1 Department of Nephrology, Shanghai Jiaotong University Medical School affiliated Shanghai Sixth People's Hospital, 200233 Shanghai, China
2 Division of Nephrology, Kidney Institution of Chinese People’s Liberation Army, Changzheng Hospital, 200003 Shanghai, China
3 Department of Nephrology and Rheumatology, Shanghai Tenth People's Hospital, Tongji University School of Medicine, 200003 Shanghai, China
4 Department of Biochemistry and Molecular Biology, Mayo Clinic, Rochester, MN 55902, USA
Academic Editor: Josef Jampílek
Abstract
Background: Autosomal dominant polycystic kidney disease (ADPKD) is a
ciliopathy characterized by abnormal tubular epithelial proliferation and fluid
secretion. Anoctamin 1 (ANO1) is a calcium-dependent chloride channel. However,
how ANO1 contributes to ADPKD is largely unexplored. Methods: Kidney
tissues from ADPKD patients, Pkd1
Keywords
- autosomal dominant polycystic kidney disease
- anoctamin 1
- primary cilium
- polycystin
Autosomal dominant polycystic kidney disease (ADPKD) is the
most common hereditary kidney disease and is
a leading cause of end-stage kidney disease with an incidence of 1 in 1000
individuals [1]. ADPKD is mainly caused by mutations in PKD1 (encoding
polycystin 1, PC1) or PKD2 (encoding polycystin 2, PC2) gene, and in
GANAB (glucosidase ii alpha subunit), DNAJB11, ALG9, or IFT140 for the rest 10%
[2]. PC1 and PC2 are located in the primary cilium. Proper targeting of
polycystins to the ciliary surface is critical for polycystin function [3, 4].
ADPKD is considered a cilia-related disease [5]. According to one of the theories
in ADPKD, the complex of PC1/PC2 acts as a mechanical sensor on the ciliary
surface of epithelial cells lining renal tubules to regulate the proper response
to intra-tubular liquid flow through triggering a Ca
Renal cysts form when the level of functional PC1 or PC2 drops below a critical threshold, with the primary cilium of renal epithelial cells as a major functional site [8]. Accumulating evidence in human genetics and hypomorphic rodent models also showed that the severity of ADPKD is negatively correlated with the dosage of functional polycystins [9, 10, 11, 12]. Besides, ADPKD is characterized by aberrant proliferation of tubular epithelial cells, which is accompanied by activation of multiple cell proliferation signaling pathways such as STAT3 and ERK pathways [11, 12].
Anoctamin 1 (ANO1) is a newly identified calcium-dependent chloride channel,
which has recently been shown to be implicated in cystic fluid secretion
in vitro [13, 14, 15]. Inhibition of ANO1, either pharmacological or
genetical, effectively suppressed cystogenesis in Pkd1
Kidney specimens of seven patients (four males, three females; 57.7
Animal experiments were approved by the local institutional review board for the
care of animals and were performed in accordance with National Institutes of
Health guidelines. PKD1
Madin-Darby canine kidney (MDCK) cells, inner-medullary collecting duct (IMCD3)
cells, PKD1
To knock down ANO1, RCTE or GANAB cells were transfected with 50 nM
siRNA using Lipofectamine RNAiMAX (No. 13778075, Invitrogen, Carlsbad, USA)
following the manufacturer’s instructions. siRNA sequences targeting human ANO1
are as follows: siRNA1,5
Lentivirus system was used to construct stabilized Ano1 knock-down MDCK cell
lines. Short hairpin RNA (shRNA)-expressing lentivirus constructs were generated
using pLV-RNAi vector (Biosettia, San Diego, CA, USA. The following shRNA
sequence was used to target Canis lupus ANO1: 5
Kidneys were fixed in 10% neutral-buffered formalin and were paraffin embedded.
For immunohistological analyses, we used 4
Cells were cultured in DMEM/F-12 containing 10% FBS on glass coverslips and were starved when reaching 70% confluence. After 24–48 h, slides were fixed with ice-cold methanol or paraformaldehyde for 10 min, then were permeabilized and immune-stained with appropriate antibodies. For immunofluorescent staining, anti-ANO1 (diluted 1:500), anti-acetylated a-tubulin (diluted 1:1000, T7451, Sigma), anti-tubulin-detyrosinated (diluted 1:1000, AB3201, Sigma) and PC2 antibodies (diluted 1:200, provided by the Baltimore Polycystic Kidney Disease (PKD) Research and Clinical Core Center) were used. Binding of the primary antibody was visualized by incubation with secondary donkey anti-mouse antibodies conjugated with AlexaFluor 488 or 555 (1:1000 for each, Molecular Probes, Invitrogen, Darmstadt, Germany). IgG was used as negative controls. Confocal experiments were carried out on a Bio-Rad MRC-1024 laser scanning confocal microscope (Bio-Rad Laboratories, Hercules, CA, USA).
Total RNA was isolated from PKD1
Three-dimensional structured illumination microscopy was performed using a standard protocol in our lab. Briefly, an ELYRA Super-resolution Microscopy system (Zeiss) equipped with an alpha ‘Plan-Apochromat’ 100_/1.46 Oil DIC oil immersion objective and an AndoriXon 885 EMCCD camera was used to capture raw images. Sections were acquired at 0.125-mm z-steps. Color channels were aligned using alignment parameters from control measurements with 0.5-mm diameter multispectral fluorescent beads (Zeiss). Structured illumination reconstruction and image processing were performed with the ZEN software package (Zeiss). Final image processing was performed by using Adobe Photoshop.
Protein was extracted by using the ProteoPrep® Total Extraction
Sample Kit (Sigma, USA), and was measured with a BCA protein assay kit (Pierce,
USA). Protein sample (60
Cells were cultured with serum-free defined medium containing DMEM-F12 and
insulin (5
All data examined are presented as means
To understand the transcriptional control of Ano1 gene in kidneys, the
renal expression of ANO1 was measured in hypomorphic PKD1
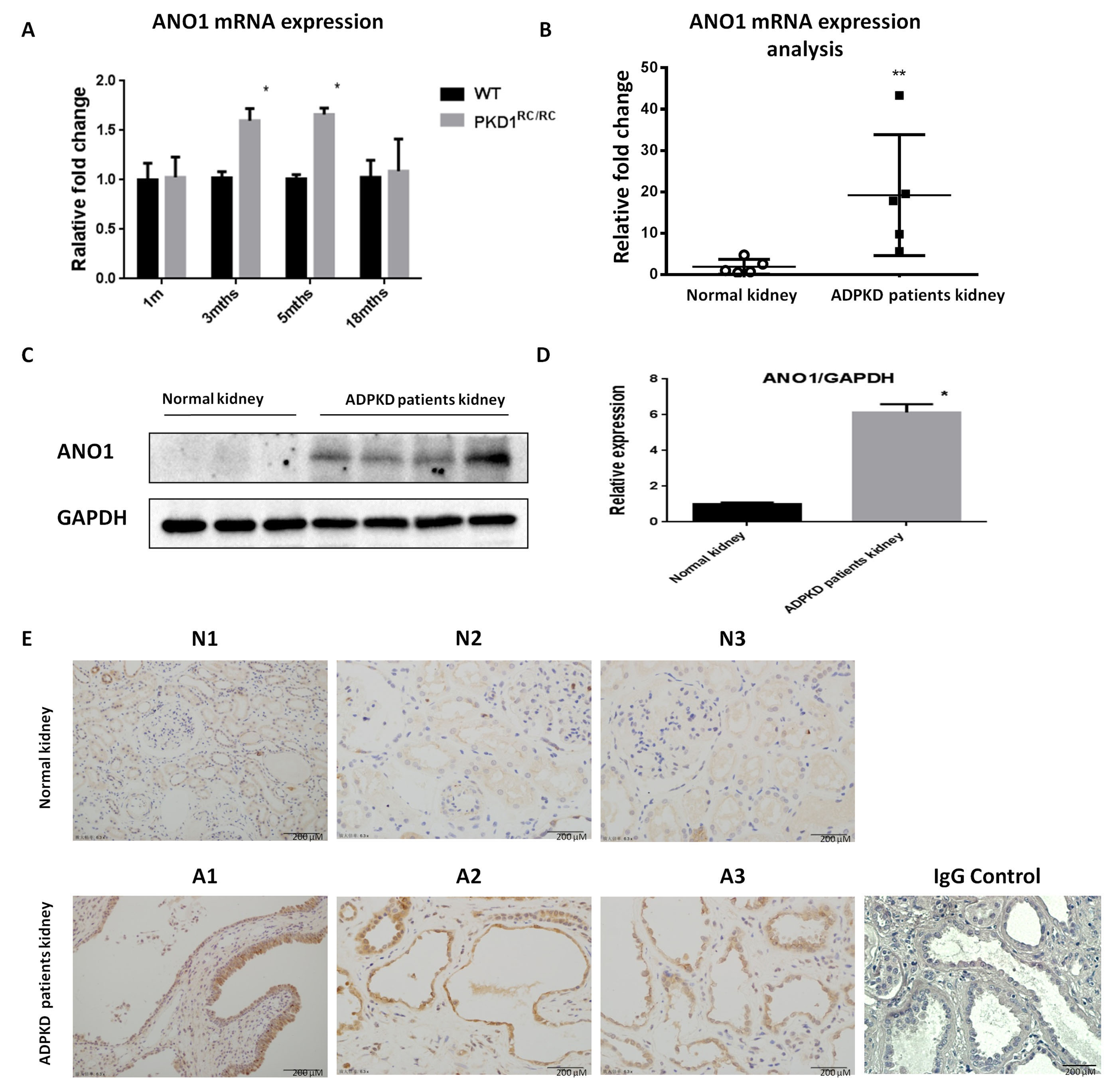
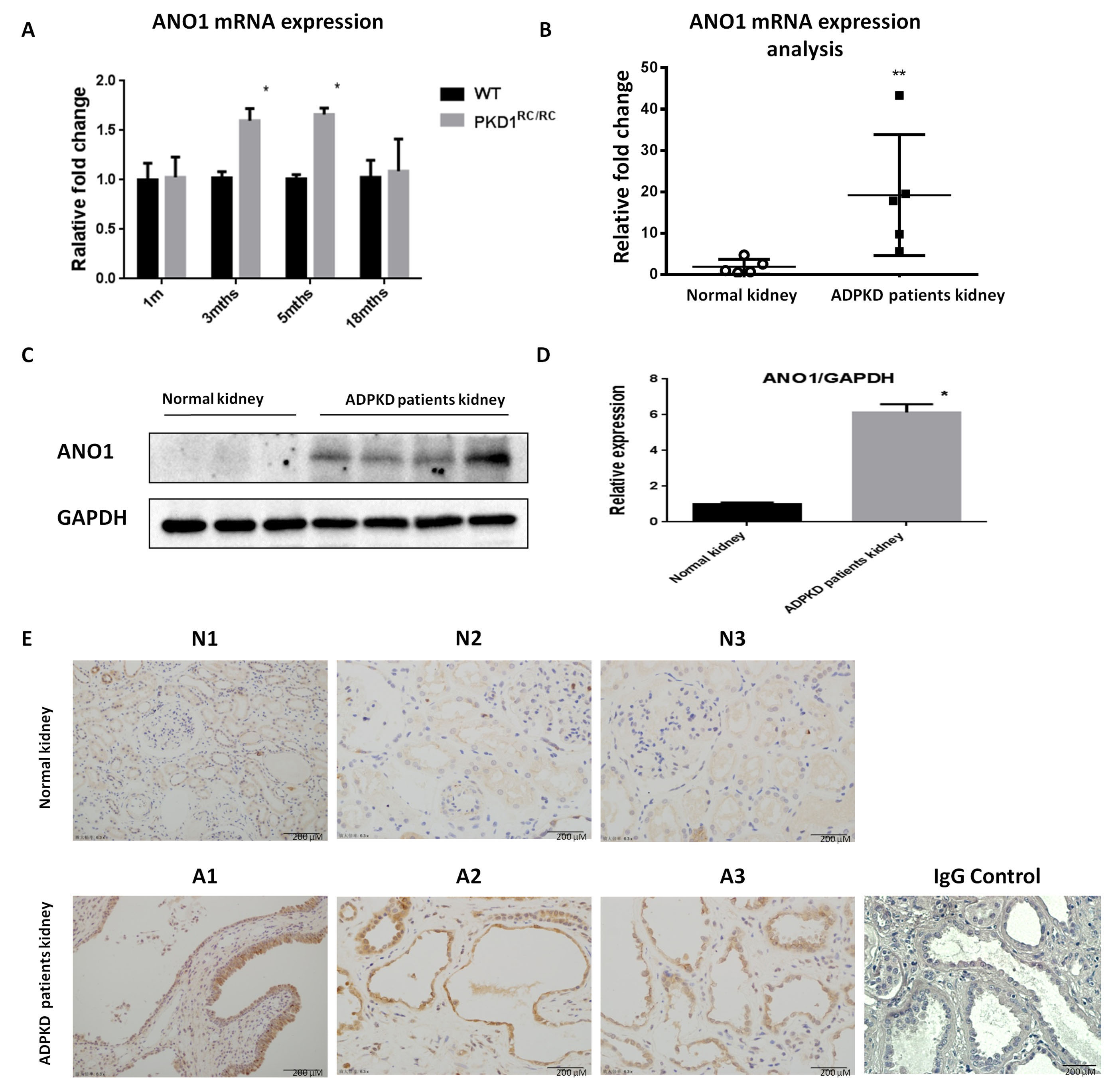 Fig. 1.
Fig. 1.ANO1 is up-regulated in mouse and human ADPKD kidneys. (A)
Quantitative PCR analysis of ANO1 expression in kidney tissues of PKD1
To examine if ANO1 upregulation is relevant in ADPKD, we analyzed ANO1 expression levels in human ADPKD kidneys (Fig. 1B). Western blotting analysis indicated that ANO1 was significantly upregulated almost 5 folds in human ADPKD kidneys as compared with normal controls (Fig. 1C,D). Immunohistochemical staining revealed strong staining of ANO1 in lining cells of cystic renal tubules, but not in normal tubules or glomeruli, of kidneys from ADPKD patients as compared with those from normal kidneys (Fig. 1E).
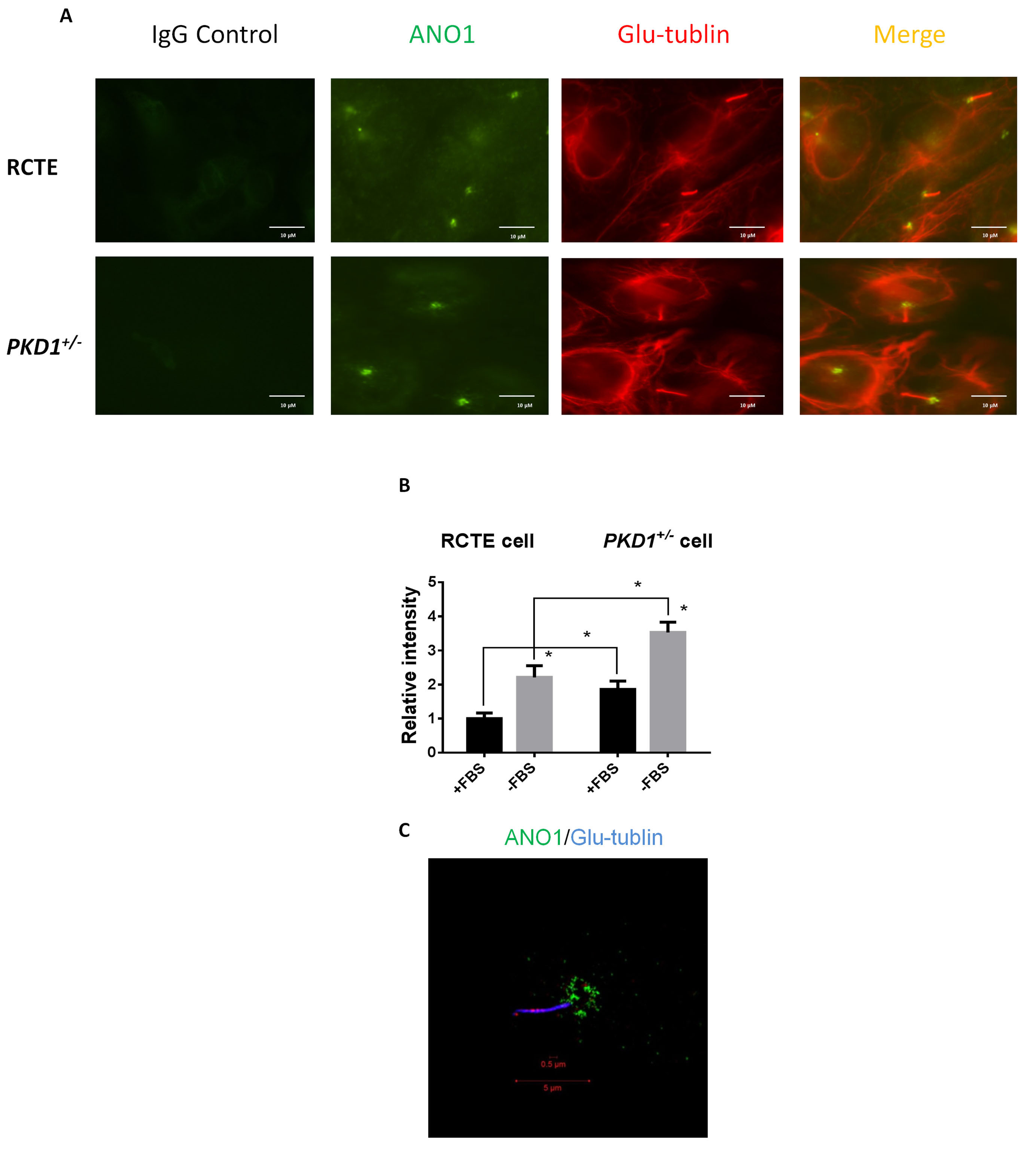
We then assessed the subcellular localization of ANO1 in human renal collecting
tubule epithelial (RCTE) cells by confocal immunofluorescence. As shown in Fig. 2A, contradictory to the assumption that ANO1 acts as a sensory receptor on the
ciliary surface, ANO1 signal was only found at the ciliary base of RCTE cells.
PKD1 deficiency did not change the localization of ANO1 (Fig. 2A). Moreover, we
found that the intensity of ANO1 staining in the primary cilium of human
PKD1
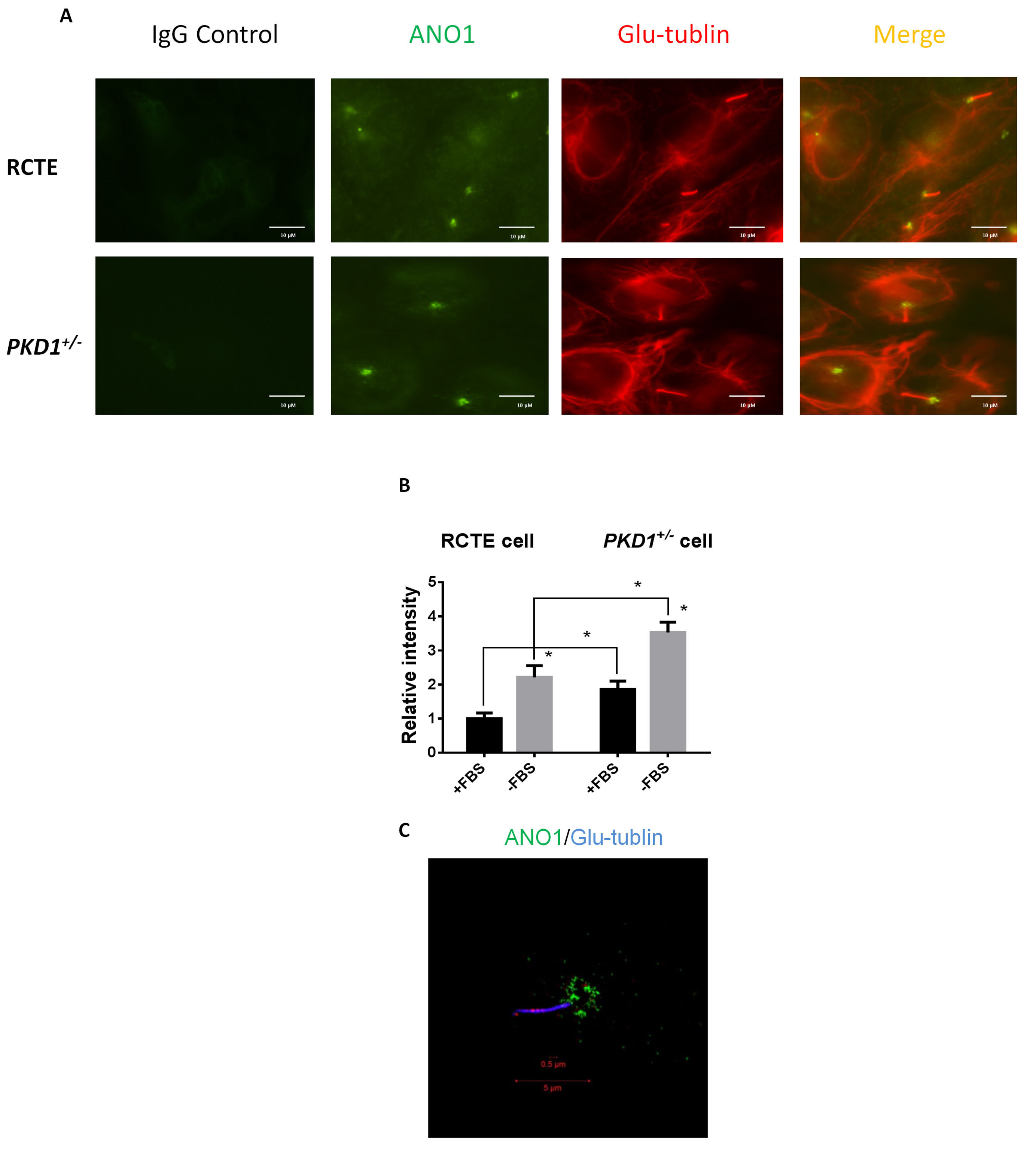 Fig. 2.
Fig. 2.ANO1 is localized in primary cilia. (A,B) Immunofluorescence
staining for ANO1 and Glu-tubulin was performed on RCTE and ADPKD cells
(PKD1
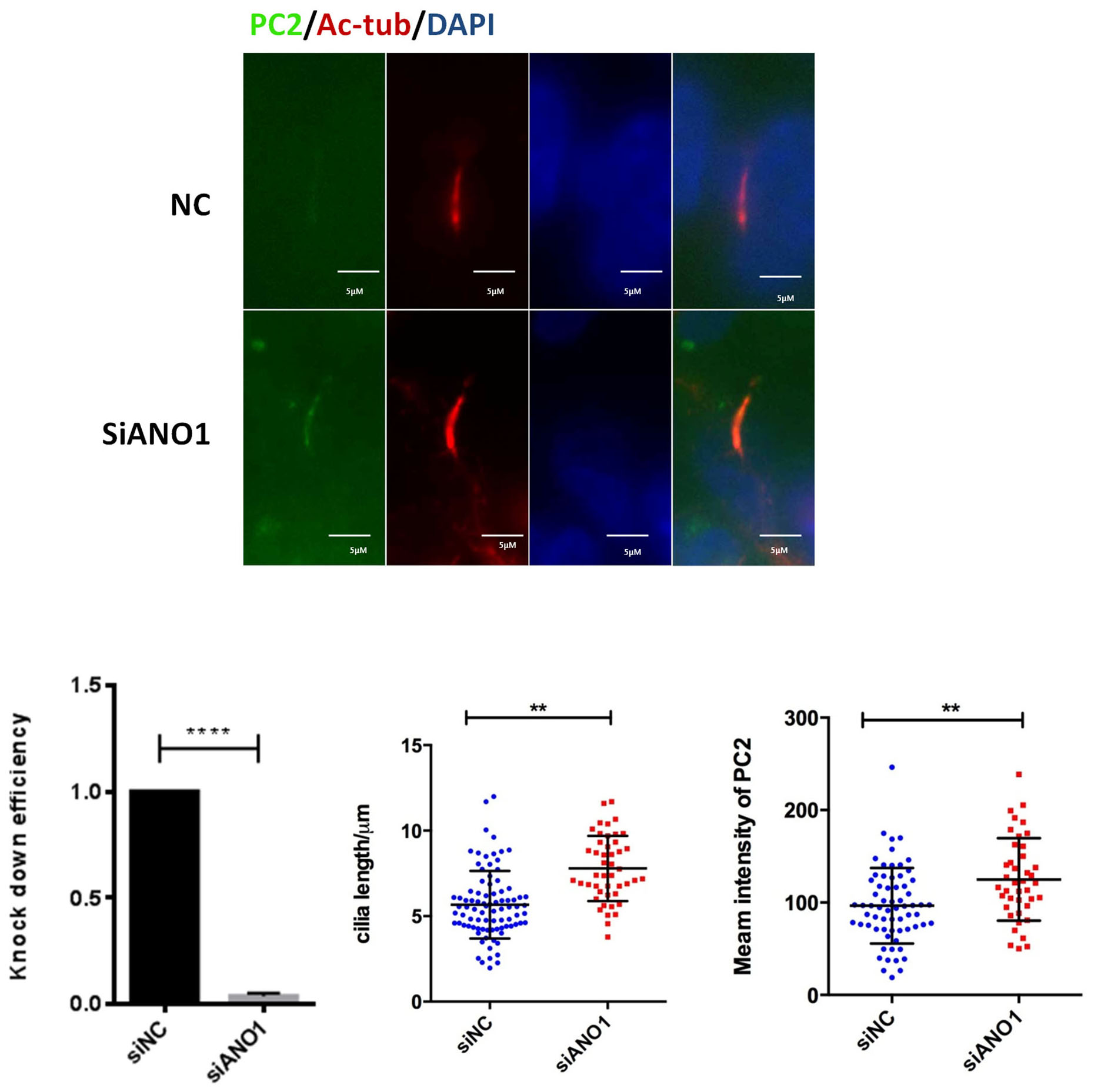
To investigate the role of ANO1 in the context of cilia, we used siRNA to knock
down the expression of ANO1 in WT9-7 human PKD1
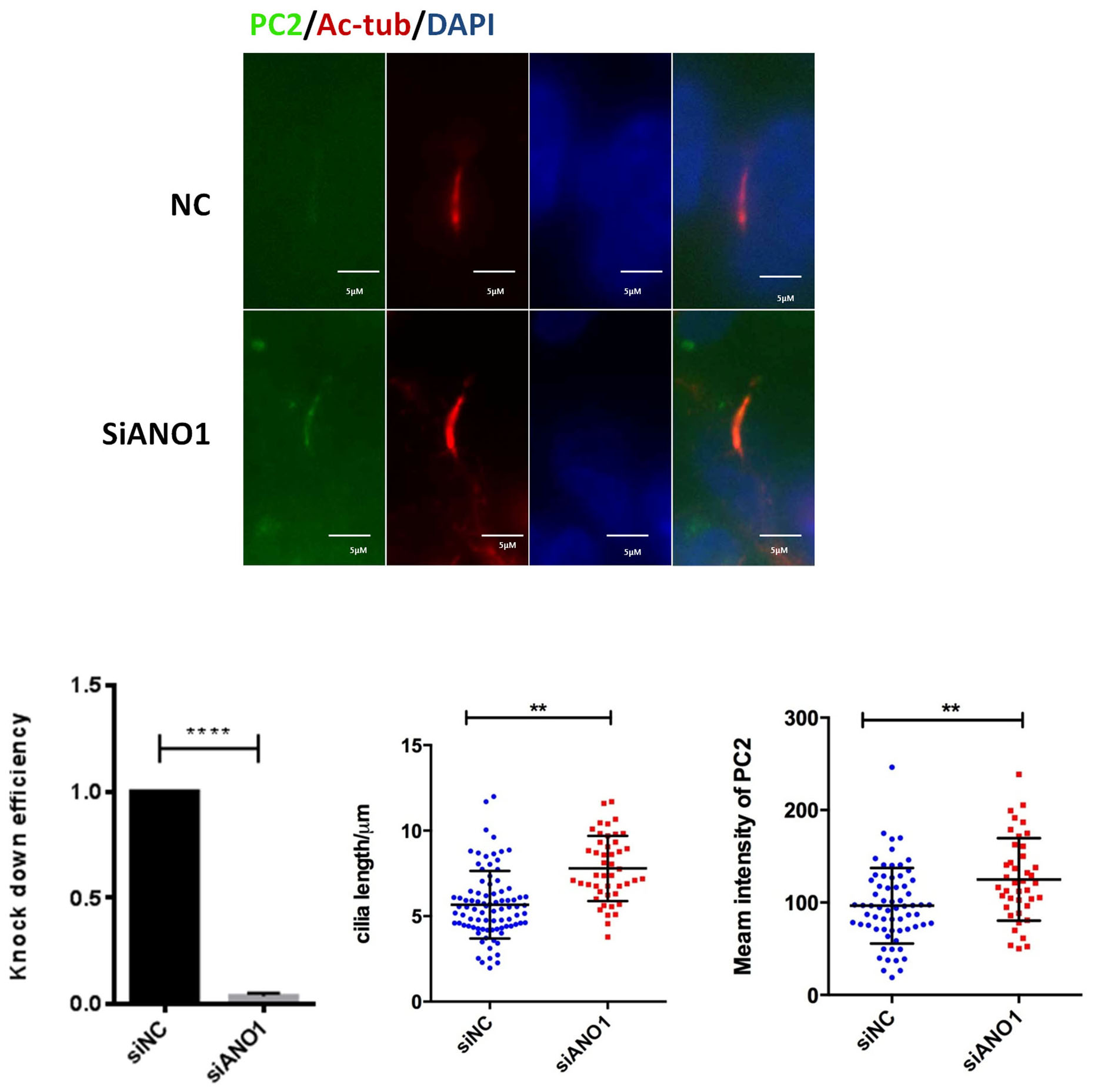 Fig. 3.
Fig. 3.Knockdown of ANO1 increases the cilium length and the expression
of polycystin 2 (PC2). Immunofluorescence: nonsense control (NC) siRNA or siRNA
against ANO1was transfected in human PKD1
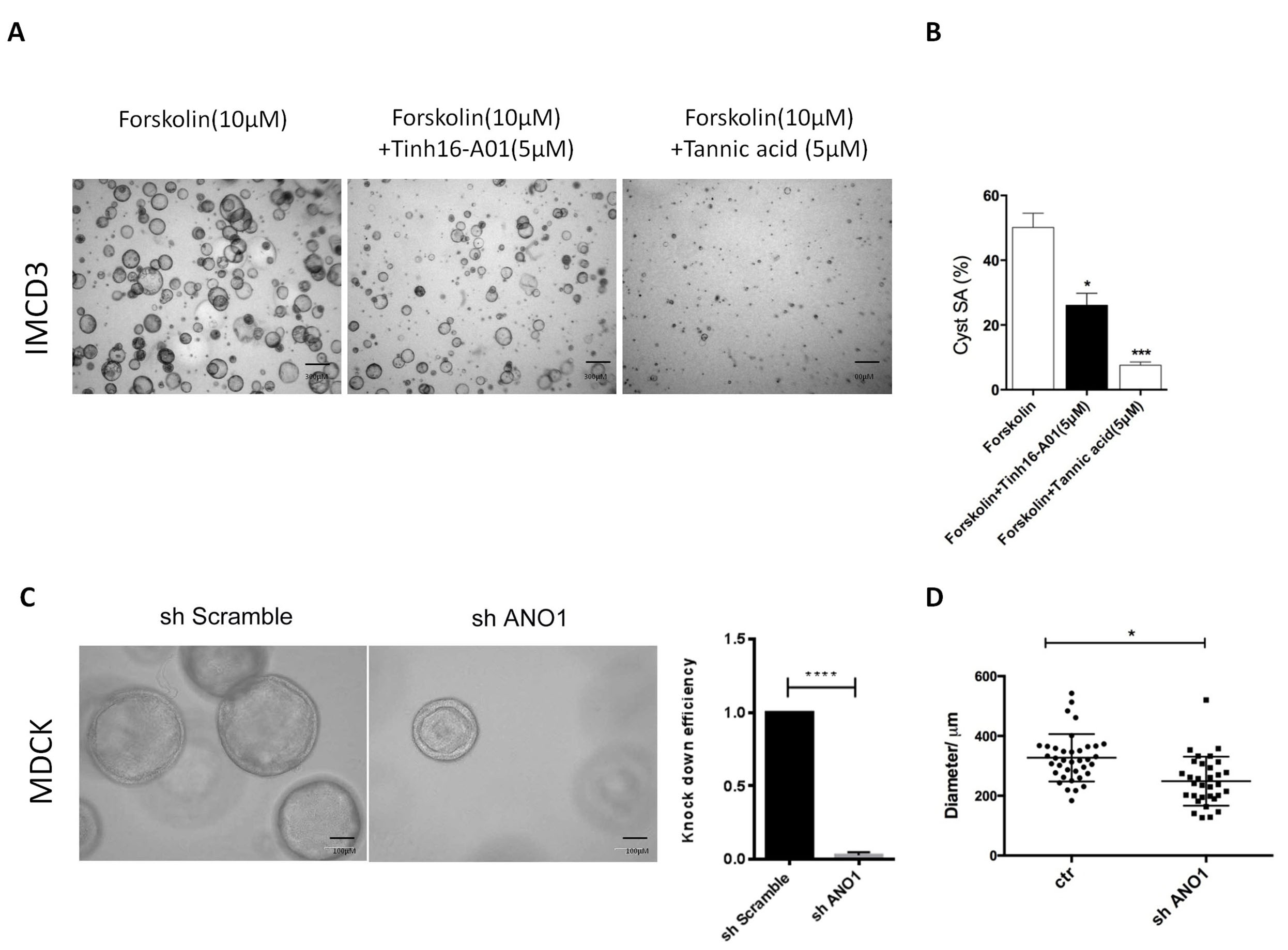
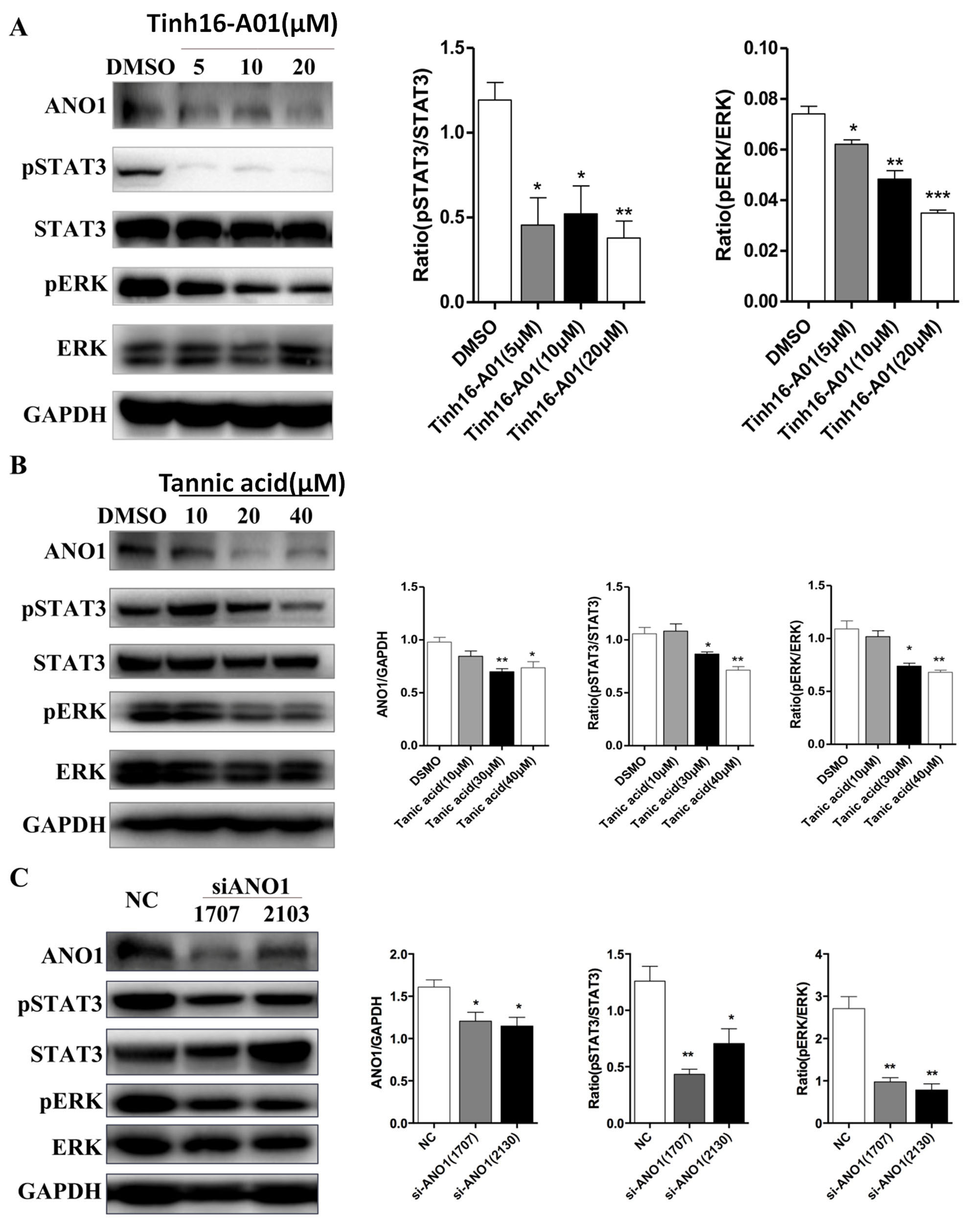
3D culture with MDCK or IMCD3 cells are classical in vitro models to study ADPKD [21]. ANO1 specific inhibitor Tinh16-A01 or Tannic acid was used to treat IMCD3 cells. Tinh16-A01 or Tannic acid significantly inhibited cyst formation in 3D-cultured IMCD3 cells (Fig. 4A,B). To further study the functional impact of ANO1 on renal cyst formation, we established MDCK cell line with steady knockdown of Ano1 gene. As expected, genetic knockdown of Ano1 significantly inhibited cyst formation in MDCK cells (Fig. 4C,D).
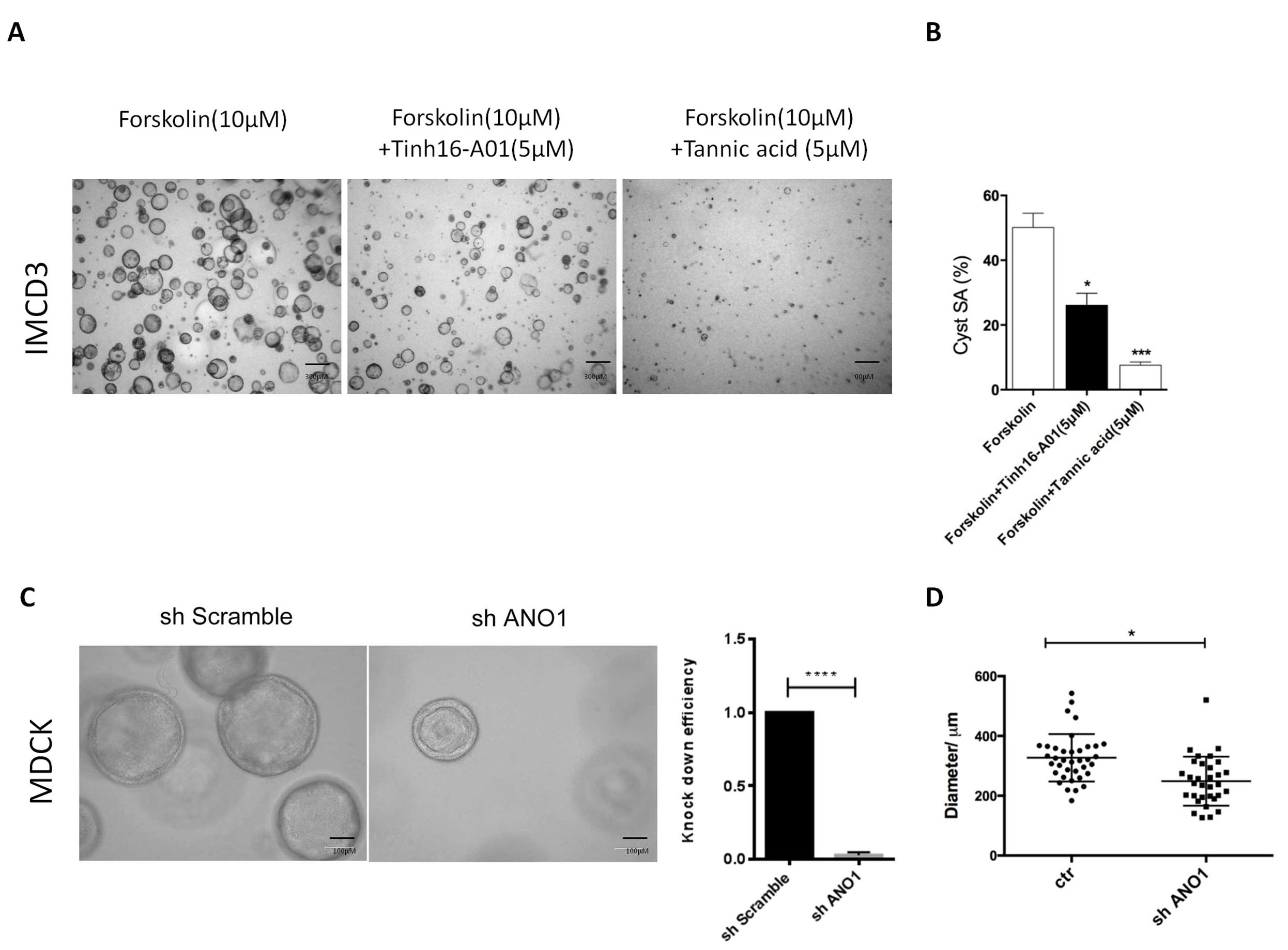 Fig. 4.
Fig. 4.Knock-down of ANO1 inhibits renal cyst formation in 3D culture
models. (A,B) A three-dimensional (3D) culture model of cyst formation was
established using IMCD3 cells which were treated with two different
ANO1inhibitors (Tinh16-A01 or Tannic acid). After 2 weeks of culture, the Surface
area of cysts (SA) was measured. Scale bars, 300
We further examined the proliferative pathways abnormally upregulated in human
PKD cells. GANAB is a novel ADPKD gene whose mutation compromises the
protein maturity of PKD1 and PKD2 and impairs their ciliary localization [22]. As
shown in Fig. 5A, ANO1 inhibition by Tinh16-A01 subdued phosphorylation of STAT3
and ERK in human GANAB
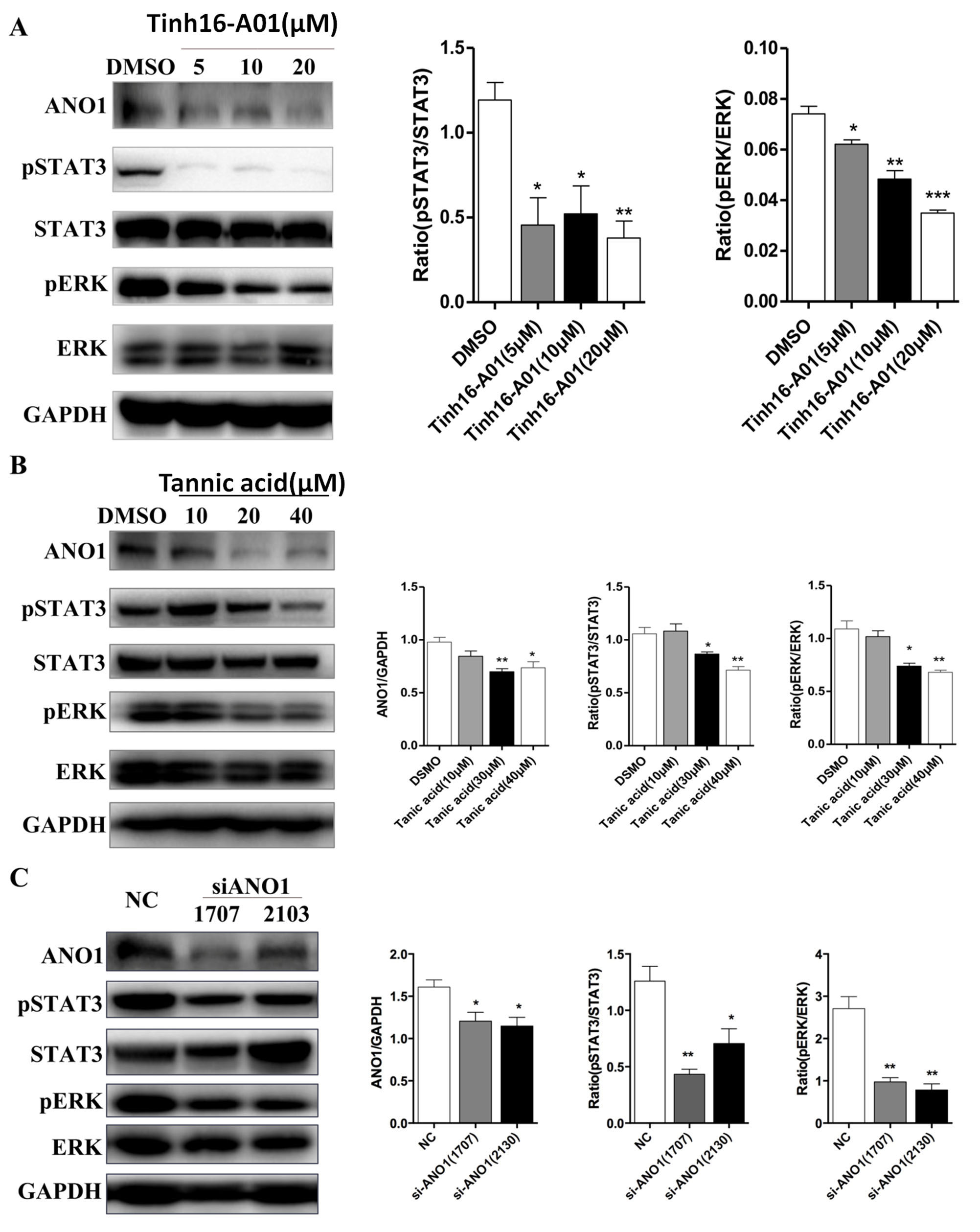 Fig. 5.
Fig. 5.The effect of ANO1 on cell proliferation signaling pathways of
ADPKD. (A) GANAB cells was treated with ANO1 specific inhibitor
Tinh16-A01.The expression of ANO1 and phosphorylation of STAT3 and ERK in
GANAB cells were analyzed by Western blot and further quantified. (B)
GANAB cells was treated with ANO1 specific inhibitor Tannic acid. The
expression of ANO1 and phosphorylation of STAT3 and ERK in GANAB cells
were analyzed by Western blot and further quantified. (C) GANAB (an
ADPKD cell line) cells were transfected with nonsense control (NC) siRNA or two
different siRNAs against ANO1. The expression of ANO1 and phosphorylation of
STAT3 and ERK in GANAB cells were analyzed by Western blot and
further quantified. *p
ANO1 drives cyst growth in PKD models but with poorly understood molecular
mechanisms [23]. In the current study, we confirmed that ANO1 is abnormally
upregulated in cyst-lining cells in both human ADPKD kidneys and hypomorphic
Pkd1
Although different lines of evidence suggested ANO1 deficiency impairs
ciliogenesis, Ano1
PC2 is localized to the primary cilium of tubular cells and ciliary exclusion of PC2 promotes renal cystogenesis in ADPKD model [27]. It has been reported that the length of PC2-negative cilia is significantly shorter than that of PC2-positive cilia [28]. We hypothesized that ANO1 inhibits cilium formation through increasing PC2 content in the primary cilium. Here, we showed that knock-down of ANO1 increased the cilia length which was correlated with increased expression of PC2 in the primary cilium of ADPKD cells. While ANO1 inhibited is not decrease expression of PKD2 in the GANAB cells.
Previous study showed that ANO1 was localized to the cilia of mature olfactory
sensory neurons [29]. In our hand, we could not detect the ciliary localization
for ANO1 even by using super-resolution SIM microscopy analysis. ANO1 is
specifically restricted to a subset of vesicles at the ciliary base, suggesting
that ANO1 is not used by renal epithelial cells as a sensory receptor or chloride
channel on the cilia surface to regulate signal transduction and/or ion exchange
between renal epithelia and tubular fluids. It is thus interesting how ANO1
regulates ion homeostasis and cilia-related function at the ciliary base in the
context of ADPKD. One plausible mechanism is that ANO1 may regulate the ciliary
import or removal of key molecules involved in cilia formation and function by
influencing local ion homeostasis and/or vesicle dynamics, as is evidenced by
upregulation of PC2 and increased cilia length in ANO1-deficient cells. ANO1
deficiency may lead to enhanced trafficking of vesicles targeted to cilia. A line
of evidence supporting this hypothesis is the electron microscopy study of
proximal tubular cells in Ano1
ADPKD is characterized by activation of multiple proliferating signaling
pathways such as STAT3 and ERK pathways [31]. It has been reported that ANO1
promotes cyst growth by increasing cystic epithelial cell proliferation [32].
Thus, we hypothesized that ANO1 promotes cyst growth by activating STAT3 and ERK
proliferating pathways. We first confirmed ANO1 inhibitors reduced cyst size in
two 3D culture models. Next, we showed that activation of STAT3 and ERK
proliferating pathways in GANAB
Our data indicate ANO1 is a negative regulator for both cilia length and cilia trafficking of polycystin-2 and provide mechanistic insights regarding the therapeutic potential of ANO1 pathway in ADPKD treatment.
CM and TX conceived and supervised the study; TX and JH designed experiments; TX, MC, QX, LF, and KL performed experiments; TX provided new tools and reagents; TX and MC analysed data; TX, CX and JH wrote the manuscript; JH and CX made manuscript revisions.
Not applicable.
Not applicable.
This study was supported by grants from the national key research and development program of china (2016yfc0901502), the national natural science foundation of china (no. 81873595, 81670612, 81300560), shanghai municipal key clinical specialty (shslczdzk02503) and pujiang talent program of shanghai, china (no. 17pj1407700).
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/j.fbl2707216.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.