1 Jiangsu Key Laboratory for Pharmacology and Safety Evaluation of Chinese Materia Medica, School of Pharmacy, Nanjing University of Chinese Medicine, 210023 Nanjing, Jiangsu, China
2 Jiangsu Joint International Research Laboratory of Chinese Medicine and Regenerative Medicine, Nanjing University of Chinese Medicine, 210023 Nanjing, Jiangsu, China
3 Jiangsu Collaborative Innovation Center of Traditional Chinese Medicine (TCM) Prevention and Treatment of Tumor, Nanjing University of Chinese Medicine, 210023 Nanjing, Jiangsu, China
4 School of Medicine & Holistic Integrative Medicine, Nanjing University of Chinese Medicine, 210023 Nanjing, Jiangsu, China
†These authors contributed equally.
Academic Editor: Josef Jampilek
Abstract
Background: The interactions between platelets and tumor cells are
well-known to play important roles in the progression of malignant tumors.
Danshensu, a main water-soluble component of Salvia miltiorrhiza, can
resist platelet aggregation and exert significant anti-tumor effects on various
types of tumors. However, whether Danshensu could inhibit the progression of
malignant tumors by suppressing the activities of platelets had not been
reported. Methods: The effects of Danshensu on the platelet activity and
epithelial-mesenchymal transformation (EMT)-like invasive phenotype of SW620
colon cancer cells were assessed by stimulating with the supernatants from
co-cultured platelets and SW620 cells with direct contact (SCP). The expression
and secretion of proteins were determined by western blot and enzyme-linked
immunosorbent assay (ELISA), respectively. Hematoxylin and eosin (H&E) staining
was performed to analyzed the histopathology of tumor tissues and
immunohistochemical staining was conducted to examine the protein expression in
tumors. Results: Co-incubation of SW620 cells with platelets directly or
SCP both generated long spindle-shaped invasive phenotype. Pretreatment of
platelets with Danshensu (25
Keywords
- platelet
- colon cancer
- Danshensu
- epithelial mesenchymal transformation
- chemoresistance
Platelets have been associated with the progression of numerous types of solid
tumors and poor prognosis in clinical practice [1]. Interestingly, there is a
pathogenic feedback loop between platelets and tumor cells. In other words, tumor
cells can activate platelets that play crucial roles in promoting the progression
of cancer, including tumor growth, angiogenesis and metastasis [2]. Besides,
there is a significant correlation between metastasis occurrence and the presence
of superabundant platelets, which has often been found in colon cancer [3]. Tumor
cells can activate platelets via a series of specific pathways involving integrin
Salvia miltiorrhiza has been employed to treat cardiovascular and
malignant diseases for a long time [9]. Danshensu is one of the main active
ingredients from Salvia miltiorrhiza that is traditional Chinese
medicine widely used for antiplatelet aggregation and anti-tumor therapy [10]. As
suggested by the screening of antiplatelet components from the aqueous extract of
S. miltiorrhiza, Danshensu could significantly reduce platelet
aggregation induced by multiple factors [11]. A large number of clinical and
preclinical studies demonstrated that platelets could promote the development of
tumor cells, and classic antiplatelet drugs including aspirin was able to
prohibit tumorigenesis and metastasis [12]. In addition, we previously reported
that Danshensu played an important role in inhibiting metastasis in spontaneous
and experimental melanoma metastasis model as well as in non-small cell lung
cancer models [13, 14]. Moreover, Danshensu suppressed the progression of
non-small cell lung cancer via inhibiting COX-2 activity and further attenuated
metastasis in vivo. Of note, Danshensu could directly exert its
inhibitory effect on tumor cell growth only at high concentration (100
Danshensu (PubChem CID: 439435) was purchased from Helin Biological Engineering Co., Ltd. (Xi’an, China). Oxaliplatin (OXA) were purchased from Aladdin Biological Technology Co., Ltd. (Shanghai, China).
SW620 human colon cancer cell line was purchased from Jiangyin Cambridge Biotechnology Co., Ltd. (Nanjing, China), and maintained in Dulbecco’s modified eagle medium (DMEM) (Invitrogen, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA).
Cell viability was determined by the MTT (3-
[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assays according to
the manufacturer’s protocol (n = 6). In short, SW620 cells (1
Platelet-rich plasma for research use only was provided by Jiangsu Province
Blood Center (Nanjing, China). Platelets were
prepared as described previously [15]. The platelets (2
SW620 cells were incubated with platelets, STP or SCP collected by centrifugation and filtration. The cells were maintained in the supernatants and observed every 1 h by IncuCyte ZOOM™ Live Content Imaging System (Essen BioScience Inc. (Essen) IncuCyte™, Michigan City, Indiana, USA).
The levels of VEGF, IL-6, IL-1
ATP levels in the supernatants were assessed using an ATP assay kit (Beyotime
Biotechnology, China) (n = 3). In brief, the platelets (2
24-well Transwell migration chambers with 8
The SW620 cells were cultured in DMEM with 10% FBS in the 6-well plates, and 500 SW620 cells were seeded into each well of the plates (n = 3). The plates were incubated at 37 °C for 14 days, and the colony formation of the cells was assessed using 0.1% crystal violet, after which the formed colonies were photographed (Leica Microsystems, Watzlar, Germany).
Total protein was extracted as previously reported [16]. Briefly, the total
protein was extracted using RIPA buffer containing 1 mM PMSF and 1:100 dilution
of protease inhibitor cocktail (BestBio, Shanghai, China). The total protein was
transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, USA) that
was then incubated with mouse anti-human E-cadherin, N-cadherin, vimentin,
TGF-
All tissue specimens were embedded in paraffin according to standard
histological procedures, sectioned (4
The whole blood sample was placed at room temperature for 2 h, centrifuged at 2500 R/min for 10 min under the condition of 4 °C, and the upper serum was separated. The full-automatic biochemical analyzer (Hitachi 7020) was used. After debugging, according to the glutamic oxaloacetic transaminase determination kit and alanine aminotransferase assay kit (Wako Pure Chemical Industries, Ltd, R1: el157; R2: el158; R1: ej715; R2: eh029) (n = 4).
All procedures were approved by the Institutional Animal Care and Use Committee
of Nanjing University of Chinese Medicine, and performed in accordance with the
use of laboratory animal guidelines of Nanjing University of Chinese Medicine
(Nanjing, China, Approval No. ACU170905). The SW620 cells were pre-incubated in
the SCP or the supernatants collected from co-cultured tumor cells and platelets
that treated with 25
The data were represented as mean
It has been well accepted that platelets can induce EMT-like transition and promote metastasis in vitro [17]. We observed the morphological changes of SW620 cells by IncuCyte Zoom, a real-time dynamic imaging system. SW620 cells underwent EMT-like morphological changes following the treatment of SCP, STP or platelets for 24 h (Fig. 1A). SW620 cells incubated with SCP had pronounced EMT-like phenotypic changes compared with those incubated with STP. Further, the expression of E-cadherin was down-regulated while the levels of N-cadherin and Vimentin in the SW620 cells were significantly up-regulated following the treatment of SCP for 24 h compare with control (Fig. 1B,C). These data indicated that SW620 exerted significant effects on activating secretion ability of platelets and platelet secretion stimulated the formation of an EMT-like invasive phenotype.
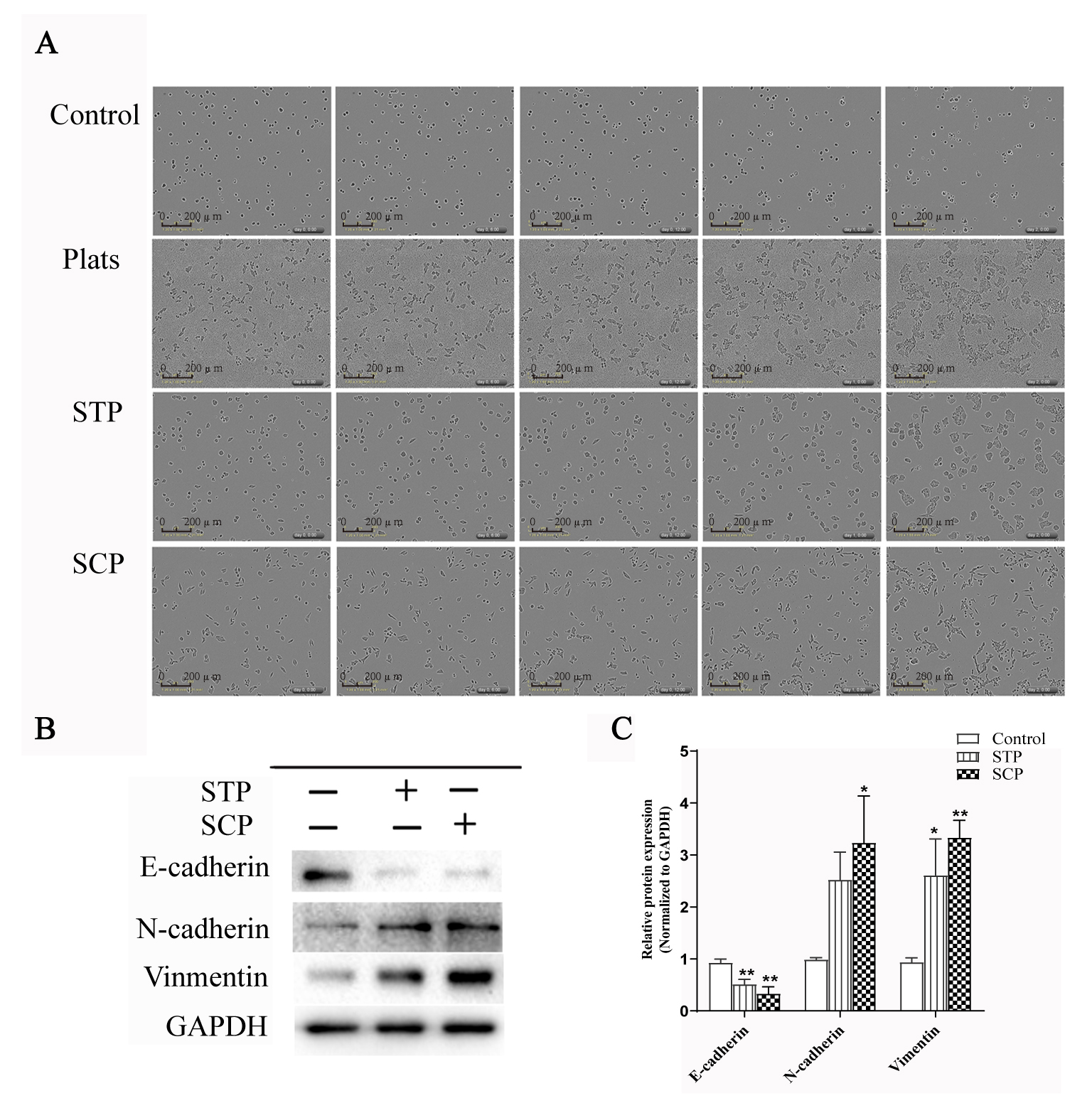 Fig. 1.
Fig. 1.SW620 cancer cells treated with SCP induced an EMT-Like invasive
phenotype. (A) Morphological changes of SW620 cancer cells incubated with
platelets, STP or SCP for 48 h. Scale bar indicates 200
Danshensu was reported to suppress platelet adhesion and aggregation by
inhibiting cyclooxygenase-2 (COX-2) and thromboxane B2 [10] (Fig. 2A). We first
explored the effects of Danshensu on the proliferation and membrane permeability
of SW620 cancer cells by MTT and LDH release
assays (Supplementary Fig. 2 and Fig. 2B). Strikingly, 25
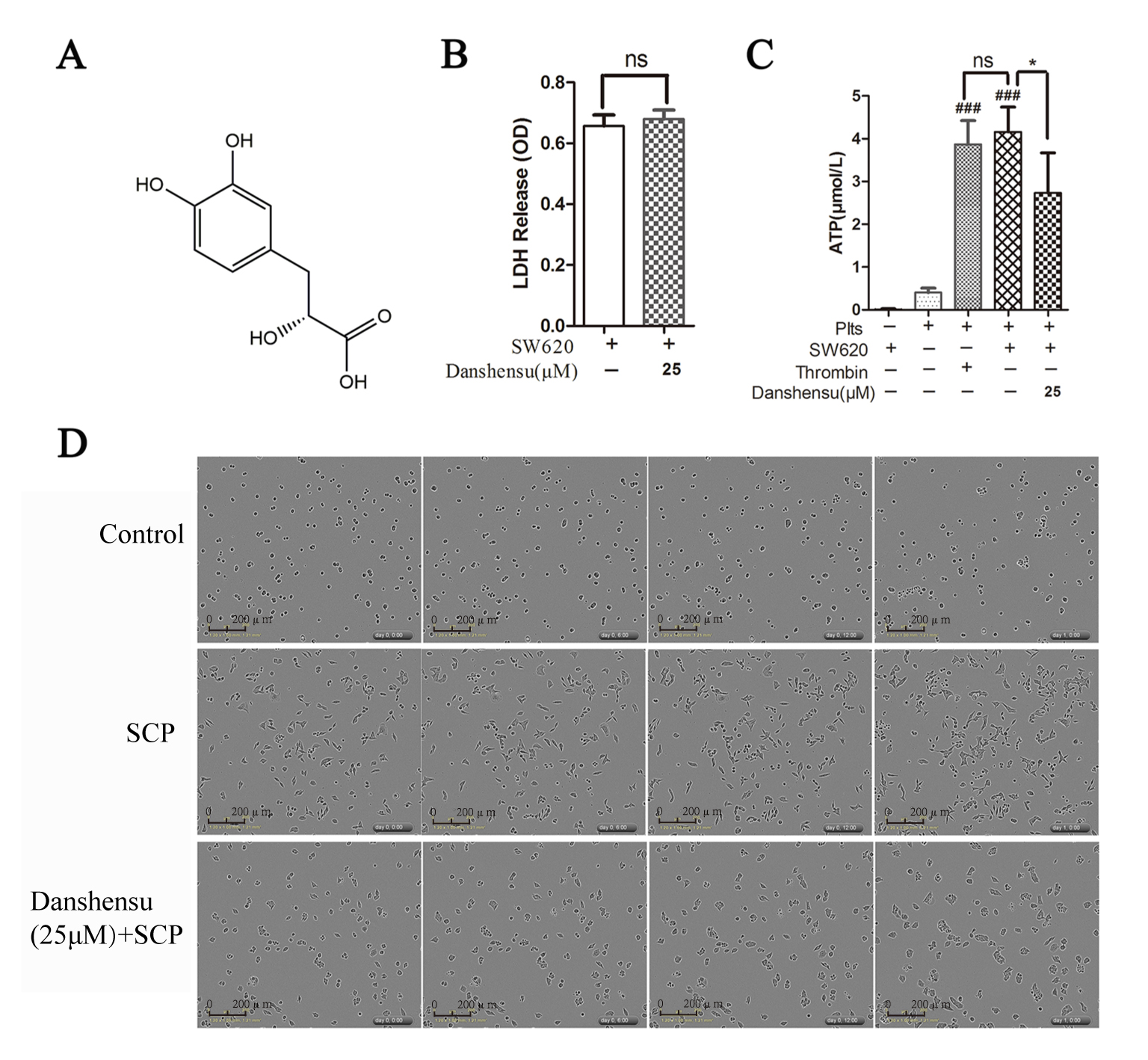 Fig. 2.
Fig. 2.Danshensu interfered with the interactions between platelets and
colon cancer cells. (A) The structure of Danshensu. (B) LDH release assay
showing the effects of Danshensu on the cytotoxicity of SW620 cells (n = 3). (C)
ATP levels in the supernatants were assessed using an ATP assay kit (n = 3). (D)
Microscopic images of the SW620 cells in the absence or presence of SCP. Data
were represented as mean
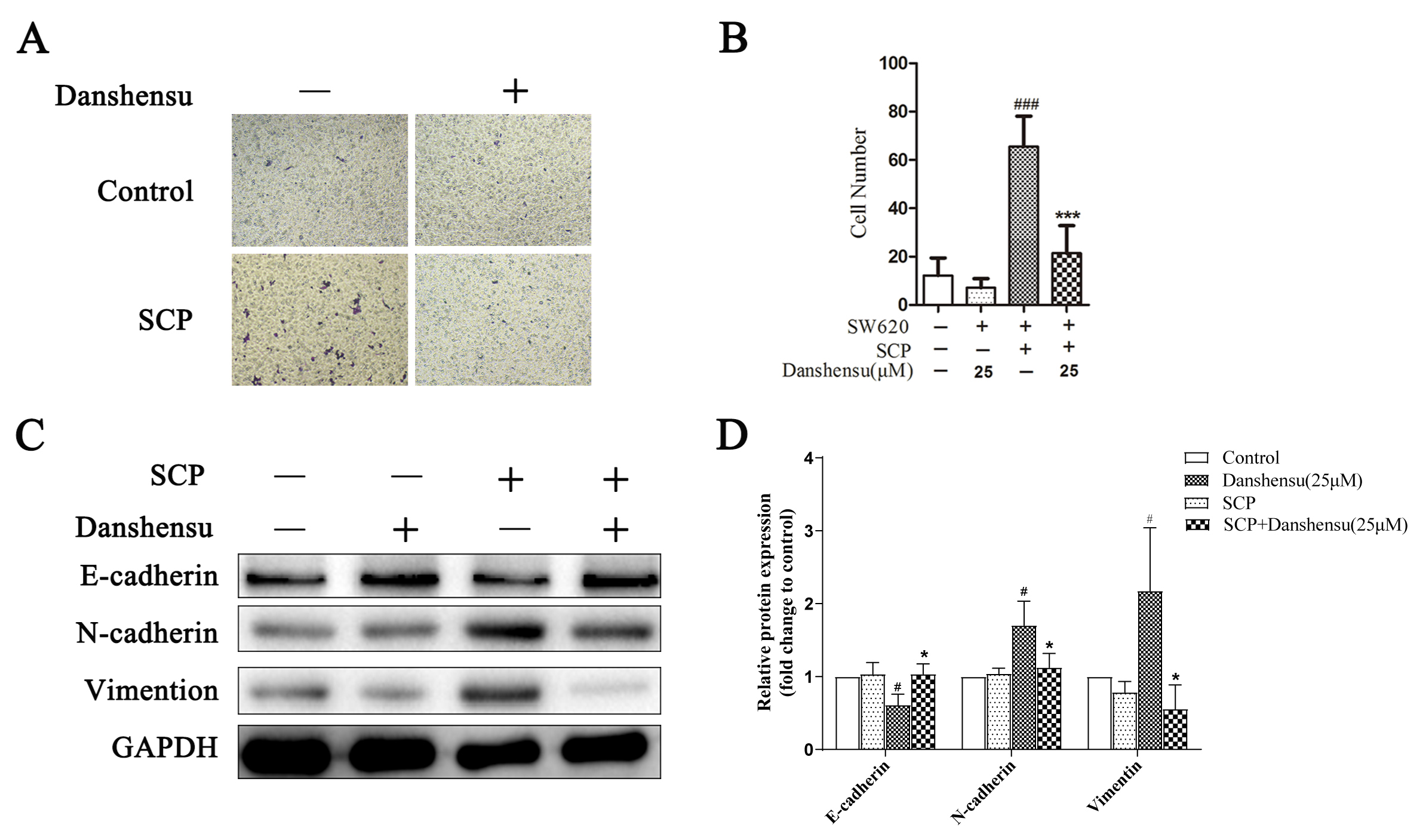 Fig. 3.
Fig. 3.Danshensu attenuated the migration of SW620 cells mediated by
platelets. (A,B) The effect of Danshensu on migration of SW620 cells using
Transwell. The results were represented as mean
Chemoresistance is a common problem in cancer treatment. Fischer et al.
[18] reported that EMT phenotype increased tumor cell chemoresistance, eventually
leading to tumor development and difficulty in cancer treatment. OXA is used for
colorectal cancer treatment, but the development of chemoresistance is inevitable
in clinical practice [19]. Drug efflux protein MDR1 is a main marker for the drug
chemoresistance in the colorectal cancer cells [20]. Therefore, we examined the
role of SCP in the chemoresistance of SW620 cells. Compared with the control
group, SCP group significantly increased the clonogenic survival of SW620 cancer
cells. However, prophylactic administration of Danshensu onto platelets
significantly decreased the clonogenic survival (p
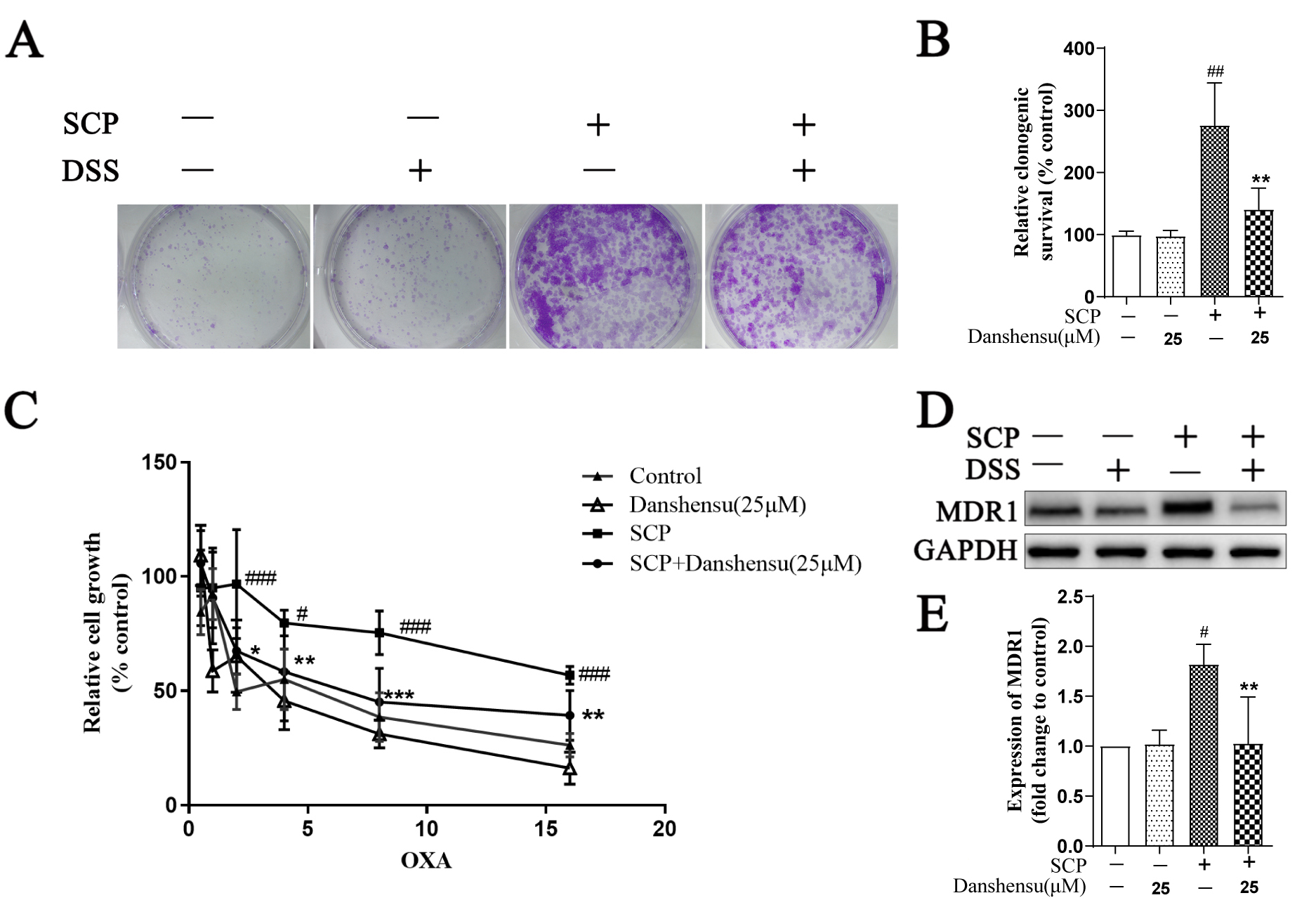 Fig. 4.
Fig. 4.Danshensu diminished SCP-induced clonogenic survival and
chemoresistance. (A,B) The effect of Danshensu on SW620 cell survival using
colony formation assay (n = 3). (C) MTT assay showing the chemoresistances of
SW620 cells to OXA following the stimulation of SCP (n = 6). (D,E) Western
blotting analysis of MDR1 expression level in the SW620 cells, GAPDH was used as
a loading control (n = 3). Data were represented as mean
It has been known that platelets contain numerous growth factors and cytokines,
and the secretions of co-cultured tumor cells and platelets play a crucial role
in the process of EMT. Notably, we previously reported that a variety of secreted
factors from platelets were involved in the malignant progression of tumor cells
[15]. In the present study, we examined the
levels of various cytokines in the
supernatants by ELISA assay. Our results demonstrated that the levels of
IL-6, TNF-
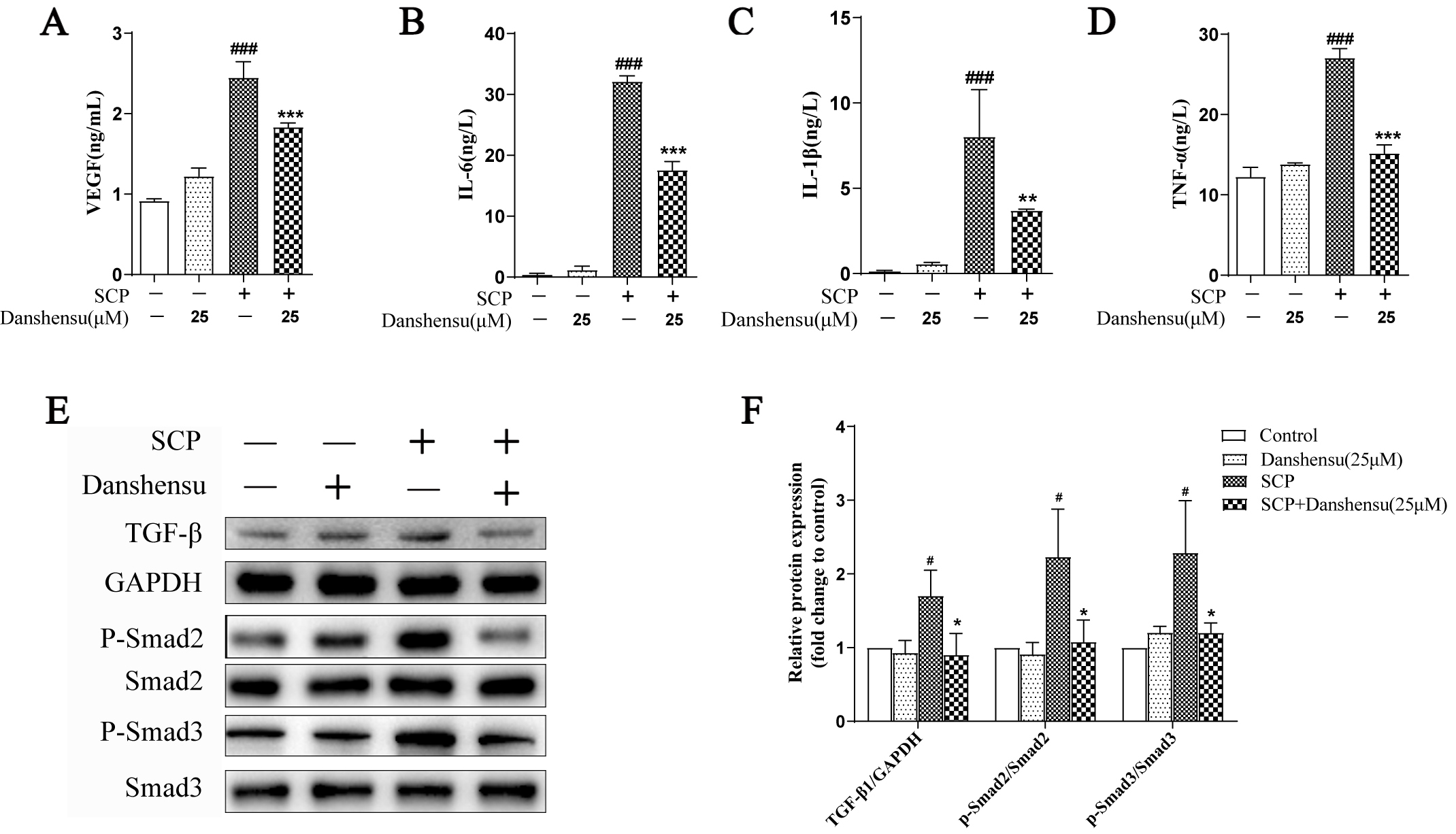 Fig. 5.
Fig. 5.Danshensu modulated the cytokine secretion from platelets
induced by SW620 cancer cells. (A–D) The levels of VEGF (A), IL-6 (B),
IL-1
To demonstrate the role of Danshensu in the progression of SW620 tumors
in vivo, SCP-stimulated SW620 cancer cells were subcutaneously injected
into mice, and the growth of tumors after intraperitoneal administration of
oxaliplatin (OXA) was monitored accordingly
(Fig. 6A). It was observed that SCP-stimulated SW620 cancer cells were
insensitive to OXA and the volume of SCP-stimulated tumors were significantly
increased compared to that of the control group (Fig. 6B). However, prophylactic
administration of Danshensu boosted the sensitivity of SW620 cancer cells to OXA,
and the tumor volume was strikingly decreased following the intervention of
Danshensu compared with SCP alone (p
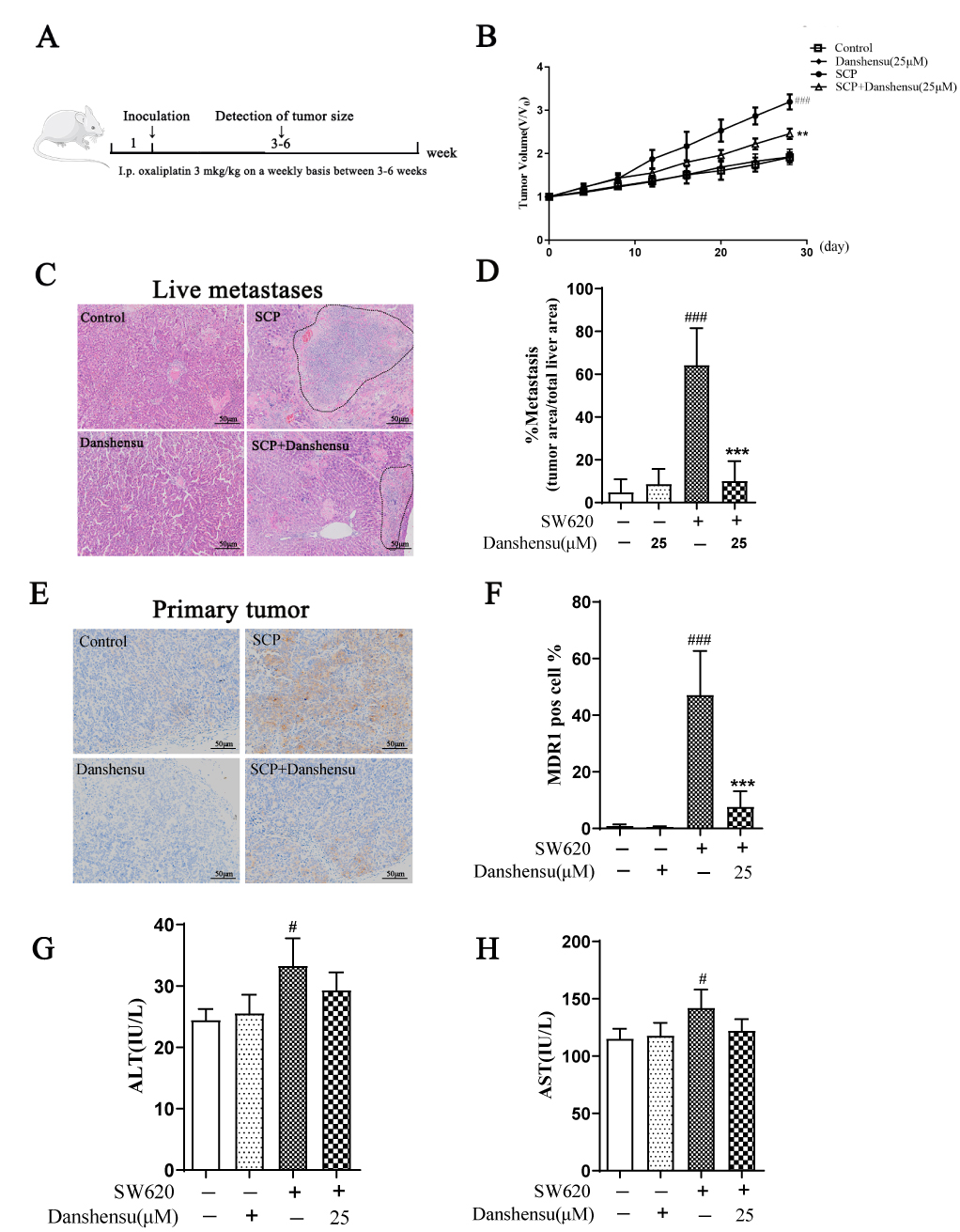 Fig. 6.
Fig. 6.Danshensu retarded the progression of SCP-stimulated SW620
tumors in vivo. (A) Schematic diagram of animal experimental procedure.
(B) Growth curve of SW620 tumors following different treatments (n = 4). (C) H&E
staining of the livers. (D) Quantification of liver metastasis (n = 4). (E)
Immunohistochemical staining of MDR1 expression in the primary tumors (n = 4).
Scale bar indicates 50
Platelets are small, enucleated cells which are abundant in blood, participating
in various physiological and pathological processes of organisms, such as
hemostasis, wound healing, inflammatory response, thrombosis, organ transplant
rejection and tumor development [21]. Platelet granules, including
As an aromatic carboxylic acid, Danshensu is one of the most abundant active phenolic acids in the dried root of S. miltiorrhiza Bunge (Lamiaceae), which is a widely used traditional Chinese medicine. The effects of Danshensu on platelet aggregation and thrombosis have been well documented [10], and it exerts potential antitumor and anti-angiogenic effects by inhibiting platelet adhesion and promoting tumor microcirculation [13, 24]. Our group also demonstrated the inhibitory effects of multiple components from S. miltiorrhiza on tumor progression [25]. More specifically, Danshensu significantly prevented tumor metastasis in vivo [14]. Although Danshensu presented remarkable antiplatelet and antitumor effects, whether it was involved in platelet-mediated development of malignant tumors still required in-depth studies.
In the present study, we found that Danshensu inhibited the effects of SCP on
the EMT and migration of tumor cells by intervening with platelets (Fig. 3), and
the secretions of VEGF, IL-6, IL-
It was reported that EMT enhanced the
chemoresistance of tumor cells [18]. The
lever of TGF-
Aspirin is currently used in clinical tumor prevention and incorporated into clinical oncology guidelines due to its striking antiplatelet activity. Moreover, it was also documented to inhibit platelet-induced EMT of circulating tumor cells [29]. Since aspirin could significantly inhibit the malignant development of colon cancer in the case of long-term and low-dose administration, antiplatelet activity was thought to be extremely important for the adjuvant treatment of malignant tumor development [30]. Targeted inhibition of platelet activity has certain inhibitory effects on the EMT and chemoresistance of tumor cells [31, 32].
In our study, although 25
In conclusion, our study showed that Danshensu resulted in the reduced
production of a series of pro-inflammatory cytokines (e.g., IL-1
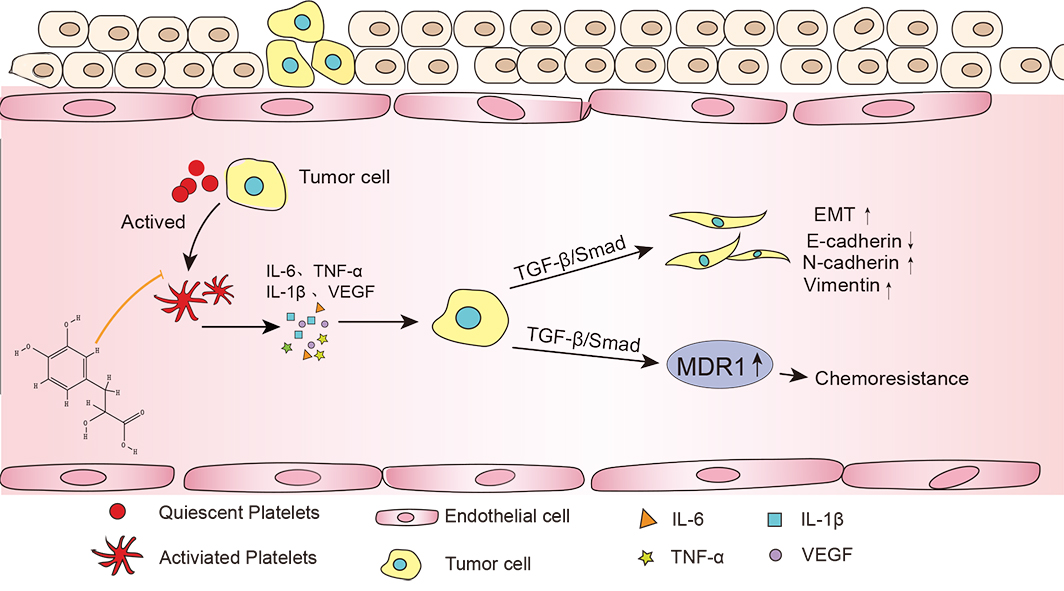 Fig. 7.
Fig. 7.Tanshinol reduced the interaction between platelets and tumor
cells and inhibits tumor metastasis. The interaction between tumor cells and
platelets can secrete a large number of cytokines (IL-6, IL-1
AW and YZ conceived, designed and led the project. YC, KL, YX and YW performed the experiments. YC and AW analyzed the data. AW contributed reagents, materials, and analysis tools. YZ and YC wrote the manuscript with input from all authors. All authors have read and approved the final manuscript.
All procedures were approved by the Institutional Animal Care and Use Committee of Nanjing University of Chinese Medicine, and performed in accordance with the use of laboratory animal guidelines of Nanjing University of Chinese Medicine.
The authors thank Yuanyuan Wu and Xiaoman Li contributed reagents, materials, and analysis tools.
This project was supported in part by National Natural Science Foundation of China (No. 81573859), China Postdoctoral Science Foundation (No. 2014M551639, No. 2016M601865), Natural Science Foundation of Higher School of Jiangsu Province (No. 17KJA360003, No. 18KJA360007), Postdoctoral funding in Jiangsu Province (No. 1401138C), Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (No. PPZY2015A070), and a project of the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), Jiangsu College graduate research and innovation projects (No. KYLX_0972).
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/j.fbl2705160.







