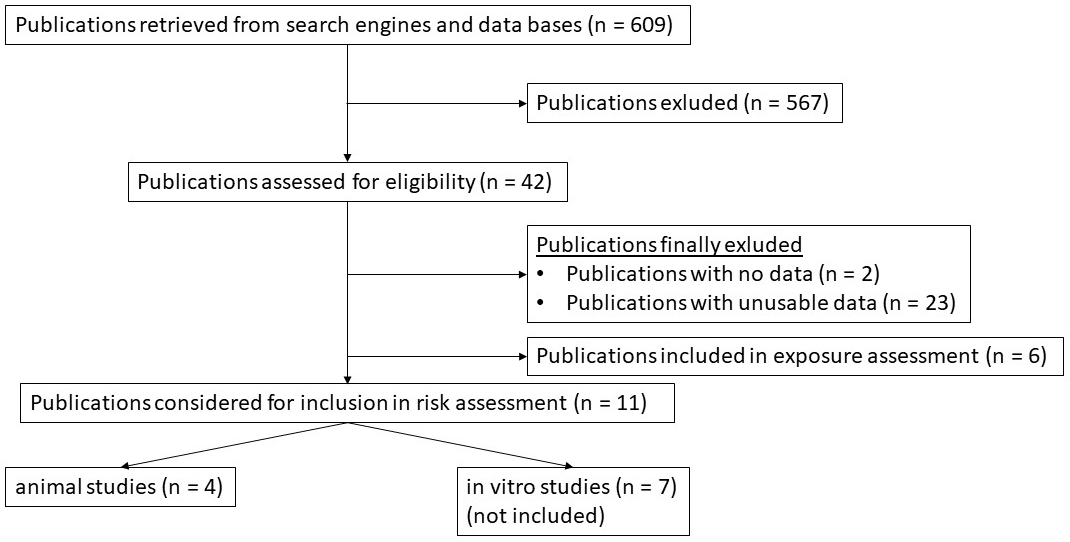 Fig. 1.
Fig. 1.Flow-chart diagram of all studies reviewed for this risk assessment. Publications were finally included after published dose-response data were considered suitable for a risk assessment using the BMD approach.
This article presents the lowest BMD and BMDL values obtained by conducting BMD
analyses with dose-response data from animal studies that administered CBD or
| Author | Model | Study type | Compound | Route of administration | Doses [mg/kg bw] | Endpoint | BMD | BMDL | p-value |
| Marx et al. [34] * |
rats | 90-day study; daily administration | CBD | Oral | 0 (Control), 100, 360, 720 | Uterus weight | 79 | 26 | 0.7 |
| Steger et al. [33] | rats | acute | CBD | Single oral dose | 0 (Control), 0.1, 1, 10 | Norepinephrine turnover | 6.8 | 4.3 | 0.06 |
| Siqueira et al. [35] * |
rats | acute | Intravenous | 0 (Control), 1, 2, 5, 10 | Change in mean arterial blood pressure | 3.05 | 0.22 | 0.4 | |
During research for retrieving published data for deriving an RfD, different
toxicological endpoints regarding physiological and psychological parameters were
considered. However, not all parameters are considered equally relevant. To
achieve an appropriate evaluation of the safety of CBD and
This study might present one of the first approaches for a risk assessment for
CBD and
This finding indicates different susceptibilities of tissues and organs to
inhaled CBD and
Uterus weight in animal models was altered by a relatively low dose of CBD (RfD 4 mg/kg bw). This endpoint may be relevant, as hormones and therefore hormone producing glands, tissues and organs have an essential function in the development of an organism, especially during prenatal development and adolescence.
From Steger et al. [33], an RfD for
Another effect observed after
Data used for estimating an RfD for inhalation of 0.04 mg/kg bw
In 2015, the EFSA postulated an oral ARfD for
A published review of risk assessments [46] derived a LO(A)EL of 2.8 mg/d for
cannabis inhalation from a study by Ramaekers et al. [47]. After
conducting a dose conversion, the theoretical NOAEL would yield an RfD for the
inhalation of cannabis of a total of 0.028 mg/d. In comparison, the RfD for
inhalation of pure
It is shown above that the RfD for
In conclusion, the RfD of 0.006 mg/kg bw, which has been derived from dose-response data published by Siqueira et al. [35], was found to be less conservative than the (A)RfD derived from EFSA, which might be due to the respective BMDL (if converted to an oral BMDL) resulting from a BMD analysis with a 5% confidence interval.
It is recommended to further investigate whether a BMDL
The BMDL and RfD values estimated for hematological endpoints and endpoints regarding clinical chemistry observations were found to be on average much higher than the acute and chronic BMDL and RfD values found for more susceptible endpoints. This observation indicates that the respective organs and systems were less susceptible to CBD. Although endocrinologically active organs were found to be more susceptible to CBD, considerable deviations in estimated BMDL and RfD values for endocrinologically active organs were observed during this study. Endpoints that indicate high tolerability of CBD were assumed to be unreliable and were excluded from the further evaluation because adverse effects have already been observed at significantly lower doses of CBD in the literature [34]. Devinsky et al. [3] reported elevated levels of aspartate (AST) and alanine aminotransferase (ALT) in more than 30 seizure patients who received an oral dose of 10 or 20 mg/kg bw of CBD per day in a 14-day trial [3]. For comparison with the RfD values obtained from the BMD analysis of dose-response data obtained from Marx et al. [34] for the respective endpoints, the oral dose of 10 mg/kg bw of CBD could be converted into an inhalation dose of approximately 1.7 mg/kg bw. In comparison an RfD of 107 mg/kg bw for AST-levels was derived from Marx et al. [34] by using the BMD approach. Other adverse events reported in this study were diarrhea, vomiting, mild upper respiratory tract infection, and pyrexia. CBD concentrations in the study by Devinsky et al. [3] were far below the corresponding RfD values estimated by Marx et al. [34]. This discrepancy might be due to the use of different test subjects. As Devinsky investigated administration in humans [3], it should be assumed that humans have a higher sensitivity to CBD than rats. It appears in this case that the used uncertainty factor of 100 did not account for this difference in sensitivity.
For CBD, some limited NOAEL values have been published. The study by Marx et al. [34], which was also included in this risk assessment, derived an oral NOAEL in hemp extract of 100 mg/kg bw in male rats, which can be converted into an RfD for inhalation of 17 mg/kg bw in humans, which corresponds to a NOAEL for CBD of approximately 1 mg/kg bw after dose conversion. In 2019, the European Medicines Agency (EMA) [48] published a NOAEL for i.v. application of CBD in beagle dogs of 15 mg/kg bw/d for hepatological endpoints in a 14-day study, which can be converted via dose conversion to an RfD of approximately 0.5 mg/kg bw/d. In comparison, the lowest RfD estimated from the study in rats by Marx et al. [34] was 2 mg/kg bw for a statistically significant change in liver weight. The BMD model might therefore estimate a larger BMDL to be a more appropriate POD than NOAEL values obtained from literature. To account for consumer safety, it is recommended to prefer the more conservative NOAEL published by EMA [48] or the NOAEL from Marx et al. [34] as a more reliable POD over the BMDL derived in this study.
Unfortunately not enough adequate dose response data could be retrieved from online sources in ordert to conduct BMD modelling for further toxicological endpoints like local (airway) effects or cognitive and psychological effects. Most animal studies retrieved for this BMD analysis only reported acute systemic effects. A change in uterus weight in rats [34] was the only long-term effect reported in this study, as this was found to be the lowest RfD for a relevant endpoint.
It should be emphasized that this comparison between extrapolated RfDs from BMDL
values and published PODs or dose-response data in humans and animals can only
represent an attempt to elucidate, whether BMD modelling of the data available
provided RfD values, which may be comparable to literature data. Therefore, RfDs
obtained in this study should not be considered as alternative doses to already
established threshold doses of CBD or
To suggest an initial approach for a risk assessment of CBD applicable to e-liquids, a theoretical consumption scenario will be considered. E-liquids contain different amounts of CBD in a glycerol/propylene glycol matrix. During our analyses, e-liquids with the highest concentrations of CBD typically contained about 100 g/L CBD in a 10 ml container. The consumer safety of these products should be assessed in a hypothetical consumption scenario with the following considerations:
The pulmonary system will be exposed to the entire amount of CBD contained in the e-liquid, and the hypothetical consumer will be exposed to an entire 10 mL of CBD-liquid. Exposure to 10 ml of e-liquid per day was also assumed in this case, considering the worst-case exposure to vaping e-liquids ranging between 5 and 25 mL/day.
When considering bioavailability after inhalation of 31% [31], a total of approximately 31 mg CBD should reach the blood circuit of consumers. With an assumed average body weight of 70 kg, a human would inhale approximately 1.43 mg/kg bw/d CBD.
The amount of CBD in the respective e-liquid would exceed the lowest estimated
RfD for inhalation of approximately 1 mg/kg bw/d of CBD by a factor of two. This
approach refers to an RfD for inhalation derived from a study in Sprague-Dawley
rats [33]. The lowest RfD for inhalation derived from a study in humans was found
to be approximately 87 mg/kg bw/d. This dose would allow a safe inhalation of the
mentioned CBD-liquid. It should be emphasized, however, that endocrinological
endpoints appear more susceptible to lower doses of both CBD and
A major challenge in conducting this risk assessment was the limited
availability of sufficient usable toxicological data on inhaled CBD and
The metabolism of CBD and
Furthermore, it should be considered that
Regarding the knowledge about the toxicology of
Regarding
CBD has been reported to be an antagonist of the type 1 vanilloid receptor in protein binding studies. Both CBD and 7-OH-CBD inhibit fatty acid amid hydrolase in rats [66]. CBD and 6-alpha/beta-OH-CBD and other hydroxylated metabolites are capable of inhibiting microsomal CYP 2C and 3A in in vivo mouse model studies [54]. Kraemer et al. [67] discovered that CBD can be metabolized to its decarbonylated form in humans. However, toxicological data on decarbonylated CBD have not been found.
In conclusion, the regarded metabolites may not be of toxicological relevance
for the results obtained in this risk assessment, but it should be assumed that
metabolism might affect the concentration of CBD and
Note that the human metabolism of CBD and
Furthermore, it should not be excluded that other factors, which cannot be
easily assessed, may impact the toxicology of CBD and
This study presents a risk assessment for inhalation of
Generally, the RfDs obtained from BMD modeling were found to be higher than
doses from the literature converted for comparison. To account for additional
consumer safety, it is not advised to consider the RfDs from this study as
reliable due to uncertainties arising from the insufficient amount and quality of
dose-response data, which have been included in BMD modeling. Note that the BMD
approach benefits from dose-response data, which cover a broad dose range. This
would allow risk assessors to conduct a retrospective meta-analysis, which
combines dose-response data from individual studies. As BMD modeling does not
require a large number of test subjects per dose group, this would allow to
combine dose-response data, especially from individual studies in human test
subjects, to efficiently use limited dose-response data for a risk assessment.
Considering that inhalation of vaporized
Further useful dose-response data could be provided by a subsequent study to Marx et al. [34] in rats, which uses additional or intermediate dose groups with fewer animals per dose group. The results should then be evaluated using BMD modeling.
CBD, Cannabidiol;
Conceptualization—DWL; methodology—DWL; software—AS; validation—PH; formal analysis—PH; investigation—PH, AS; resources—SGW; data curation—PH, DWL; writing original draft preparation—PH, AS; writing review and editing—DWL, ER, SGW; visualization—PH, AS; supervision—SGW, DWL, ER; project administration—DWL. All authors have read and agreed to the published version of the manuscript.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest. DWL is serving as one of the Guest Editor of this journal. We declare that DWL had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to MI.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.