1 Department of Hepatobiliary and Pancreatic Surgery, The First Affiliated Hospital, Zhejiang University School of Medicine, 310000 Hangzhou, Zhejiang, China
2 Zhejiang Provincial Key Laboratory of Pancreatic Disease, The First Affiliated Hospital, Zhejiang University School of Medicine, 310000 Hangzhou, Zhejiang, China
3 Zhejiang University Cancer Center, Zhejiang University, 310000 Hangzhou, Zhejiang, China
4 Department of Gastroenterology, The First Affiliated Hospital, Zhejiang University School of Medicine, 310000 Hangzhou, Zhejiang, China
5 Department of Medical Oncology, The First Affiliated Hospital, Zhejiang University School of Medicine, 310000 Hangzhou, Zhejiang, China
6 College of Energy Engineering and State Key Laboratory of Fluid Power and Mechatronic Systems Zhejiang University, 310027 Hangzhou, Zhejiang, China
†These authors contributed equally.
Academic Editors: Yoshikatsu Koga, Kenji Takashima and Takahiro Anzai
Abstract
Hypoxia is a typical characteristic of most solid malignancies, which has multiple effects on malignant phenotypes and biological behaviors of tumors including epithelial-mesenchymal-transition (EMT), invasion, migration, metastasis, autophagy, stem cell maintenance, pathological angiogenesis, drug resistance, and immunosuppression. Rcentlyumoand reversing the tumor hypoxic environment via nanotechnology has emerged as a novel therapeutic approach for the treatment of malignancies. The main strategies related to nanotechnology to alleviate or ameliorate hypoxic environment are as follows: (1) Bringing external oxygen to tumor hypoxic microenvironment; (2) Generating oxygen based on nanotechnology in situ; (3) Regulating the structure of the tumor microenvironment; (4) Decreasing oxygen consumption in the tumor microenvironment. In this review, we will discuss these nanotechnologies in detail.
Keywords
- nanotechnology
- tumor microenvironment
- hypoxia
- metal-organic frameworks
- photodynamic therapy
Most solid malignancies are characterized by hypoxic tumor microenvironment (TME) due to the malformed blood vessel that cannot provide adequate oxygen or the imbalance between oxygen support and consumption in tumor cells [1, 2, 3, 4, 5, 6]. Intratumoral hypoxia leads to increased activity of hypoxia-inducible factors (HIFs), which plays an important role in tumor progression and affect malignant tumor hallmarks including but not limited to cell proliferation, differentiation, apoptosis, genetic instability, tumor metabolism, vascularization/angiogenesis, immunosuppression, and metastasis [7]. In addition, tumor hypoxic microenvironment is a significant obstacle to oxygen-dependent cancer therapy such as radiotherapy, chemotherapy, photodynamic therapy (PDT), immunotherapy, and so on [8].
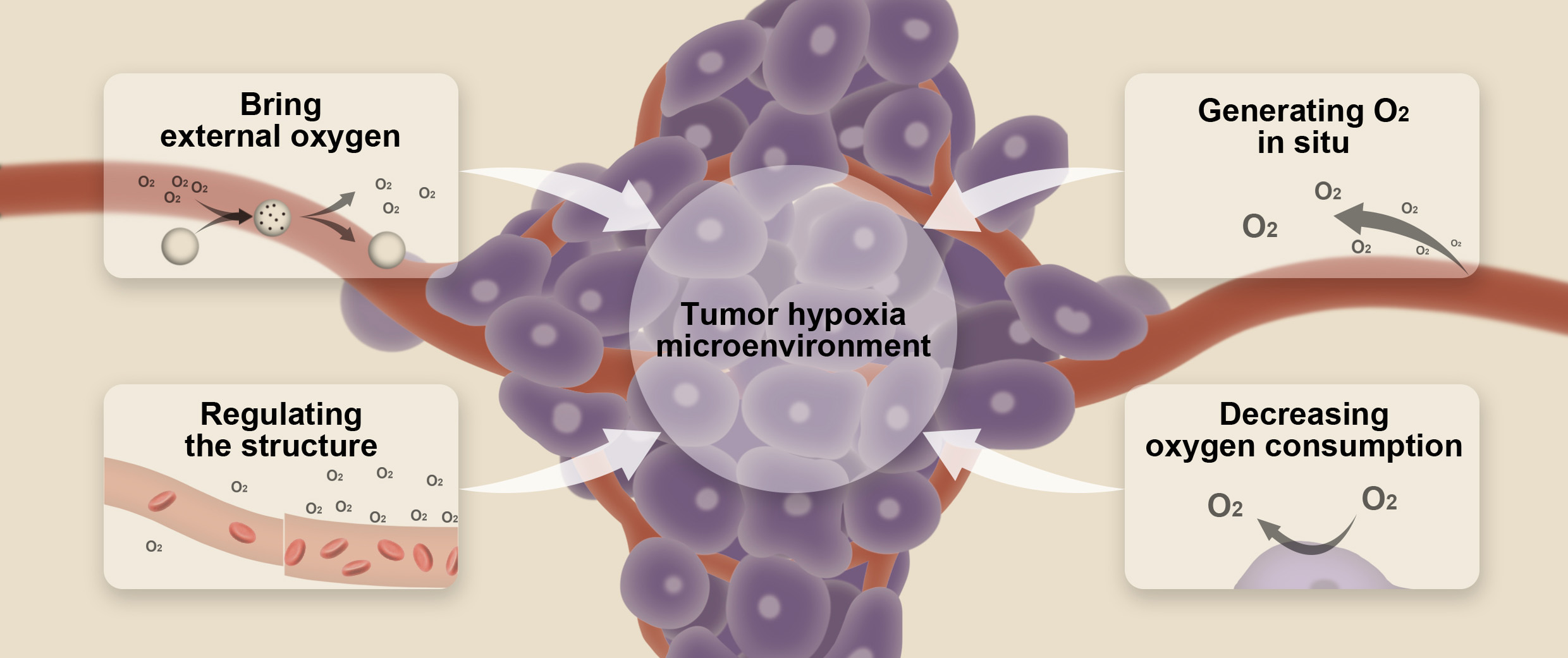
Overall, hypoxia is regarded as a critical role in tumor progression and resistance to tumor therapy, which makes it a novel target for cancer therapy [9]. Recently, many researchers have tried to target hypoxia TME via nanotechnology. In this review, we focused on the recent advancements of nanotechnology-based strategies for overcoming hypoxia in TME. The main strategies related to nanotechnology to alleviate or ameliorate hypoxic environment are as follows: (1) Bringing external oxygen to tumor hypoxia microenvironment; (2) Generating oxygen based on nanotechnology in situ; (3) Regulating the structure of the TME; (4) Decreasing consumption of oxygen in the TME (Fig. 1). These strategies are shown in Table 1 (Ref. [10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35] and will be discussed in detail in the following sections.
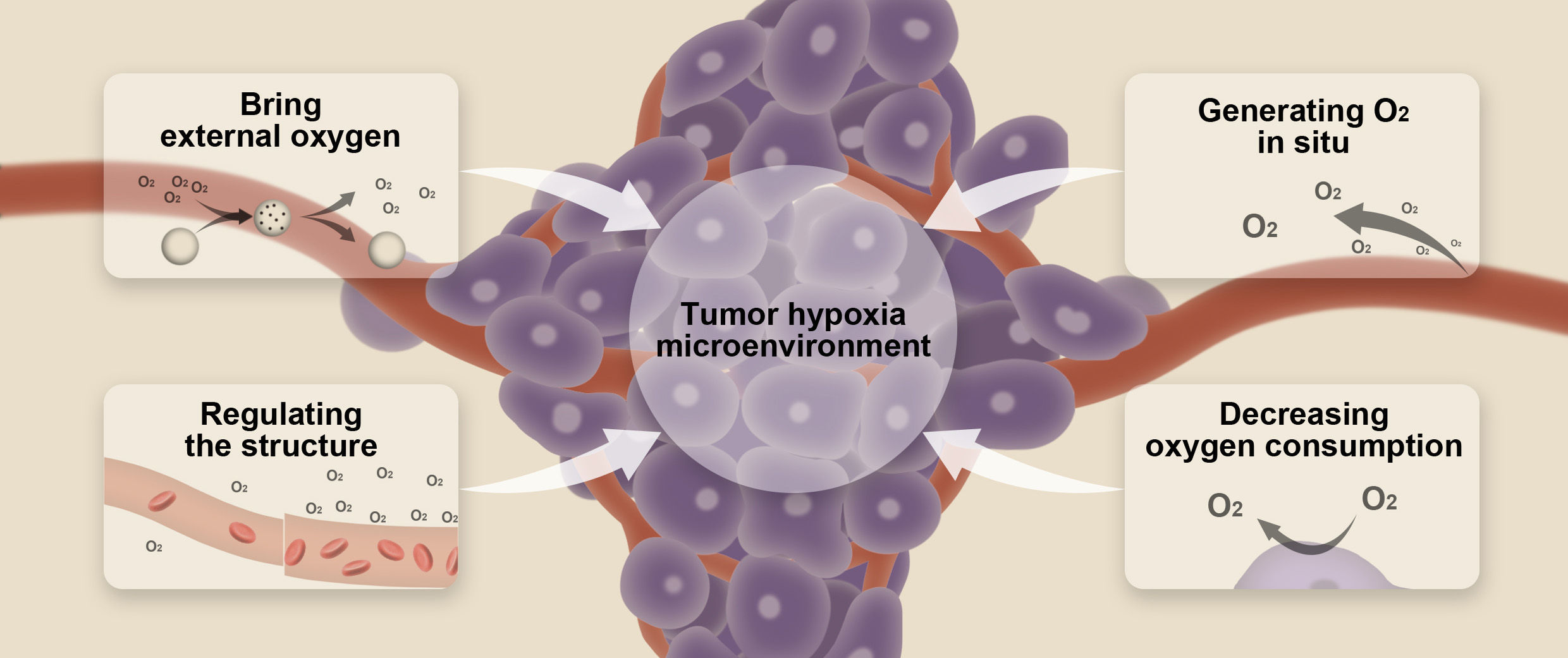 Fig. 1.
Fig. 1.Schematic illustration of the strategies based on nanotechnology for overcoming tumor hypoxia microenvironment.
| Strategy | Nanoparticles | Tumor model (cell line) | References |
| Bring external oxygen to tumor hypoxia microenvironment | Oxy-PDT | Colon carcinoma (CT26) | [10] |
| HMSNs-GOx-Ce6@CPPO-PFC/O |
Melanoma lung metastasis (B16 F10) | [11] | |
| PFC@PLGA-RBCM | Breast carcinoma (4T1) | [12] | |
| IPH@RBC | Colon carcinoma (CT26) | [13] | |
| PCN-224-Pt | Liver carcinoma (H22) | [14] | |
| O |
Breast carcinoma (MCF-7) | [15] | |
| IR780@O |
Breast carcinoma (4T1) | [16] | |
| ZDZP@PDA–PEG | Breast carcinoma (4T1) | [17] | |
| Polydopamine-nanoparticle-stabilized oxygen microcapsules | Pancreatic carcinoma (KPC) | [18] | |
| Generating O |
LipoMB/CaO |
Breast carcinoma (4T1) | [19] |
| A-MnO |
Breast carcinoma (EMT6) | [20] | |
| BSA-MnO |
Esophageal carcinoma (KYSE 30) | [21] | |
| HSA-MnO |
Breast carcinoma (4T1) | [22] | |
| Fuco-MnO₂ | Pancreatic carcinoma (BxPC-3) | [23] | |
| CAT@Pt (IV)-liposome | Breast carcinoma (4T1) | [24] | |
| HA-CAT@aCe6 | Breast carcinoma (MDA-MB-231) | [25] | |
| mCMSNs | Breast carcinoma (MCF-7) | [26] | |
| PDA-Pt-CD@RuFc | Breast carcinoma (4T1) | [27] | |
| PCCN | Breast carcinoma (4T1) | [28] | |
| FA-WN-Ce6 | Colon carcinoma (CT26) | [29] | |
| Regulating the structure of TME | SPION@hMSN | Breast carcinoma (4T1) | [30] |
| Breast carcinoma (MCF-7) | [31] | ||
| DiR-hCe6-liposome | Breast carcinoma (4T1) | [32] | |
| Decreasing oxygen consumption in the TME | PV-TS | Breast carcinoma (4T1) | [33] |
| Mito-OxE | Breast carcinoma (4T1) | [34] | |
| SORgenTAM | Breast carcinoma (4T1) | [35] |
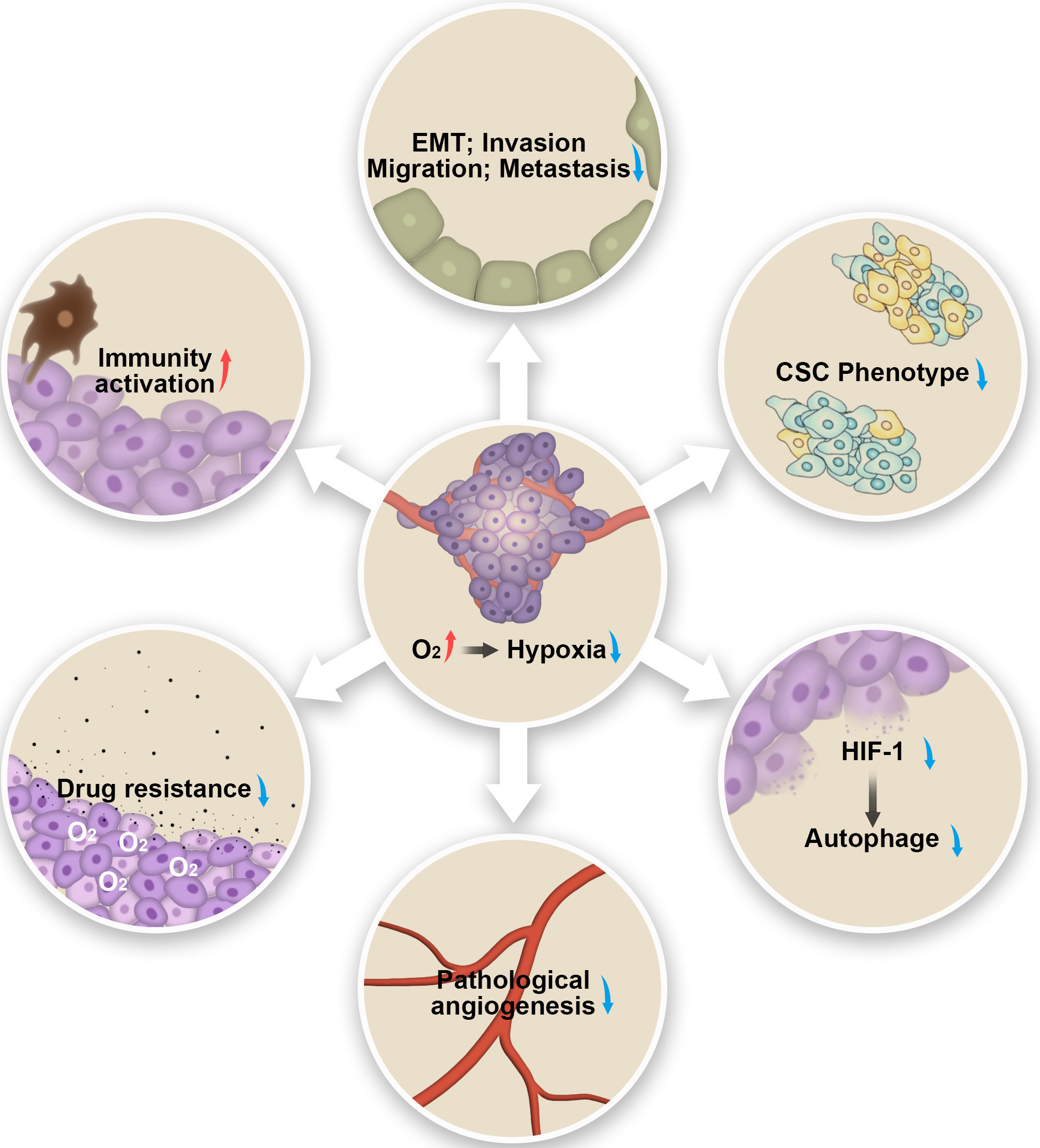
The hypoxic condition has pleiotropic effects on malignancy phenotypes and
biological behaviors of tumors including such as EMT, invasion, migration,
metastasis, cancer stem cell maintenance, autophagy, pathological angiogenesis,
drug resistance, and immunosuppression [36, 37, 38, 39, 40]. For example, Hypoxia
plays an important role in causing the EMT, invasion, migration, and metastasis
of pancreatic ductal adenocarcinoma (PDAC) [37]. Tumor cells acquire and maintain
cancer stem cell (CSC) phenotype under hypoxic conditions, which has increased
self-renewal and invasion potential [41, 42]. Autophagy is associated with
cellular response to stresses such as hypoxia in the TME [43, 44, 45, 46]. Evidence
suggested that enhanced autophagy promoted tumor survival [45]. Angiogenesis is
another important component in the TME under hypoxic conditions [47], and
HIF-1
Delivering oxygen to a hypoxic microenvironment by nanomaterials is the most common method to alleviate tumor hypoxia. Recently, various nanotechnology-based methods have been developed to bring external oxygen to the tumor hypoxic microenvironment.
2.2.1.1 Artificial Red Blood Cells Substitutes (RBCSs)
The most important function of natural Red Blood Cells (RBCs) is to transfer
oxygen (O
Many researchers are devoted to assembling semi-synthetic RBCs with Hb as the
oxygen carrier, which are called Hb-based oxygen carriers (HBOCs) (Fig. 2i).
There are several different HBOC systems such as cell-free Hb [55, 56] and
particle-encapsulated Hb [57, 58, 59]. Cell-free Hb has some disadvantages including
short circulation time, poor stability, and potential side effects, due to the
dissociation of Hb tetramer, binding to plasma haptoglobin, and clearance by
liver, kidney, and spleen [60, 61]. By chemical modification or encapsulation
with biodegradable materials, Hb-based O
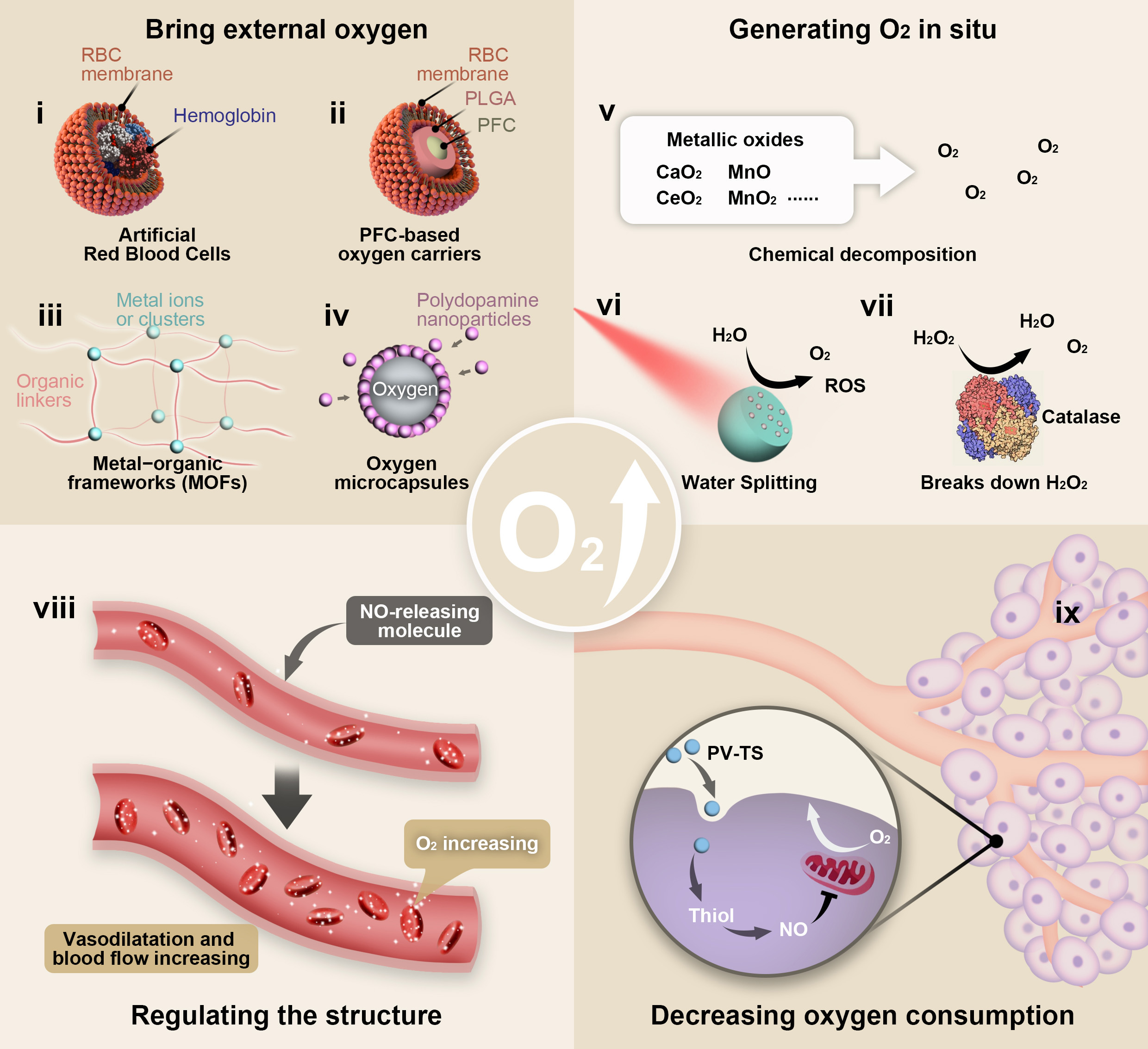
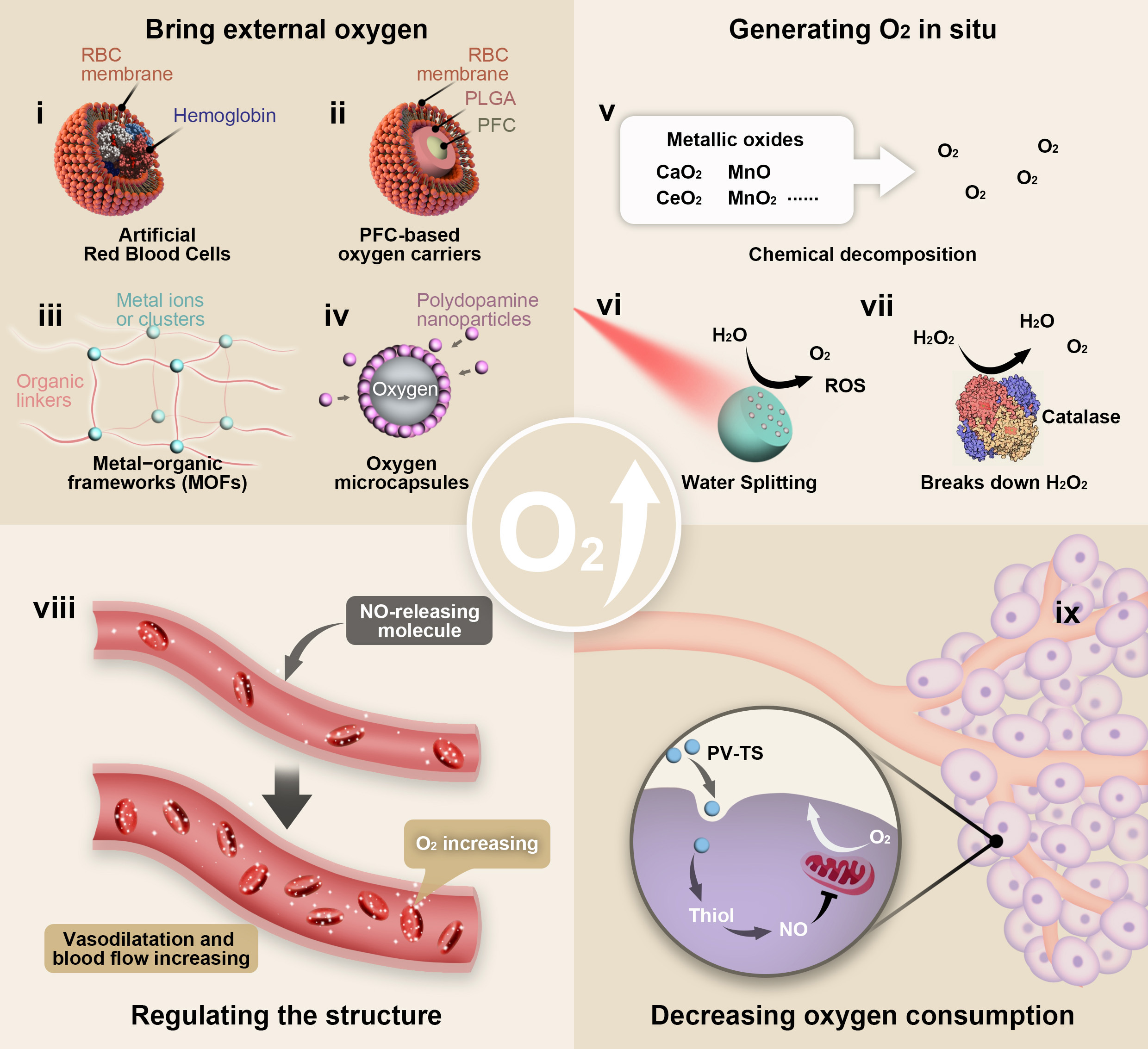 Fig. 2.
Fig. 2.Schematic illustration of the strategies based on nanotechnology
for overcoming tumor hypoxia microenvironment. (i) The morphology and structure
of artificial RBCs. (ii) The morphology and structure of PFC-based oxygen
carriers. PFC was wrapped in PLGA shell and then coated with RBCM. (iii) The
illustration of construction of a MOF and its building components. (iv) The
morphology and structure of polydopamine-nanoparticle-stabilized oxygen
microcapsules. (v) The illustration of Chemical decomposition like metallic
oxides producing oxygen. (vi) The schematic diagram of water-splitting strategy
breaks down water to produce oxygen. (vii) The schematic diagram of catalase
breaks down H
Compared with natural RBCs, Hb-based O
2.2.1.2 PFC-Based Oxygen Carriers (PFOCs) and Fe
To alleviate the disadvantages of HBOCs, many investigators have focused on synthetic molecules to replace the function of natural Hb. Perfluorocarbons (PFCs) have a great potential to carry gases and are used as oxygen carriers due to their stability and biosafety [10, 11].
PFCs are inert liquids, and the shape can be cyclic or linear so that PFCs can
easily dissolve tremendous amounts of O
Because of the rapid growth of the tumor, the center of the tumor is often hypoxic, which prohibits the production of reactive oxygen species (ROS) and leads to the tolerance of tumor cells to radiotherapy [70, 71]. PFOCs can promote oxygen concentration at the hypoxic site of the tumor to increase the sensitivity of tumor cells to radiotherapy [8, 72, 73]. For example, Gao et al. [12] reported that biocompatible PFC@poly (d,l-lactide-co-glycolide) (PLGA) nanoparticles, which were further coated with a red-blood-cell membrane (RBCM) to create PFC@PLGA-RBCM nanoparticles (Fig. 2ii).
PFC@PLGA-RBCM nanoparticles can availably deliver oxygen to the oxygen-starved interior of the tumor, which greatly relieves tumor hypoxia and thus enhance the treatment efficacy of radiotherapy [12, 74]. Furthermore, PFOCs can be used in enhancing the efficacy of PDT and chemotherapy. Although PFOCs have the outstanding oxygen-carrying ability, the hydrophobic features of PFOCs block their application in tumors [11]. In addition, extensive and enduring exposure to PFOCs may lead to some adverse reactions, including elevated central venous pressure, cutaneous flushing, pulmonary hypertension, chest tightness, fever, and hypotension [75].
Fe
2.2.1.3 Metal–Organic Frameworks (MOFs)
In recent years, metal-organic frameworks (MOFs) (Fig. 2iii) are a useful material in many domains for their catalytically active sites, large porosity, and flexible structure [77, 78]. In addition, MOFs are appropriate candidates for gas storage and separation due to the large surface area and uniform pore size.
DeCoste et al. [79] systematically explored MOFs for the adsorption of oxygen. The working capacity of MOFs was determined by high-pressure oxygen isotherms. Simulations were performed on the oxygen capacity of 10,000 hypothetical MOF materials in a database created using established high-speed MOF generation techniques, NU-125 was selected as prime candidate based on its superior ability to absorb oxygen and currently known synthesis techniques, and NU-125 was proved to have more capacity for oxygen than Norit activated carbon and zeolite NaX. These MOFs were proved to be useful for oxygen storage in different fields such as aerospace, military, and medicine [79]. Recently, Moghadam et al. [80] introduced a visualization concept to clarify the relationships between structural properties and oxygen adsorption ability. They employed high-throughput screening (HTS) techniques to explore oxygen storage in a database of 2,932 existing MOFs and discovered a top MOF material (UMCM-152). They further proved that UMCM-152 delivered 22.5% more oxygen than the material known.
Furthermore, MOFs have been explored to improve the therapeutic efficacy of PDT
in cancers in combination with photosensitizers (PS). Zhang et al. [14]
reported a nanoplatform that could promote the production of O
Gao et al. [15] reported a PDT nanoplatform that took full advantage of
MOFs. As an oxygen carrier, Zirconium (IV)-based MOF (UiO-66) was combined with
indocyanine green (ICG) and further loaded with the cytomembrane of RBCs. After
laser irradiation, ICG would degrade the cytomembrane of RBC. Meanwhile, the
photothermal characteristic of ICG could accelerate the release of oxygen from
UiO-66 in the hypoxic TME. Subsequently, the production of O
2.2.1.4 Oxygen Microcapsules
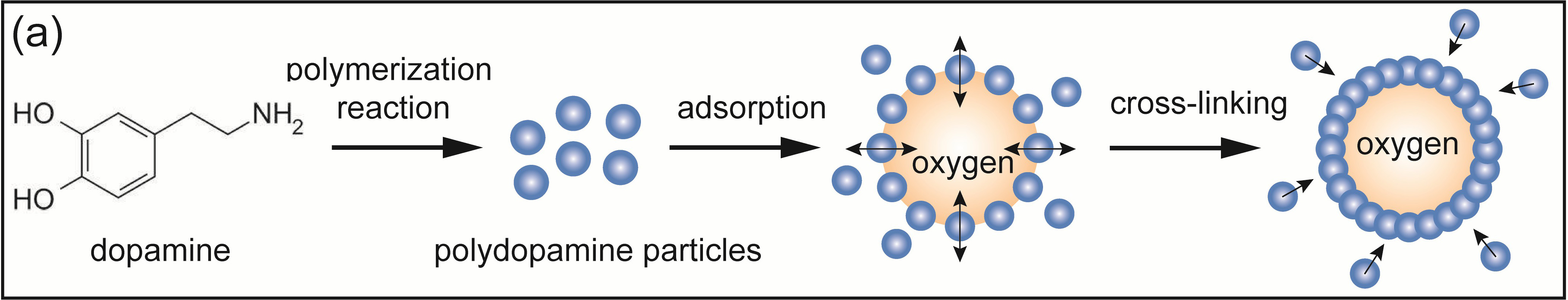
Recently, with the help of interfacial polymerization, our group created an effective oxygen delivery vehicle of polydopamine-nanoparticle-stabilized oxygen microcapsules (Fig. 2iv).
To prepare nanoparticle-stabilized oxygen microcapsules, at first, under vigorous and rapid shearing, dopamine, a neurotransmitter that assists cells to transmit pulses, was oxidized to form polydopamine nanoparticles at the oxygen/water interface. With the help of amino-rich polylysine and chitosan, uniform polydopamine nanoparticles were achieved and the overoxidation of polydopamine nanoparticles was prevented. By optimizing the interfacial adsorption kinetics of polydopamine nanoparticles, chitosan increases the life of oxygen microbubbles. Due to the decline of the interfacial tension, polydopamine nanoparticles temporarily assembled at the surface of oxygen microbubbles. Then, to achieve stable polydopamine-nanoparticle-stabilized oxygen microcapsules, glutaraldehyde was added to permanently crosslink polydopamine nanoparticles. After removing excess solvents and residual polydopamine nanoparticles, nanoparticle-stabilized oxygen microcapsules were well dispersed in oxygen-enriched water. Due to the solid shells availably enclosing oxygen in the core and effectively keeping oxygen microbubbles from coalescing, the oxygen microcapsules keep stable without observable changes for at least one week (Fig. 3, Ref. [1]). We created an effective oxygen delivery vehicle of polydopamine-nanoparticle-stabilized oxygen microcapsules, which effectively improves the hypoxic microenvironment. Gemcitabine (GEM) is a first-line chemotherapy drug for pancreatic cancer. Oxygen microcapsules combined with GEM drugs achieve a synergetic therapeutic effect in pancreatic cancer treatment [18].
 Fig. 3.
Fig. 3.Preparation of polydopamine-nanoparticle-stabilized oxygen microcapsules. Reproduced with permission [1].
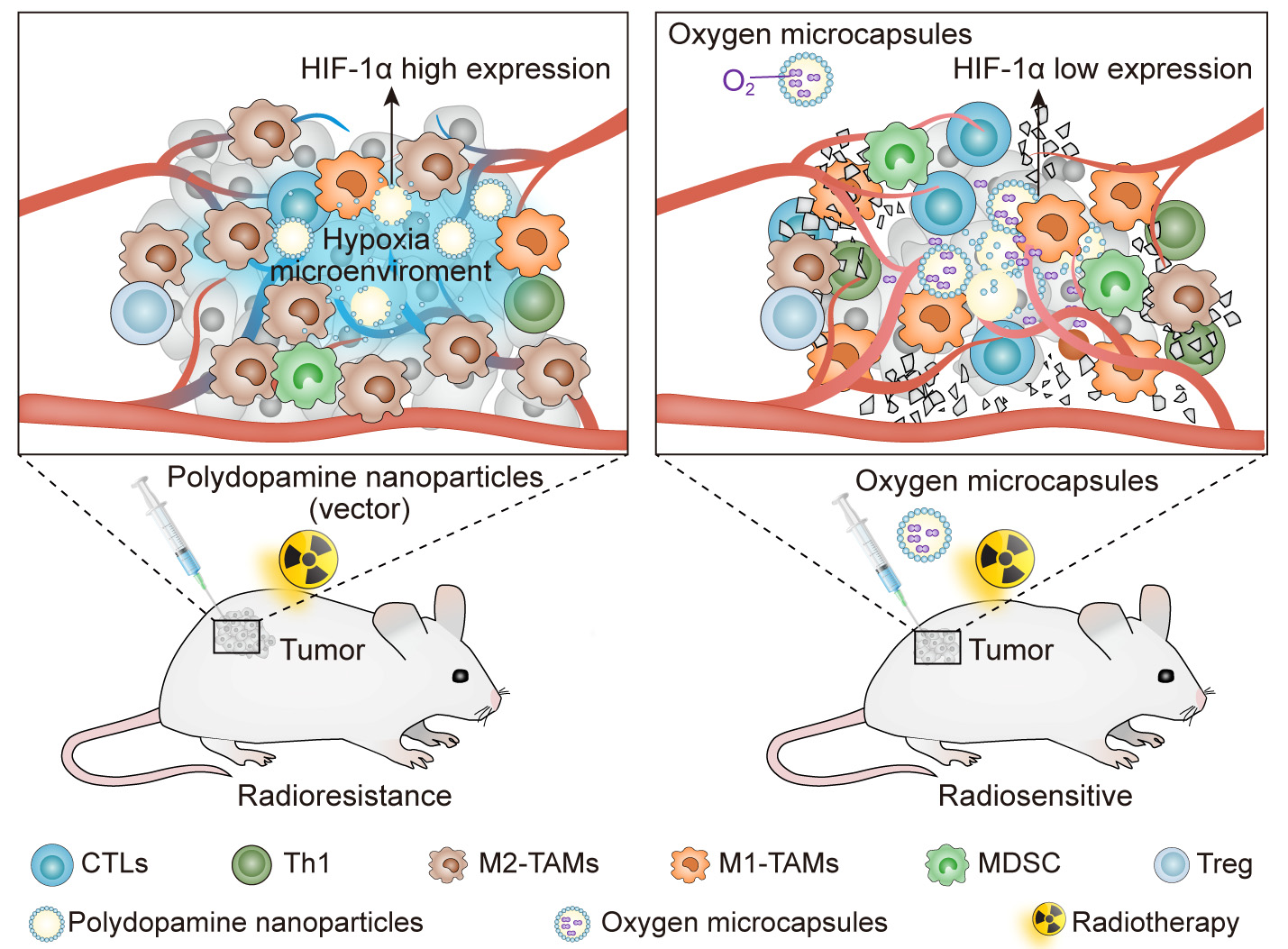
In addition, we reported that oxygen microcapsules can markedly improve radiotherapy efficacy in a mouse model of hepatocellular carcinoma (HCC). Combined treatments of oxygen microcapsules and radiotherapy can effectively decrease tumor-associated macrophages (TAMs) infiltration in tumors while promoting the transformation of pro-tumor type M2 TAMs to anti-tumor type M1 TAMs, which activates the immune responses of T cell against the tumor (Fig. 4, Ref. [2]) [81].
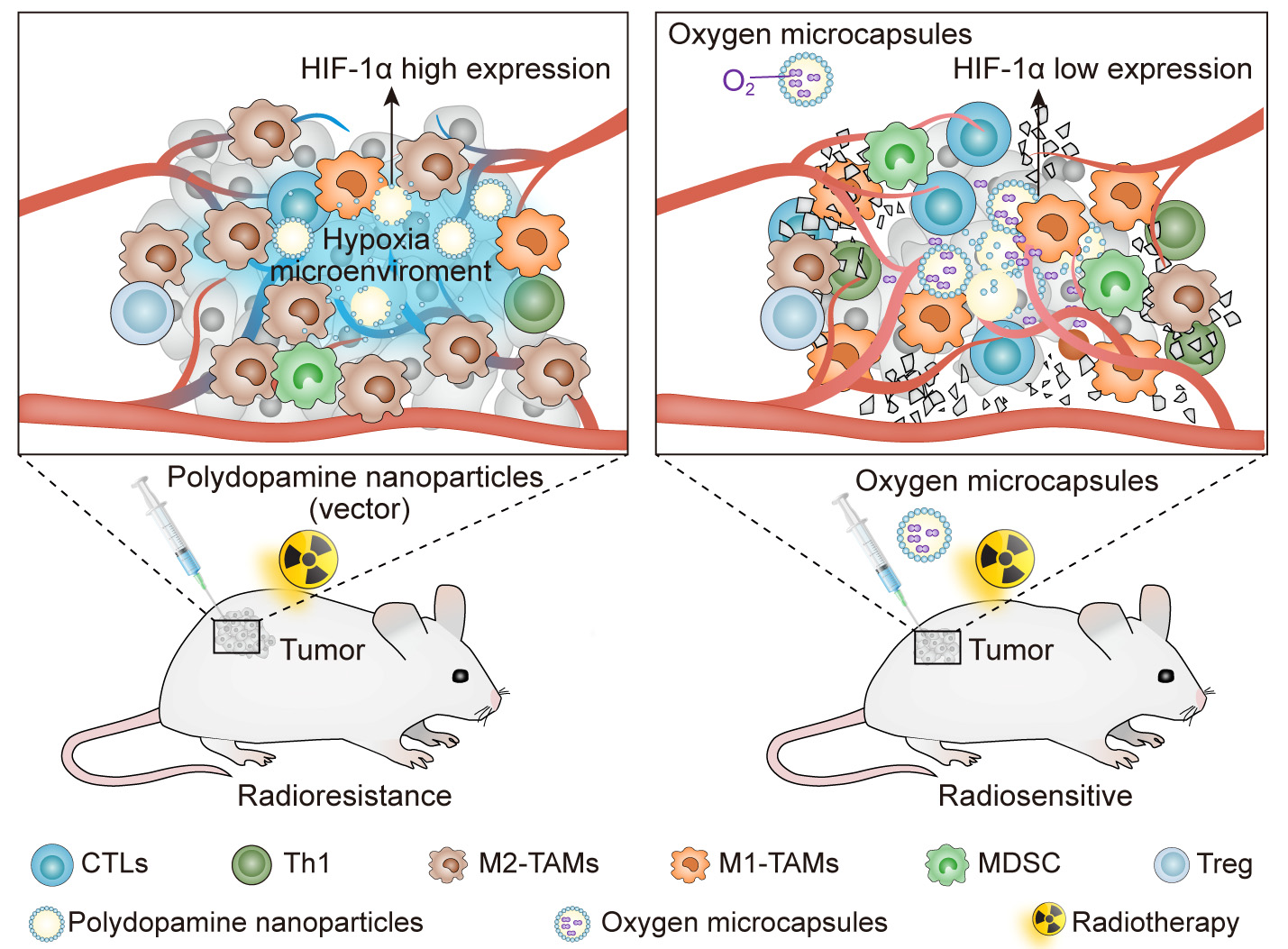 Fig. 4.
Fig. 4.Improving the efficacy of radiotherapy and anti-tumor immunity via using oxygen microcapsules. Reproduced with permission [2].
Another way to improve hypoxia in the TME is to produce oxygen in situ. Three major strategies are discussed here, including chemical decomposition system, catalase, and water splitting.
2.2.2.1 Chemical Decomposition System
The chemical decomposition system involves a highly reactive compound that can
decompose H
Yang et al. [94] developed a multifunctional nanoplatform
(MONs-GOx@MnO
2.2.2.2 Catalase Breaks Down H
In living cells, catalase is a critical enzyme that prevents cells from
oxidative damage via catalyzing H
A lot of researchers are dedicated to improving the stability of catalase via
encapsulating them to various nano-platforms [24, 99, 100]. For example, Phua
et al. [25] created catalase-encapsulated hyaluronic-acid (HA)-based
nanoparticles loaded with an adamantane-modified photosensitizer to improve the
therapeutic effect of PDT. The adamantane-modified Ce6 (aCe6) was created by
combining Ce6 with adamantane. The HA-CAT@aCe6 nanoparticles containing catalase
can increase the therapeutic effect of PDT by decomposing endogenous H
Liu et al. [101] wrapped the catalase in outer layers of MOFs. The
integrated MOF system with characteristics of tandem catalysts can decompose
endogenous H
Li et al. [100] created a tumor-targeted cascade bioreactor (mCGP) which
can increase targetability and retention capacities in the tumor. Once mCGP
entered the tumor cell, it can catalyze the endogenous H
Recently, a multifunctional nano-platform PDA-Pt-CD@RuFc was developed, which
consisted of platinum-decorated and cyclodextrin (CD)-modified polydopamine (PDA)
nanoparticles combined with a ferrocene-appended ruthenium complex (RFC).
PDA-Pt-CD@RuFc nanoparticles combined PDT with photothermal therapy (PTT), which
can kill tumor cells in an anoxic microenvironment in several aspects. Firstly,
the PDA-Pt-CD@RuFc nanoparticles can decompose H
2.2.2.3 Generating O
Catalase and catalase-like material can effectively decompose H
In addition to directly carrying oxygen to the tumor hypoxic microenvironment or producing oxygen in situ, regulating the structure of TME can elevate oxygen levels in the tumor hypoxic microenvironment [89].
Increasing Blood Flow
Due to the abnormal blood vessels in the area of the tumor, inadequate blood flow is a common characteristic of solid tumors. Therefore, improving blood flow would be an effective method to increase the oxygen level in the hypoxic TME [108]. For example, Nitric oxide (NO) can mediate the relaxation of the blood vessel. Blood flow can be regulated by controlling the release of NO (Fig. 2viii).
Jin et al. [30] synthesized a novel BNN-type NO-releasing molecule (NORM) activated by ultrasound and a nano-carrier of superparamagnetic iron oxide-encapsulated mesoporous silica nanoparticles (SPION@hMSN) to create a novel nanomaterial (BNN6-SPION@hMSN). BNN6-SPION@hMSN demonstrated excellent tumor-killing ability and ultrasound-triggered NO release to mediate relaxation of the blood vessel. BNN6-SPION@hMSN can efficiently control the release of NO to improve the hypoxia of the TME.
Analogously, Deng et al. [31] invented a nanocarrier with GSH-sensitive
NO prodrug. The nanocarrier was fabricated by LEGO-like self-assembly for
efficient intracellular delivery of NO, NO released by the nanocarrier can not
only improve hypoxia in the tumor by mediating vasodilation, but also increase
the concentration of ROS generated by PDT through GSH depletion and generate
cytotoxic peroxynitrite anions (ONOO
It is well known that the blood flow of cancer can be augmented by mild heating [109, 110]. Brizel et al. [110] created a nanomaterial that can increase blood flow in a hypoxic tumor environment by mild photothermal heating. This is achieved through a near-infrared (NIR) exposure combined with intravenous injection of DiR-hCe6-liposome and then leads to mild photothermal heating, which would increase blood flow in a hypoxic tumor environment and enhance the therapeutic effect of PDT in the hypoxic TME [32].
Diminishing oxygen consumption is also an alternative treatment strategy to
increase oxygen levels in hypoxia of the TME [111]. Therefore, strategies for
inhibiting oxidative respiration to decrease tumor oxygen consumption also have
been designed to solve the hypoxia issues in tumors [108] (Fig. 2ix). For
example, Denko et al put forward an “O
For strategy of bringing external oxygen to tumor hypoxia microenvironment, due to the existence of membrane protein complexes that are crucial for long-term blood circulation, the RBCM-encapsulated nanoparticles have significantly prolonged blood half-life and biocompatibility. They can be used as long-circulating oxygen carriers to alleviate tumor hypoxia and enhance therapeutic effects [11, 66, 67, 68]. Studies have shown that hypoxia affects the function and phenotype of immune cells in the TME, and RBCs are closely related to immune cells in the liver, such as Kuffer cells. Therefore, RBCM-coated nanoparticles with both immune regulation and oxygen-carrying functions shows research potential for anti-tumor immunotherapy in liver cancer [112, 113]. The advantages of MOFs are high porosity, large surface area and tunable structure, various modifying groups have been introduced into the system to improve the performance and functions of this particle. In addition, single therapeutic way of MOFs doesn’t work very well. Therefore, it’s an urgent need for more combination therapy. For example, MOFs can be combined with more other therapies like immunotherapy to acquire a synergistic effect. Furthermore, the synthesis processes of these well-designed MOFs are often difficult to control. In different tumor microenvironments, different modifying component may alter the kinetic behavior of delivery and release of MOFs, which need to be further clarified in the future [114].
For strategy of Generating O
For strategy of regulating the structure of TME. Nanoparticles mainly mediate vasodilation in tumors through NO releasing and tissue heating to increase blood flow and oxygen supply in TME. In addition, the release of NO from nanoparticles can also enhance the effect of photodynamic therapy through GSH depletion and reactive nitrogen species generation [30, 31]. It is well known that intravenous injection is one of the main application methods of anti-tumor drugs. The increased blood supply in TME helps improve drug infiltration into the tumor, which offers the possibility of combining these nanoparticles with antitumor drug treatments such as chemotherapy. However, the increased blood flow may lead to adjacent dissemination and distant metastasis of the tumor, this risk requires further study and evaluation.
For strategy of decreasing oxygen consumption in the TME. The nanoparticles
mainly target the respiration process of tumor cells, thereby reducing the
consumption of oxygen in the tumor and enhancing the effect of PDT. Metabolic
reprogramming is a key hallmark of cancer, in which aerobic glycolysis is an
important feature [116], so targeting aerobic respiration to reduce oxygen
consumption in tumors may have limited effect. In addition, O
The tumor hypoxic microenvironment is a major obstacle to oxygen-dependent
cancer therapy such as radiotherapy, chemotherapy, PDT, immunotherapy, and so on.
Currently, the strategies of nanotechnology for increasing the oxygen level
mainly include the following aspects: (1) Bring external oxygen to tumor hypoxia
microenvironment. (2) Generating O
 Fig. 5.
Fig. 5.Schematic illustration of the possible antitumor mechanism by alleviating hypoxia.
Although nanotechnology targeting hypoxia has been researched and developed for a long time, the clinical applications associated with it have lagged behind. A variety of nanotechnology have been tried to improve tumor hypoxia, however, there are no FDA-approved treatments to reverse tumor hypoxia. On the one hand, tumor heterogeneity is the most important factor because different tumors have phenotypic features and genetic mutations. This requires that different tumors should be treated in different ways. On the other hand, the size, shape, surface charge and mechanical properties, as well as unstable biocompatibility and safety, also negatively affect the clinical application of these nanoparticles. Further research is needed to improve these aspects. In addition, multifunctional composite nanoparticles with complementary mechanisms have shown encouraging research prospects. The existing nanoparticles targeting hypoxic TME mainly focus on radiotherapy, chemotherapy and PDT and other treatments. The immunomodulatory effects of these nanoparticles and the prospect of combination with immunotherapy deserve further attention.
PDT, photodynamic therapy; PTT, photothermal therapy; MRI, magnetic resonance imaging; EMT, epithelial-mesenchymal-transition; TME, tumor microenvironment; PDAC, pancreatic ductal adenocarcinoma; HCC, hepatocellular carcinoma; HIF, hypoxia-inducible factor; HBOC, Hb-based oxygen carrier; PFOC, PFC-based oxygen carrier; MOF, Metal–organic framework; NU-125, Northwestern University-125; UMCM-152, University of Michigan Crystalline Material-152; GSH, glutathione; MONP, mesoporous organosilica nanoparticle; ROS, reactive oxygen species.
JW, JSo wrote the manuscript and contributed equally. XY, JT, JZ, XW, YJ, YZ, and DC provided help and advice on the manuscript, JSh, XB, and TL reviewed the manuscript and secured the funding. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicable.
The authors would like to thank Zhou Meisheng for administrative help.
This work was funded by Zhejiang Provincial Natural Science Foundation, grant LR22H160009, National Key Research and Development Program of China (grant number 2019YFA0803000, grant 2019YFC1316000), the National Natural Science Foundation of China, grant U20A20378, the National Natural Science Foundation of China, grant 82173078, the National Natural Science Foundation of China, grant 81871925.
The authors declare no conflict of interest.