1 Department of Biology, Chemistry, and Environmental Sciences, College of Arts and Sciences, American University of Sharjah, 26666 Sharjah, United Arab Emirates
2 Department of Biomedical Science, College of Health Sciences, Member of QU Health, Qatar University, 2713 Doha, Qatar
3 Biomedical Research Center, Qatar University, 2713 Doha, Qatar
Academic Editor: Amedeo Amedei
Abstract
Natural products with known safety profiles are a promising source for the discovery of new drug leads. Berberine presents an example of one such phytochemical that has been extensively studied for its anti-inflammatory and immunomodulatory properties against myriads of diseases, ranging from respiratory disorders to viral infections. A growing body of research supports the pluripotent therapeutic role berberine may play against the dreaded disease COVID-19. The exact pathophysiological features of COVID-19 are yet to be elucidated. However, compelling evidence suggests inflammation and immune dysregulations as major features of this disease. Being a potent immunomodulatory and anti-inflammatory agent, berberine may prove to be useful for the prevention and treatment of COVID-19. This review aims to revisit the pharmacological anti-inflammatory and immunomodulatory benefits of berberine on a multitude of respiratory infections, which like COVID-19, are known to adversely affect the airways and lungs. We speculate that berberine may help alleviate COVID-19 via preventing cytokine storm, restoring Th1/Th2 balance, and enhancing cell-mediated immunity. Furthermore, the role this promising phytochemical plays on other important inflammatory mediators involved in respiratory disorders will be underscored. We further highlight the role of berberine against COVID-19 by underscoring direct evidence from in silico, in vitro, and in vivo studies suggesting the inhibitory potential berberine may play against three critical SARS-CoV-2 targets, namely main protease, spike protein, and angiotensin-converting enzyme 2 receptor. Further preclinical and clinical trials are certainly required to further substantiate the efficacy and potency of berberine against COVID-19 in humans.
Keywords
- berberine
- COVID-19
- SARS-CoV-2
- anti-inflammatory
- immunomodulatory
- cytokine storm
- Th1/Th2 balance
Coronaviruses (CoVs) are enveloped viruses containing non-segmented, positive-stranded RNA genomes [1]. These viruses are known to cause a broad spectrum of diseases, including bronchitis, gastroenteritis, systemic disease, and even death in both animals and humans [2]. CoVs were identified as the causative agent of the Middle-East respiratory syndrome (MERS-CoV) and the severe acute respiratory syndrome (SARS-CoV) outbreaks, which occurred in Saudi Arabia in 2012 and in China in 2002, respectively. Recently, another CoV outbreak, namely severe acute respiratory syndrome 2 (SARS-CoV-2), the causative agent for the novel 2019 coronavirus disease (COVID-19), was first identified in Wuhan, China in December 2019 and is still ongoing, resulting in many fatalities worldwide. As of August 2021, the total number of confirmed COVID-19 cases exceeds 400 million and the total number of deaths is around 5 million worldwide [3]. Like MERS-CoV and SARS-CoV, SARS-CoV-2 is a respiratory pathogen. Most COVID-19 patients present with mild to moderate symptoms and recover without the need for hospitalization [4]. Nevertheless, severe respiratory complications are not uncommon, especially in high-risk populations including the elderly and those immunocompromised [4]. The most commonly reported clinical symptoms of COVID-19 include fever, dry cough, fatigue, myalgia, and dyspnea [5, 6]. A small fraction of patients may also present with acute respiratory distress syndrome (ARDS), possibly as a result of cytokine storm development and over-exaggerated immune responses, leading to acute respiratory failure and/or multiorgan failure [7, 8]. Currently, multiple vaccines are available with the promise of protection against COVID-19 [9]. Nevertheless, none of these vaccines ensure complete immunity against this dreaded disease, with many contracting COVID-19 despite being double-vaccinated [10]. Developing and maintaining strong immunity, even with vaccination, is therefore still essential, and there is ample evidence supporting the exceptional pharmacological benefits that natural products may have against myriads of viral infections [11], of which COVID-19 is no exception.
Berberine is a quaternary ammonium salt derived from the protoberberine group of
benzylisoquinoline alkaloids, with a molecular formula of C
The reported in vivo and in vitro effects of berberine on cytokine storm are summarized in Table 1 (Ref. [29, 30, 31, 32, 33]).
| Main effects | Experimental model | Dosage | Administration mode | Administration duration | Reference |
| Reduction of OVA-induced secretion of IL-4, IL-5, IL-6, IL-13, IL-17, and IL-1 |
Male Wistar rats | 100 and 200 mg/kg | Oral | 28 days | [29] |
| Reduction of CSE-induced IL-6 and TNF |
C57BL/6 mice | 25 mg/kg (low dose) and 50 mg/kg (high dose) | Oral | Treatment for 6 days a week for 60 days | [30] |
| Suppression of LPS-induced rise in IL-6 and IL-8 protein and gene expression levels in 16HBE cells | 16HBE cells | 2.5, 5, and 10 |
- | Pretreatment for 4 hrs | [31] |
| Reduction of LPS-induced rise in IL-6 and KC levels in mice | Male C57BL/6 mice | 10 mg/kg | Intraperitoneal | Pretreatment twice (at 24 and 2 hrs before LPS stimulation) | |
| Reduction of CS-induced rise in MPO activity | Male C57BL/6 mice | 50 mg/kg | Intragastric | 1 hr before CS exposure on 4 consecutive days | [32] |
| Reduction of CS-induced rise in TNF |
|||||
| Inhibition of LPS-induced glycocalyx shedding in HUVECs and in mice | HUVEC cells | 1.25, 2.5, 5 |
- | Pre-treatment for 1 hr before LPS stimulation | [33] |
| Suppression of LPS-induced rise in ROS and oxidative stress in HUVECs | Male C57BL/6 mice | 50, 100, and 200 mg/kg | Oral | Treatment for 7 days | |
| Suppression of LPS-induced rise in IL-1 |
Maladaptive immune responses in face of infections can result in an inflammatory
response going out of control and pro-inflammatory cytokines being excessively
released. Cytokine storms are associated with a wide array of infectious and
non-infectious diseases and are suspected to be the main reason for mortality in
many patients [34]. Multiple lines of evidence suggest the possible intervening
role berberine may have against cytokine storm development in various lung
pathologies. For example, in an in vivo study, berberine (low dose: 100
mg/kg; high dose: 200 mg/kg) was found to exert protective effects against asthma
in ovalbumin (OVA)-induced male Wistar rats via suppressing the levels of the
pro-inflammatory cytokines IL-6, IL-1
The reported in vivo and in vitro effects of berberine on Th1/Th2 balance are summarized in Table 2 (Ref. [29, 36, 37, 38]).
| Main effects | Experimental model | Dosage | Administration mode | Administration duration | Reference |
| Reduction of IL-6 and CCL11 levels | BEAS-2B cells | 1 |
- | 16–18 hrs | [36] |
| Suppression of STAT-6 pathway | |||||
| Reduction of OVA-induced secretion of IL-4, IL-5, and IL-13 | Male Wistar rats | 100 and 200 mg/kg | Oral | 28 days | [29] |
| Induction of IL-12 P40 production | Mouse splenic macrophages | 0.1, 0.5, 1 |
- | Incubation for 48 hrs | [37] |
| Activation of p38 MAPK pathway | |||||
| Induction of IL-12 production in mouse macrophage and dendritic cells | Mouse macrophage and dendritic cells | 0.1, 0.5, 1 |
- | Pre-treatment for 6 hrs | [38] |
| Elevation of IFN-y levels and suppression of IL-4 levels in antigen-primed CD4 |
Female DBA/2 mice | 200 |
Intraperitoneal | Treatment for 24 hrs | |
| Induction of IL-12 production in macrophages derived from female DBA/2 mice |
T-helper cells are regarded as the most prolific cytokine producers [39]. Consequently, these cells are believed to play a fundamental role in modulating immunity in different disease pathologies by directing different immune responses [40]. T-helper cells can be subdivided into type 1 helper (Th1) and type 2 helper (Th2) T cells. Th1-related cytokines are known to promote pro-inflammatory responses against intracellular pathogens whereas Th2-related cytokines are believed to play more of an anti-inflammatory role [39]. Striking a balance between Th1 and Th2 responses depending on the immune challenge in question is key to orchestrate well-coordinated immune responses to eliminate pathogens [41]. Studies suggests that an immunomodulatory agent like berberine may be capable of restoring Th1/Th2 balance in various pathological conditions, including respiratory ailments, and can help alleviate lung pathologies via increasing Th1responses and decreasing Th2 responses.
In an in vitro investigation, Ma and colleagues found that berberine
possesses promising anti-inflammatory effects in pro-inflammatory
cytokine-activated human bronchial epithelial cells (BEAS-2B) mainly via
modulating Th1- and Th2-cytokine production [36]. Pro-inflammatory cytokine like
IL-4 released from t helper cell type 2 (Th2) activation, can trigger the release
of eotaxin-1 (CCL11), the most potent chemokine that can enhance eosinophil
movement and consequently exacerbate asthma symptoms [36]. Berberine treatment (1
Interestingly, in another in vitro analysis, berberine was found to be
capable of inducing the production of IL-12, which has a role in promoting the
development of Th1 immune responses via inducing IFN
The reported in vivo and in vitro effects of berberine on cell-mediated immunity are summarized in Table 3 (Ref. [29, 30, 31, 32, 43, 44, 45, 46]).
| Main effects | Experimental model | Dosage | Administration mode | Administration duration | Reference |
| Reduction of OVA-induced eosinophil, neutrophil, macrophage, and lymphocyte levels in BALF | Male Wistar rats | 100 and 200 mg/kg | Oral | 28 days | [29] |
| Suppression of CS-induced inflammatory-cell infiltration in alveolar lung tissue | Male C57BL/6 mice | 50 mg/kg | Intragastric | 1 hr before CS exposure on 4 consecutive days | [32] |
| Reduction of CS-induced rise in macrophage, neutrophil and total cells in BALF | |||||
| Attenuation of CS-induced airway histopathological changes | Male BALB/c mice | 5 and 10 mg/kg | Intraperitoneal | 30 min before CS exposure (twice daily, 6 days per week for 4 weeks) | [43] |
| Reduction of CS-induced goblet cell hyperplasia in lungs | |||||
| Reduction of CS-induced Muc5ac synthesis | |||||
| Reduction of CS-induced inflammatory cell influx to BALF | |||||
| Reduction of CSE-induced histopathological changes of lung tissue | C57BL/6 mice | 25 mg/kg (low dose) and 50 mg/kg (high dose) | Oral | Treatment for 6 days a week for 60 days | [30] |
| Reduction of CSE-induced rise in total and differential cell count in BALF | |||||
| Attenuation of bleomycin-induced histopathological changes in lungs | Male Wistar rats | 200 mg/kg/day | Intraperitoneal | Treatment from days 1–14 (preventive group) and from days 14–28 (therapeutic) | [44] |
| Suppression of bleomycin-induced inflammatory cell infiltration in BALF | |||||
| Suppression of bleomycin-induced MPO levels | |||||
| Suppression of bleomycin-induced mast cell deposition and histamine release | |||||
| Greater reduction of LPS-induced rise in lung W/D ratio with THBru than with berberine in mice | THP-1 cells | 1, 5, and 10 |
- | Pretreatment for 1 hr prior to LPS stimulation | [45] |
| Greater reduction of LPS-induced rise in protein content in BALF with THBru than with berberine in mice | Male ICR mice | 50 mg/kg (Berberine) 2, 10, and 50 mg/kg (THBru) | Oral | Pretreatment for 1 hr prior to LPS stimulation | |
| Greater reduction of LPS-induced rise total cell count and MPO level in BALF with THBru than with berberine in mice | |||||
| Reduction of LPS-induced lung structure damage with THBru and berberine in mice | |||||
| Reduction of LPS-induced rise in TNF |
|||||
| Downregulation of LPS-induced rise in JNK, AKT/p65, and p38 expression with THBru in THP-1 cells | |||||
| Suppression of LPS-induced rise in macrophage, neutrophil, and total cell count in BALF in mice | Male C57BL/6 mice | 10 mg/kg | Intraperitoneal | Pretreatment twice (at 24 and 2 hrs before LPS stimulation) | [31] |
| Reduction of LPS-induced rise in MPO activity | |||||
| Attenuation of pulmonary fibrosis | Male Wistar rats | 200 mg/kg/day | Intraperitoneal | Treatment for 14 days | [46] |
| Suppression of BLM-induced rise in phagocytic and inflammatory cells infiltration |
Berberine is believed to play a critical role in strengthening adaptive immune response. Cell-mediated immunity, a type of adaptive immune response, depends largely on the function of T cells, both helper (with CD4 coreceptor) and cytotoxic (with CD8 coreceptor) [47]. Both of these cell types aid in pathogen elimination via activating other immune cells and assisting in the elimination of pathogens and other infected host cells [42]. As indicated in the previous section, berberine may be a useful agent in restoring Th1/Th2 balance in various lung pathologies. More specifically, berberine was shown to be useful for elevating Th1 immune responses in diseases characterized by elevated Th2 immune responses like COPD and asthma [48]. Because Th1 immune responses and cell-mediated immunity are two processes believed to be highly interrelated [49], berberine is expected to exert similar enhancing effects on cell-mediated immune responses.
In one in vivo study, berberine was found to exhibit potent
anti-inflammatory and immunomodulatory properties against asthma in OVA-induced
male Wistar rats [29]. In particular, berberine (low dose: 100 mg/kg; high dose:
200 mg/kg) was found to reverse the OVA-induced rise in immune cell count in
rats, whereby it resulted in a significant and dose-dependent drop in eosinophil,
neutrophil, lymphocyte, and macrophage levels in BALF, indicating reduced
inflammatory infiltration in the airways. In accordance, in another in
vivo investigation, berberine was found to ameliorate CS-induced lung injury via
exerting potent anti-inflammatory effects [32]. CS is considered as one of the
main risk factors involved in the pathogenesis of COPD and is known to trigger
lung inflammation by initiating the infiltration of innate and adaptive
inflammatory cells into the airways [50]. Berberine treatment (50 mg/kg) of
C57BL/6 male mice was found to suppress the CS-induced rise in macrophage,
neutrophil, and total cell count in BALF fluid, and was even found to
significantly inhibit their infiltration into alveolar spaces, indicating reduced
interstitial edema and inflammation. The role of berberine in attenuating
CS-induced lung inflammation was also confirmed in another in vivo study
by Xu and colleagues [43]. Specifically, treatment of CS-induced male BALB/c mice
with berberine (5 and 10 mg/kg) significantly attenuated the CS-induced airway
histopathological changes, whereby it reversed the thickening of the airway
epithelium, the obstruction of airway lumen by mucus and cell debris, and
inhibited the infiltration of inflammatory cells, including neutrophils, which
are regarded as the main inflammatory cells involved in the pathogenesis of COPD
[51]. Additionally, berberine (5 and 10 mg/kg) dose-dependently inhibited the
CS-induced increase in goblet cell hyperplasia and further molecular analysis
revealed a significant drop in Muc5ac, the predominant mucin gene expressed in
goblet cells, indicating reduced mucus production in mice airways. Importantly, a
significant attenuation in CS-induced inflammatory cell influx and inflammatory
cytokine release in BALF was also observed with berberine treatment (5 and 10
mg/kg). In particular, berberine treatment reduced CS-induced recruitment of
total cells and differential cells to BALF and suppressed the influx of the
proinflammatory cytokines and chemokines like TNF
In another combined in vivo and in vitro investigation, Yu and
colleagues explored the potential use of tetrahydroberberrubine (THBur), a
berberine derivative with higher oral bioavailability, in alleviating ALI in male
ICR mice and in the human monoblastic leukemia cell line, THP-1 [45]. As
indicated previously, ALI and its more severe form, ARDS, are characterized by
lung edema and neutrophil and inflammatory cell accumulation in the lung
interstitium, possibly as a result of alveolar-capillary barrier disruption [55].
Treatment with an anti-inflammatory agent like berberine or its derivative,
THBur, was found to improve ALI outcomes via decreasing various inflammatory
mediators and signaling pathways that lead to the expression of inflammatory
cytokines and chemokines. In particular, in vivo, treatment of
LPS-induced mice with berberine (50 mg/kg) or THBur (2, 10, and 50 mg/kg) was
found to curb down the LPS-induced damage caused to lungs, whereby a reduction in
interstitial edema, thickening of the alveolar wall, and infiltration of
inflammatory cells was observed. Additionally, analysis on inflammatory indices
revealed a reduction in LPS-induced rise in wet to dry (W/D) ratio, protein and
total cell count in BALF, and MPO content in mice lungs. Interestingly, these
indices were found to be more potently reduced with THBur than with berberine,
possibly due to its higher oral bioavailability. Similar promising results were
also observed in vitro whereby THBur (1, 5, and 10
The reported in vivo and in vitro effects of berberine on other inflammatory mediators involved in respiratory disorders are summarized in Table 4 (Ref. [29, 30, 31, 44, 58, 59, 60]).
| Main effects | Experimental model | Dosage | Administration mode | Administration duration | Reference |
| Reduction of OVA-induced IgE secretion | Male Wistar rats | 100 and 200 mg/kg | Oral | 28 days | [29] |
| Reduction of DNP-IgE/HSA-induced rise in B-HEX and histamine levels | RBL-2H3 cells | 0.3, 3, and 30 |
- | Pretreatment for 1 hr prior to DNP/IgE-HSA stimulation | [58] |
| Reduction of DNP-IgE/HSA-induced rise in TNF |
|||||
| Reduction of DNP-IgE/HSA-induced FcɛRI-mediated signaling | |||||
| Reduction of DNP-IgE/HSA-induced JNK, ERK, and p38 signaling | |||||
| Reduction of PMA plus A23187-induced rise in TSLP production and mRNA expression levels | HMC-1 and BMMC cell lines | 0.1–10 |
- | Pretreatment for 2 hrs | [59] |
| Reduction of PMA plus A23187-induced rise in NF-ĸB expression in HMC-1 cells | |||||
| Reduction of PMA plus A23187-induced activation of caspase-1 activity | |||||
| Reduction of PMA plus A23187-induced rise in TSLP production in BMMC cells | |||||
| Promotion of Nrf2 nuclear translocation and phosphorylation in 16HBE cells | 16HBE cells | 2.5, 5, and 10 |
- | Pretreatment for 4 hrs | [31] |
| LPS-induced drop in HO-1 levels | Male C57BL/6 mice | 10 mg/kg | Intraperitoneal | Pretreatment twice (at 24 and 2 hrs before LPS stimulation) | |
| Elevation of LPS-induced drop in Nrf2 levels in mice | |||||
| Reduction of ER stress levels in mice | |||||
| Upregulation of Nrf2 transcription factor and an increase in antioxidant ability | Male Wistar rats | 200 mg/kg/day | Intraperitoneal | Treatment from days 1–14 (preventive group) and from days 14–28 (therapeutic) | [44] |
| Suppression of NF-ĸB transcription | |||||
| Suppression of TNF |
|||||
| Downregulation of CSE-induced rise in TGF- |
C57BL/6 mice | 25 mg/kg (low dose) and 50 mg/kg (high dose) | Oral | Treatment for 6 days a week for 60 days | [30] |
| Reduction of bleomycin-induced structural modification in lung tissue | Male Wistar albino rats | 200 mg/kg body weight | Intraperitoneal | Treatment once daily for 27 days | [60] |
| Reduction of bleomycin-induced smad 2/3 expression | |||||
| Reduction of bleomycin-induced TGF- |
Besides the modulatory effects of berberine on cytokine storm development,
Th1/Th2 balance, and cell-mediated immunity, studies suggest that berberine may
also help alleviate inflammation in various respiratory disorders via exerting
inhibitory and modulatory effects on other aspects of innate and adaptive
immunity. For example, in an in vivo investigation, berberine (100 and
200 mg/kg) was found to result in a significant and dose-dependent drop in
immunoglobulin-E (IgE) levels in OVA-induced male Wistar, indicating reduced
hyperinflammation [29]. In another in vitro study, berberine was found
to suppress IgE-mediated hypersensitivity reactions in allergic disorders like
asthma in rat basophilic leukemia cells (RBL-2H3) [58]. Specifically,
pre-treating RBL-2H3, known to possess similar characteristics to mast cells,
with berberine (0.3, 3, and 30
Multiple studies also indicated that berberine may have the potential to
attenuate cytokine storm development and progression via dampening the production
and expression levels of not just cytokines, but also other inflammatory indices
that seem to increase following lung infection. For example, in an in
vitro study, berberine was found to exert protective anti-inflammatory effects
against thymic stromal lymphopoietin (TSLP), an IL-7-like cytokine molecule
believed to play a pivotal role in allergic disorders like asthma and COPD, in
the human mast cell-line 1 (HMC-1) and in murine primary cultured bone
marrow-derived mast cells (BMMCs) [59]. In particular, berberine (0.1–10
The above sections highlight the role immune dysfunctions play in different
types of respiratory ailments and propose berberine as a promising candidate
capable of alleviating such complications. Like many of these respiratory
conditions, SARS-CoV-2 is also known to primarily target the airways and lung
tissue [64]. Although the exact pathophysiological processes underlying COVID-19
are still under elucidation, multiple lines of evidence highly implicate immune
dysregulations in COVID-19 pathogenesis and progression [65]. Indeed, studies
revealed an increase in multiple inflammatory indices including C-reactive
proteins (CRP), white blood cell count, neutrophil count, IL-6, and even ARDS in
patients infected with COVID-19 [66, 67]. The reported rise in inflammatory
indices is believed to be mediated, at least in part, via COVID-19-induced
dysregulations in immune responses [65]. Precisely, SARS-CoV-2 infection is
thought to distort the balance between Th1/Th2 immune responses [68]. As such,
the levels of Th1-related cytokines like TNF
It is clear that immune dysfunctions are highly implicated in COVID-19 and that
such an infection could potentially be prevented, ameliorated, or even treated
using anti-inflammatory and immunomodulatory agents. Despite the well-documted
anti-inflammatory and immunomodulatory effects of berberine presented above, to
date, literature exploring the immunomodulatory potential of berberine
specifically against COVID-19 is rather scarce. Nevertheless, evidence from the
studies that have been published so far on this topic are very promising and
encourage future research in this area. Specifically, evidence from an in
vitro study conducted by Wang and colleagues found that treatment of a
SARS-CoV-2-infected Calu-3 cell-line with an immunotherapeutic berberine
nanomedicine molecule named NIT-X (20 and 40
In addition to the literature published specifically on the potentially
promising immunomodulatory and anti-inflammatory effects of berberine in COVID-19
prophylaxis and treatment, substantial research has been conducted confirming the
antiviral capacity of berberine against the dreaded disease COVID-19. Since the
start of the pandemic, a multitude of antiviral drugs have been tested for their
potential application as anti-COVID drugs. Of relevance, Remdesivir, Paxlovid,
and Molnupiravir were all shown to demonstrate promising therapeutic effects
against COVID-19 [74]. Of these 3 drugs, Remdesivir is the only drug currently
approved by the FDA for the treatment of COVID-19 [74]. The latter two antiviral
drugs are currently authorized by the FDA only for emergency use for COVID-19
treatment [74]. Despite the potentially promising therapeutic potential of such
drugs in COVID-19 treatment, considerable adverse effects may accompany their
use. For example, Paxlovid is known to exhibit significant and complex drug-drug
interactions and has been shown to cause myalgia, dysgeusia, and diarrhea in
COVID-19 patients [74]. Similarly, Molnupiravir was shown to be associated with
diarrhea and nausea symptoms, in addition to a potential risk of genotoxicity in
pregnant women [74]. As for Remdesivir, some studies have shown that its use can
be linked to renal and liver toxicity, an elevation in the liver enzymes
aspartate aminotransferase (AST) and alanine aminotransferase (ALT),
hypersensitivity, and nausea [74]. Given the plethora of side effects associated
with the use of synthetic drugs, the use of a phytonutrient-derived antiviral
agent, such as berberine, may prove to be especially useful. Berberine has
previously been shown to be useful for the mitigation of a plethora of viral
infections via exerting potent anti-inflammatory and immunomodulatory effects
[28], and it is tentatively suggested that berberine may exert similar potent
effects on SARS-CoV-2 virus. Literature published on the potential therapeutic
effects of berberine against the dreaded disease COVID-19 is promising. For
example, in an in-silico study conducted by Narkhede and colleagues,
berberine was found to exert protective antiviral effects against COVID-19 [75].
In particular, berberine was found to be capable of exerting inhibitory effects
on main protease (M
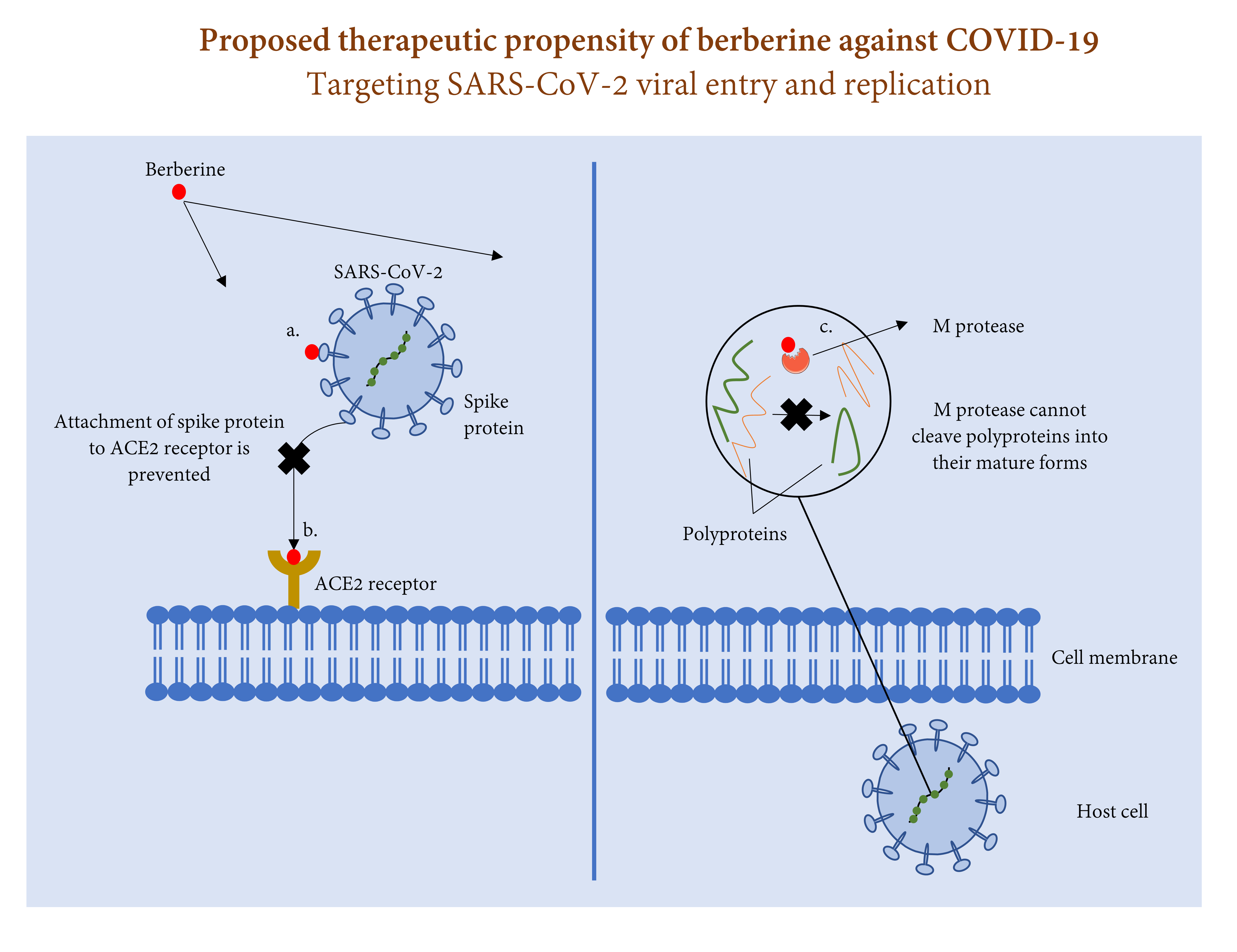 Fig. 1.
Fig. 1.Process of SARS-CoV-2 entry and replication in host cell and the therapeutic role of berberine against this process. Berberine may prevent SARS-CoV-2 entry to host cell via (a) attaching to spike protein, and/or (b) attaching to ACE2 receptor. Berberine may also halt SARS-CoV-2 replication via (c) attaching to M protease and preventing cleavage of polyproteins 1a and 1ab into their mature forms.
In another combined computational and in vitro study, Wang and
colleagues indicated the potential therapeutic mechanisms of berberine in
preventing and treating SARS-CoV-2 [70]. In particular, data from molecular
docking analysis identified berberine as an efficient inhibitor for multiple
SARS-CoV-2 protein targets, including host immune-related proteins, host
receptors, and virus proteins, which may aid in halting or suppressing
hyperinflammation, viral entry, and viral replication, respectively.
Specifically, berberine was found to exert moderate to strong binding affinities
(–6.4––9.8 Kcal/mol), with the strongest binding results obtained for MAPK-3
(binding affinity: –8.9 Kcal/mol) and MAPK-8 (binding affinity: –8.6 Kcal/mol)
for immune-related proteins, ACE2 (binding affinity: –9.8 Kcal/mol) for host
receptor proteins, and 3CLpro (binding affinity: –6.7 Kcal/mol) for viral
proteins. In vitro investigation from the same study further
substantiated the role of berberine as an efficacious drug against SARS-CoV-2. In
particular, treating SARS-CoV-2-infected Calu-3 cells with an immunotherapeutic
berberine nanomedicine molecule named NIT-X (20 and 40
Berberine as a natural agent possessing potent anti-inflammatory and immunomodulatory properties is very promising. This natural isoquinoline alkaloid has been shown to aid in the mitigation and/or treatment of various ailments including allergic and respiratory disorders. COVID-19 has emerged as a very serious threat to global health and economy. This respiratory pathogen is mainly characterized by excessive inflammation and immune dysregulations. The multiplicity of pathophysiological features induced by SARS-CoV-2 highlights the relevance of combination therapy for more effective protection against COVID-19. Thus, the use of berberine as an adjuvant therapy in addition to vaccines currently approved for use against the dreaded disease COVID-19 appears sensible. Berberine may benefit COVID-19 patients mainly by lowering inflammation and regulating immune responses. Precisely, berberine may help dampen cytokine storm, restore Th1/Th2 balance, and enhance cell-mediated immunity. Additionally, this critical natural compound may prove to be useful for modulating the levels of various other inflammatory mediators implicated in respiratory infections. Moreover, evidence from in silico and in vitro studies suggests berberine as a promising candidate for exerting inhibitory effects on three main SARS-CoV-2 targets, namely Mpro, ACE2 receptor, and S protein. As it stands, there is ample evidence supporting the role berberine may play in alleviating immune dysregulations and excessive inflammation in COVID-19. Yet, further well-planned in vivo and clinical studies are warranted for validation.
AFM, SMY, IAAY, and GKN performed the literature analysis and wrote the original draft of the article. AFM and SMY generated the tables and the figure. AFM, SMY, IAAY, and GKN critically reviewed and revised the final draft of the article.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest. GKN is serving as one of the Editorial Board of this journal. We declare that GKN had no involvement in the peer review of this article and has no access to information regarding its peer review.Full responsibility for the editorial process for this article was delegated to AA.

