1 Institut de la Vision, Sorbonne Université, INSERM, CNRS, IHU Foresight, 75012 Paris, France
2 Quinze-Vingts National Ophthalmology Hospital, IHU Foresight, 75012 Paris, France
3 University of Versailles Saint Quentin en Yvelines, 78000 Versailles, France
4 Santen SAS, 91000 Evry, France
5 Toxicology Department, Faculty of Pharmaceutical and Biological Sciences, Paris Descartes University, 75006 Paris, France
Academic Editors: Shikun He and Graham Pawelec
Abstract
Background: Benzalkonium chloride (BAK)-containing antiglaucoma therapies alter the ocular surface over the long term. We used an in vitro scraping model to compare the effects of preserved and unpreserved topical commercial prostaglandins (PGs) in a wound-healing model. Methods: Standardized mechanical scraping was performed in confluent immortalized human corneal/conjunctival epithelial cell layers. Cytotoxicity, cell migration and proliferation, as well as the percentage of closure, were analyzed 2 h and 1/2/3/6 days after a 30-min exposure to 1/10 dilutions in phosphate buffered saline (PBS) used also as control, BAK solutions at concentrations ranging from 0.0001% to 0.1%, latanoprost-0.02%BAK, travoprost-0.015%BAK, bimatoprost-0.005%BAK, BAK-free Tafluprost, latanoprost in cationic emulsion, and travoprost (Polyquad® and SofZia®). Results: PG eyedrop preparations with BAK preservative delayed corneal healing, which is primarily related to the presence of BAK, in a dose-dependent manner, especially at day 1, as evidenced through actin disorganization and decreased Ki-67-positive cell numbers. The PGs (BAK-free tafluprost, latanoprost in cationic emulsion,travoprost (Polyquad® and SofZia®)) maintained a normal healing process with results similar to those of control. Conjunctiva-derived cell layers healed more slowly than corneal cell layers and were more sensitive in in vitro cytotoxicity tests. Conclusions: This novel in vitro scraping model mimics the damaged ocular surface epithelia observed in glaucoma patients affected by ocular surface disease, such as toxic-induced dry eye (TIDE) and offers a tool to assess the potential cytotoxic effects of PG formulations with or without BAK.
Keywords
- cornea
- conjunctiva
- wound-healing
- antiglaucoma prostaglandin
In addition to efficacy, safety and tolerance are the major criteria for evaluating new eye drops in preclinical and clinical trials, especially for antiglaucoma chronic treatments due to their repeated instillations over the long term [1, 2]. Classically, experimental toxicological models of tolerance and safety are carried out on a healthy ocular surface (OS). However, in real life, long-term use of BAK-preserved glaucoma therapies is associated with or may induce ocular surface disease (OSD). The overall incidence of glaucoma in patients with OSD was reported to be of 20%, with a prevalence of glaucoma of 66% in patients with severe OSD, as defined by Tsai et al. [3] such as limbal stem cell deficiency resulting the conjunctivalization of the corneal surface. OSD is more frequent in older, polymedicated patients presenting severe, hard-to-treat glaucoma [3, 4, 5]. The frequency of topical treatment changes consecutive to treatment intolerance and the degree of glaucoma severity were found in a recent observational survey to be positively correlated with the severity of OSD [6]. Therefore, there is a real need to develop safe and effective antiglaucoma therapies in order to avoid triggering or worsening OSD on the one hand and to alter glaucoma outcome on the other hand. Ideally, such therapy should also offer OS protective properties so that eventual pre-existing OSDs can be cured at the same time. Little knowledge is available on the influence of the antiglaucoma eyedrop formulations on an abnormal or already impaired OS. One in vivo study has reported the better effect on rat corneal healing of travoprost preserved with SofZia® compared to travoprost preserved with benzalkonium chloride (BAK) [7]. No in vitro corneal wound-healing model has been established for testing the impact of antiglaucoma ophthalmic solutions. Developing an in vitro model could be interesting to rapidly screen active antiglaucoma compounds and assess the impact of their formulations while respecting animal care ethics guidelines.
Wound healing is a complex dynamic process widely described in previous studies. Zhang et al. [8, 9], found using in vitro and in vivo models, a prolonged and strengthened inflammation and a raised reactive oxygen species production after benzalkonium bromide administration contributing to delayed skin wound healing. On the OS, in the cornea, limbus and conjunctiva, many mediators through complex pathways coordinate cell migration, proliferation and differentiation in order to repair the epithelial defect [10, 11, 12]. Understanding the corneal healing process is essential for refractive surgery, such as photorefractive keratectomy (PRK) or laser in-situ keratomileusis (LASIK). Conjunctival healing is also mandatory for glaucoma filtration surgery: it is the major factor contributing to its long-term efficacy. An insufficiently healed postoperative conjunctival wound is significantly correlated with thin-walled filtration blebs, wound leakage and infection reduces the surgical success rate [13, 14]. Furthermore, an excessive scarring process is also a major cause for filtration site fibrosis and surgical failure [15].
BAK, the most widely used preservative in ophthalmic aqueous solutions, induces pro-inflammatory and pro-apoptotic effects proportional to its concentration. BAK severely influences the wound-healing process: in 1985, Kossendrup et al. [16] showed that 0.01%BAK was able to delay corneal epithelial wound healing in rabbit and pig eyes. In rats, the cytotoxicity of BAK worsened a debrided corneal epithelium with a matrix metalloproteinase (MMP) increase [7, 17]. In a previous study, with in vivo and in vitro scraping models designed to investigate the negative impacts of BAK-containing solutions on damaged OS, we demonstrated that the two scraping models correlated well [18]. In addition, the in vitro model offered the advantages of simplicity and rapidity and limits the unnecessary use of animal models. In that regard, we used this in vitro model as a validated toxicological model to test the effect of cyclosporine (CsA) in different formulations, which perfectly matched the known cytotoxicity of certain solutions in established rabbit toxicity models [19].
Following two previous in vivo and in vitro studies describing the impact of ophthalmic solutions on an altered OS, we aimed to evaluate in this in vitro wound-healing model the cytotoxicity of seven commercial antiglaucoma prostaglandin (PG) formulations, with or without preservative (i.e., BAK), or with alternative preservatives. This study is also the first to investigate the impacts of such a large number of PGs in two different OS cell lines, namely human corneal epithelial cell (HCE) and human conjunctiva-derived epithelial cell (HCjE) lines.
Under standard culture conditions (humidified atmosphere of 5% CO
The Wong-Kilbourne derivative of Chang conjunctival cells (clone 1 to 5c-4l
American Type Culture Collection [ATCC, Manassas, VA]-certified cell line [CCL],
20.2) were cultured under standard conditions (humidified atmosphere of 5%
CO
At day zero (D0) a standardized wound was created by scraping an HCE cell monolayer with a sterile micropipette tip under an inverted microscope (Leica DMIRB; Heidelberg, Germany) equipped with a digital camera (Coolpix 5000, Nikon, Tokyo, Japan). The cells were then incubated for 30 min in 1:10 dilutions (in their corresponding cell culture medium) of the different eyedrop formulations and control as previously published [20]: phosphate buffered saline (PBS), BAK solutions from 0.0001% to 0.1%, 0.005% latanoprost-0.02%BAK (Xalatan®; Pfizer, New York, NY, USA), 0.004% travoprost-0.015%BAK (Travatan®; Alcon, Fort Worth, TX, USA), 0.03% bimatoprost-0.005%BAK (Lumigan®; Allergan, Irvine, CA, USA), BAK-free 0.0015% tafluprost (Santen SAS, Evry, France), 0.005% latanoprost in cationic emulsion (Catioprost®, Santen SAS), 0.004% travoprost-0.001% polyquaternium-1 (Polyquad®), and 0.004% travoprost-SofZia® (ion-buffered solution with borate, zinc and sorbitol (TravatanZ®, Alcon).
For better understanding, only these initial concentrations will be kept for the presentation of the results. Day zero (D0) was set at the beginning of the incubation period. After 30 min, the incubation medium containing the different test items was discarded, the cell culture plates washed to remove any cells in suspension and replaced with fresh cell culture medium. Cytotoxicity, cell migration and proliferation were analyzed 2 h (H) after the end of the incubation and on day (D) 1, 2, 3, 4, 5 and 6. Since epidermal growth factor (EGF) is known to be activated during the corneal epithelial wound-healing process and induces a strong stimulus on corneal epithelial cell proliferation and migration [21, 22, 23], EGF at 100 ng/mL was used as a positive control in this in vitro wound-healing model.
The scraping model in HCjE cells was performed using the same protocol as for HCE cells. Only a subset of the different PG eye drops was selected and evaluated in HCjE cells: PBS, PG formulated with the highest BAK concentration (latanoprost-0.02%BAK) with its corresponding 0.02%BAK control solution, and latanoprost in cationic emulsion. To objectively compare the corneal and conjunctival lines, the same operator analyzed the same cell passages from P10 to P15 for both cell lines.
At the different time points, a picture of each well was taken (at
For standard immunofluorescence staining, cells were cultured in chamber slides (Lab-Tek™) and were subjected to the aforementioned protocol: the cells were incubated for 30 min with the different test items diluted 1/10, washed with PBS and culture for an additional 24 h in fresh cell culture medium. The cells were then fixed in 4% paraformaldehyde (PFA). The images were analyzed with a confocal fluorescence microscope (E800, PCM 2000; Nikon, Tokyo, Japan) for proliferation and morphological patterns determination with Ki-67 and Alexa 488-conjugated phalloidin antibodies.
Only the cells treated with the different PG formulations were used for cell proliferation evaluation given that data with the 0.02%BAK solution were already published using this model [18]. Proliferating cells were identified by indirect immunohistology with Ki-67 (Nuclear Antigen Ki-67 Immunotech, Marseilles, France) as the primary antibody, and Alexa 488-conjugated goat anti-mouse (Molecular Probes, Invitrogen, Montluçon, France; in green) as the secondary antibody. Final staining with 4’,6-diamidino-2-phenylindole (DAPI) allowed for the visualization of the scraped area under the microscope. Ki-67-positive cells at D1 were counted in the superior, middle and inferior areas near the scraping edges and compared in the different conditions.
To evidence cytoskeleton alterations we used four different concentrations of BAK (0.0001, 0.001, 0.005 and 0.01%BAK). The other BAK concentrations and PG formulations showed similar or intermediate patterns. The morphological pattern was analyzed using a confocal fluorescence microscope (E800, PCM 2000; Nikon, Tokyo, Japan). Propidium iodide (PI) at the concentration of 1:10,000 in 1X PBS was added just before mounting to localize cell nuclei.
Statistical comparisons were performed using one-way analysis of variance
(ANOVA) followed by the Fisher test (Software ID). Statistical significance was
set at p
In PBS-incubated HCE cells (Fig. 1A), at D1 and D3, the percentage of closure
reached 53.0% and 89.0%, respectively. From D3 to D6, the scraped area was
almost covered with cells following PBS treatment, as for EGF treatment; however,
the latter presented a better healing rate than with PBS:
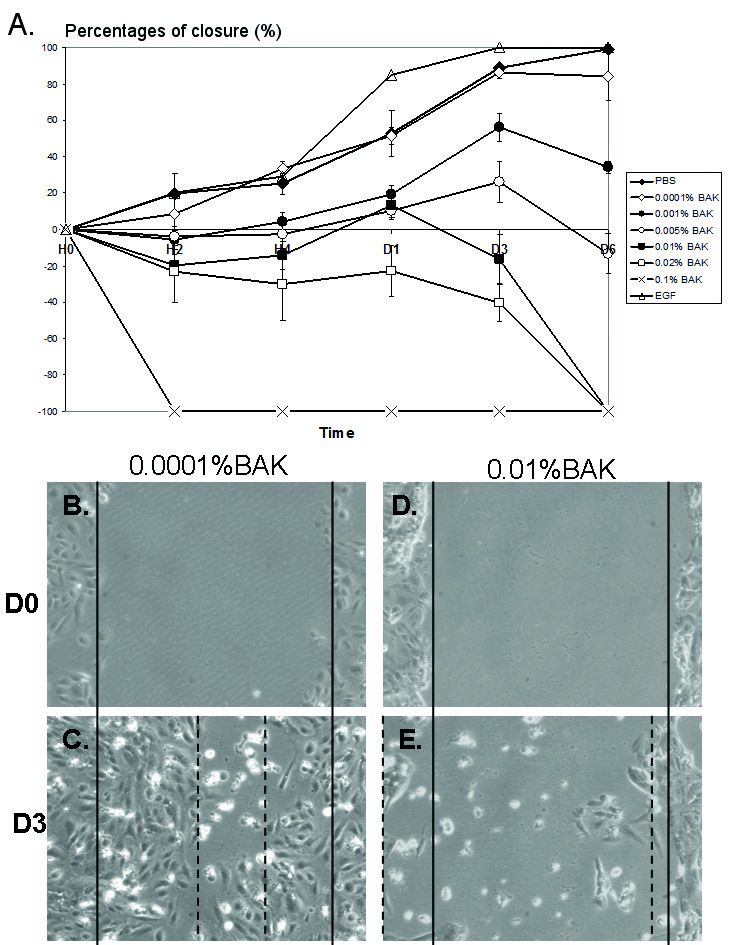 Fig. 1.
Fig. 1.Effects of different concentrations of BAK in the corneal
wound-healing model. Percentage of closure over 6 days (D) with different
concentrations of BAK ranging from 0.0001% to 0.1%, and EGF (100 ng/mL) as
positive control (A). BAK at 0.1% caused cell desquamation and no closure can be
measured (red asterisk). Pictures of scraped HCE cells at D0 (B and D) and D3
after treatment with 0.0001%BAK (C) and 0.01%BAK (E). Note: no clear healing
edges could be observed after incubation with 0.01%BAK. p
Pictures in Fig. 2 of the phalloidin staining showed F-actin fibers near the scraped area. At D1 for the 0.0001%BAK-treated cells (Fig. 2A), cells near the edges of the scraped area (identified as “scraping” on the images) presented a normal and regular actin cytoskeleton showing extension of actin fibers (arrowhead) towards the scraped area. BAK at the 0.001% (Fig. 2B) and 0.005% concentrations (Fig. 2C) presented moderate anisocytosis with shortened phalloidin-stained cytoskeleton marks. HCE cells treated with 0.01%BAK (Fig. 2D) showed cell loss with considerable anisocytosis. The phalloidin staining for F-actin fibers was less intense with no extension towards the scraped area. Cells also showed significant anisocytosis and the PI-stained nuclei were much smaller, with many nuclear bodies.
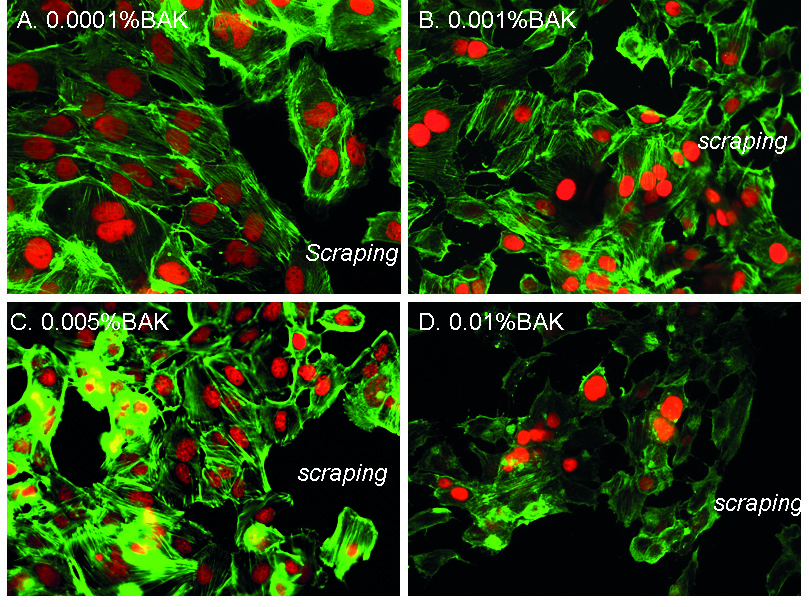 Fig. 2.
Fig. 2.F-actin fiber organization near the scraped area following
treatments with BAK solutions. Phalloidin immunohistology staining (in green) at
D1 after treatment with 0.0001%BAK (A), 0.001%BAK (B), 0.005%BAK (C) and
0.01%BAK (D). “Scraping” localizes the area where the cellular monolayer was
scraped. Nuclei are stained in red with propidium iodide (magnification
Following the characterization of the various BAK concentrations in the HCE scraping model, we assayed all the commercially available anti-glaucoma PGs over a 3-day time period.
The percentage of closure in the different conditions is presented in Fig. 3A. At D1, no migration to a very slight migration was observed for the all BAK-containing PG formulations. The other PG formulations (BAK-free Tafluprost/Catioprost®/travoprost (Polyquad® and SofZia®) already presented significant wound closure at D1. At D2, we found –4.3% for latanoprost-0.02%BAK, while the percentage of closure ranged from 5.0 to 20.0% for the other two BAK-containing PG formulations. In contrast, between D1 and D2 the percentage of wound closure increased steadily for the BAK-free Tafluprost/Catioprost®/travoprost (Polyquad® and SofZia® with a percentage of closure ranging from 45.0 to 56.0%. At D3, this closure ranging from 74.0 to 82.0% was observed, which was similar to the closure observed in the PBS group. Interestingly, BAK-containing PGs showed a better closure rate than the corresponding concentrations of BAK alone (Fig. 3B). Latanoprost-0.02%BAK presented a higher closure percentage than that of 0.02%BAK alone; and bimatoprost-0.005%BAK also had a better rate than 0.005%BAK alone.
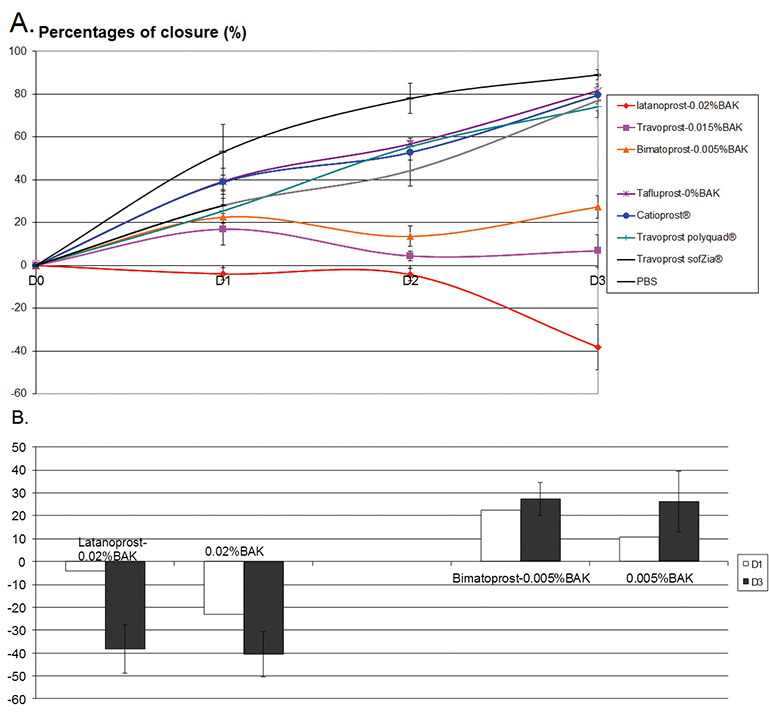 Fig. 3.
Fig. 3.Effects of PG formulations on corneal wound-healing model. (A) Percentage of closure over time (up to D3) following the treatment with the different PG formulations. (B) Comparison of two BAK-containing PG formulations with their corresponding BAK concentrations at D1 and D3.
Ki-67 was used to detect the proliferating cells at the edges of the scraped
areas at D1, as shown in Fig. 4A–H. Following PBS treatment, 666
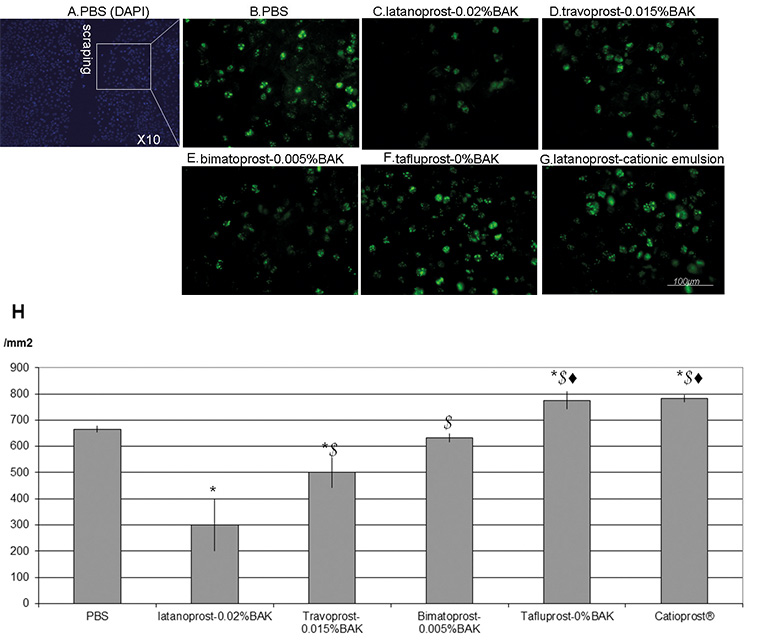 Fig. 4.
Fig. 4.Immunohistology of Ki-67 proliferating cells and cell counts at
D1. Immunohistology for the proliferation marker Ki-67 (in green) near the
scraped area (A) at day 1 (D1) after treatments with PBS (B),
latanoprost-0.02%BAK (C), travoprost-0.015%BAK (D), bimatoprost-0.005%BAK (E),
BAK-free Tafluprost (F), latanoprost in cationic emulsion (G) (bar = 100
HCjE cells were scraped according to the protocol used for the HCE scraping
model (Fig. 5) and treated with the PBS control to characterize the difference in
response, if any, between HCE and HCjE cells. At D0 following the scraping and
PBS treatment, both cell types presented similar patterns (Fig. 5A,B). However, a
delay in the closure process was observed with HCjE cells. At D5, while HCE cells
reached completed closure (
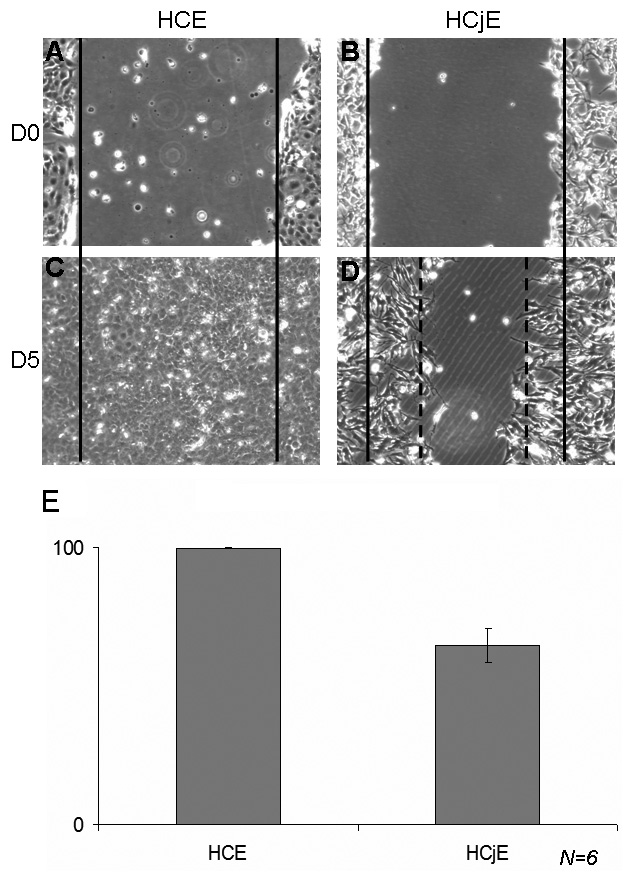 Fig. 5.
Fig. 5.Comparison of the scraping model with HCE and HCjE cells. Comparison of the normal healing process in human corneal epithelial cells (HCE) and human conjunctiva-derived epithelial cells (HCjE) at D0 (A for HCE, B for HCjE) and D5 (C for HCE, D for HCjE). While the HCE healing process was complete at D5 (C with 99% wound closure in E), HCjE cells reached only 64.60% closure at D5 (n = 6).
HCjE cells were scraped and treated with different BAK-containing and PG formulations, as in the HCE scraping model. The percentage of closure in Fig. 6. At D1 all the HCjE cells were floating with no identifiable edges and no measurable distance, clearly suggesting that the 0.02%BAK and latanoprost-0.02%BAK were more toxic for HCjE cells than for HCE cells. Interestingly, the closure process was much slower in HCjE cells: at D1, the percentage of closure was only 30.6% for PBS, 40.7% for latanoprost in cationic emulsion, without any significant difference between them. At D3, this percentage of closure was 36.1% for PBS and 49.3% for latanoprost in cationic emulsion, with no significant differences between them. From D4, the solutions became too toxic for the cultured cells, and no clear edges could be measured (data not shown).
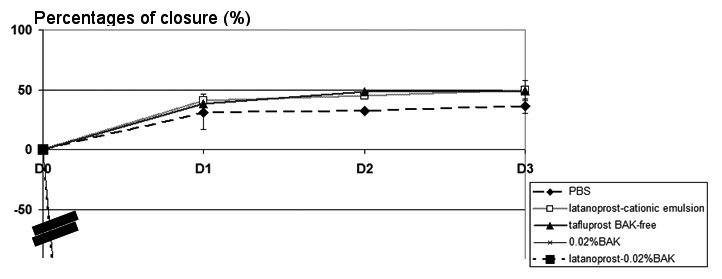 Fig. 6.
Fig. 6.Effects of PG formulations in the HCjE wound-healing model.
In this study we used an established in vitro wound-healing assay as a dynamic and relatively simple in vitro model to test eye drop PG formulations, not only their safety/cytotoxic profile but also their impact on the epithelial cell’s proliferative capabilities. In this study, we compared commercially available antiglaucoma PGs using the same model. Our results demonstrated that in the HCE cells: (1) BAK-containing PGs inhibited the healing process in a BAK concentration-related manner; (2) none of the BAK-free Talfuoprost/Catioprost®/travoprost (Polyquad® or SofZia®) altered the normal healing process without altering the capacity of two OS cells to multiply despite using different preservative systems; (3) the BAK inhibition and cytotoxic effects were associated with an alteration of F-actin fiber organization and the decrease or loss of proliferating Ki-67-positive cells; (4) BAK-containing PG formulations progressed faster than their corresponding concentration of BAK, suggesting a positive, protective effect of the PGs and/or formulation. These toxic effects of BAK seem to affect both cell survival and cell motility, as a possible consequence of a more general metabolic toxicity. BAK is known to inhibit the cornea’s healing due to its pro-apoptotic cytotoxicity, which has been largely demonstrated in the literature [1, 24, 25, 26, 27, 28]. Both conjunctival and corneal epithelial DNA synthesis was inhibited in a dose-dependent manner by a 15-min exposure to 0.001%, 0.01% or 0.1%BAK solutions [29]. In a 3D-reconstituted corneal epithelial model, expression of Ki-67 decreased in a BAK concentration-dependent manner, in accordance with the decreased cell viability observed by MTT tests and the increased apoptosis evidenced with the TUNEL assay [27, 28]. The BAK inhibited the cornea epithelium wound healing in a concentration-dependent manner. This inhibition started at H4, and increased after one day. Indeed, BAK is known to induce adverse effects on the ocular surface and is, at the same time, considered useful in promoting the permeation of active molecules. But, it is interesting to note, that, the formulation of prostaglandin analogs as an ester prodrug, with a high lipophilicity, further hydrolyzed by cellular esterases into the acid form of prostaglandin, allows a better absorption by the cornea [30]. Additionally, several studies comparing prostaglandin analogs, with or without BAK showed similar efficacy especially on lowering IOP [31, 32, 33] suggesting that a penetration enhancer might not be helpful.
BAK induces cell apoptosis, necrosis and inhibition of proliferation, but the
PGs seem to have a positive effect on the proliferation process. PGF2alpha
agonists have been shown to induce a significant stimulation of bovine corneal
endothelial cell (BCEC) proliferation [34]. Latanoprost and brimonidine increased
the number of proliferating subconjunctival fibroblasts [35]. In
latanoprost-incubated conjunctival specimens from glaucoma patients, the
upregulation of matrix MMPs, MMP-1 and MMP-3 reduced extracellular matrix
accumulation in the conjunctival stroma [36]. Daily treatments with exogenous
PGF2alpha agonist (
Two different epithelial cell types of the OS, namely corneal and conjunctiva-derived epithelial cells, were used to evaluate the effects of the PG analog formulations on their healing capacity.
Even though there is some discussion that HCjE was originally derived from Hela cells, HCjE remains an acceptable and simple tool for testing toxicity without biopsy or primary culture. Indeed, two conjunctival cell lines ‘IOBA-NHC’ and ‘Chang cells’ showed similar toxicological profiles in in vitro studies [38]. In the future, primary cultures of conjunctival epithelial cells could be used in this model. The conjunctival cells seemed to heal more slowly than corneal cells and are apparently more sensitive to BAK, with inhibitory effects seen at lower concentrations. Lin et al. [39] have shown that corneal epithelium cells expressed EGF receptor (EGFR), while only minimal to no staining for EGFR was observed in the superficial conjunctival and limbal epithelia. This reduced EGFR expression may explain the differences observed in the healing capacity between the two cell lines.
The safety and ocular tolerance of any eye drops are major criteria that are usually investigated on healthy ocular tissues via the Draize test [40] in animal models or during clinical trials that exclude severely afflicted patients, i.e., the patients whose disease severity is susceptible to leading to drug intolerance or to an increased patient dropout rate. Therefore, diseased preclinical models (in vitro and in vivo) might be of help [18, 41, 42]. The biological difference observed in the corneal/conjunctival wound-healing response results most probably from a difference in cytokine, growth factor , and chemokine expression, which would warrant further exploration and the development of appropriate models.
It is becoming increasingly evident that it will no longer be acceptable to lower elevated intraocular pressure (IOP) in glaucoma patients at the detriment of the OS, or without protecting the OS, while at the same time controlling elevated IOP. In that regard, cationic emulsion vehicles seemed to be the appropriate answer, because they have been shown to offer OS protective properties improving signs and symptoms of dry eye in clinical trials [43]. This innovative technology was used to develop a new latanoprost formulation: 0.005% latanoprost in cationic emulsion (Catioprost®) [44, 45]. These cationic emulsions used 0.005% cetalkonium chloride (CKC), a C16 alkyl derivative of BAK, for its cationic property and its binding capacity to the oil nanodroplets rather than for any preservative effect [18, 19, 46]. For antiglaucoma treatments, it is recommended to reduce the use of BAK in eye drops as much as possible or even avoid it, and avoid use of any other excipients which may be deleterious to the ocular surface on the long term [27, 41]. Therefore, removing BAK will allow for the exploration of other therapeutic strategies, such as goblet cell activation and growth factor stimulation, evidencing the possible beneficial effects of the PG analogs for the OS.
Using an in vitro scraping model aimed at mimicking a damaged OS and using two OS epithelial cell lines, we demonstrated that BAK-containing formulations delayed the wound-healing processes while the antiglaucoma active compounds maintain the healing capacity of the epithelial cells, and as such may offer a therapeutic interest for glaucoma patients already suffering from OS disorders. Outcomes of this in vitro study are in line with similar observations described in other preclinical models (in vivo, ex vivo). 2D and 3D reconstructed corneal epithelial models exposed to BAK solutions or BAK-containing prostaglandin formulations confirmed the toxicity through a delayed corneal healing [18, 27, 41, 42]. Furthermore, a BAK-free travoprost preparation showed positive effects on tear secretion and corneal protection [42]. The next steps for antiglaucoma treatments might be to target elevated IOP, OSD, and its associated inflammation, the latter of which, if left untreated, may lead to trabecular meshwork complications and antiglaucoma treatment failure. Therefore, for the greater benefit of glaucoma patients, the concomitant treatment of ocular inflammation and elevated IOP might be of great value.
HL—Design, experiments and main manuscript writing; CB, FBB—Design and manuscript correction; PD, JSG—Figures and manuscript correction.
Not applicable.
Not applicable.
Financial support: Unrestricted grants from Quinze-Vingts National Ophthalmology Hospital, Vision Institute, INSERM UMR S968, Paris, France and Santen SAS, Evry France.
The authors declare no conflict of interest.






