1 CNR-ICMATE Institute of Condensed Matter Chemistry and Technologies for Energy, via De Marini, 6, 16149 Genova, Italy
2 Institute of Nanoscience and Nanotechnology– IN2UB, University of Barcelona, Avda. Diagonal, 645, 08028 Barcelona, Spain
3 Department of Biochemistry and Physiology, Physiology section – Faculty of Pharmacy and Food Science, University of Barcelona, Avda. Joan XXIII, 27-31, 08028 Barcelona, Spain
Academic Editor: Graham Pawelec
Abstract
Substrates composition and surface features of materials rule adhesion control of cells to surfaces. As a result, most of the aspects of cell functions, such as spreading, migration, proliferation, and differentiation, can be significantly influenced in biomedical applications. Cell cultures make possible to understand cell biology, tissue morphology, mechanisms of diseases, drug action, and tissue engineering development, among others. Recent techniques related to culturing 3D cell aggregates in the presence of very low wettable surfaces represent an innovative field for in vitro experimentation aimed at more reliable conditions to investigate both tumor and non-tumor cell lines. Matching in particular cell biology to innovative materials, this work reviews the recent literature available on promoting cell aggregates formation strongly influenced by the high surface hydrophobicity. In particular, for spheroid formation, the highest water repellent coatings seem to be required for the significant effectiveness of the process. In this way, 3D cell culture has become a reliable method for reproducing in vitro cellular growth in more realistic physiological conditions.
Keywords
- 3D spheroids
- drug screening
- hanging drop
- omniphobic
- sitting drop
- superhydrophobic
- wettability
In biomedical applications, most of the aspects of cell function, such as spreading, migration, proliferation, and differentiation, are significantly determined by substrates materials ruling adhesion control of cells to surfaces. Key material features like surface chemistry and topography are known to affect cell behavior, and the relationship between cell size and surface properties, or more generally, cell-surface interaction, has been widely investigated [1, 2, 3, 4, 5].
Since the first experiments in the early 20th century, in vitro studies have grown to investigate cell biology processes or test drugs outside the body. However, the classical 2D conditions, simple and low cost, with some key drawbacks like the monolayer itself, are not very representative of the structure and functions of the natural structures of tissues or tumors. Since the late 70s, 3D cultures methods have rapidly spread worldwide as more reliable tools to mimic in vivo conditions, an alternative to animal models, ethically not favored [6, 7, 8, 9, 10]. 3D models are then considered a promising approach to investigating biomarkers and treatment strategies, aiming at the final goal of personalized medicine. However, only recently, the development of adhesion control techniques for non-wettable substrates has fostered cell biologists to exploit liquid-repellent surfaces as a platform to grow and control the development of three-dimensional cell aggregates.
The assessment of physiology and physiopathology largely depends on animal models or in vitro alternatives such as the use of cell culture systems [11]. Focusing our attention on the in vitro cell culture, the cells are directly isolated from living organisms containing different cell types and populations from the selected tissue, when considering primary cultures. The difficulties in cell isolation and the short life span of the derived cells are the main characteristics of the primary cell cultures. However, they closely mimic the in vivo genetic features of tumors or other physiopathological conditions, which is advantageous for performing some functional experiments. To overcome these problems, established cell lines became an alternative option. Bioresource centers, such as the ATCC (American Type Culture Collection) and the ECACC (European Collection of Authenticated Cell Cultures), offer characterized models of various types of tumor and non-tumor cell lines that are routinely used in research [12].
In vitro cell cultures make it possible to assess cell biology, mechanisms of diseases, drug action, and tissue engineering development, among others [13, 14]. The cultures can be performed under adherent conditions where the attached cells grow as a monolayer in a culture flask [15]. Some of the benefits of 2D cultures are their low-cost and straightforward maintenance and high functional tests performance. Adherent cultures, however, have numerous disadvantages. For instance, in 2D cultures, cells do not mimic the natural assemblies of tissues or tumors. The cell-cell interactions and cell-extracellular environment are not represented, as they happen in the real tissue. Cell differentiation and viability, proliferation, expression of genes and proteins, and responsiveness to stimuli and drug metabolism are mediated by these interactions [16, 17, 18, 19].
Moreover, it is well reported how the cell isolation from the tissue and the growth in the 2D conditions would alter the cell morphology and cell division mode. The loss of diverse phenotypes is also a result of 2D culturing [20, 21]. Changes in the morphology of the cells affect their function [22, 23], the organization of the structures inside the cell, and cell secretion and cell signaling [24, 25]. The lack of proper interactions with the external environment induces the loss of polarity of adherent cells [26], which modifies the response of those cells to various phenomena, such as the apoptotic process [27, 28].
The access to the medium ingredients, such as oxygen, nutrients, metabolites, and signal molecules, strongly differed from 2D cultures to in vivo conditions. Whereas cells in 2D monolayers have unlimited access to the medium ingredients, the availability of nutrients and oxygen for cancer cells in vivo is more variable because of the own architecture of the tumor mass [16]. In addition, adherent cultures are usually used as monocultures allowing for the study of only one cell type [29], which results in a lack of tumor microenvironment if the presence of cancer-initiating cells are considered as would happen in vivo [30, 31].
The many disadvantages of 2D systems made it necessary to find alternative models to mimic better natural tumor environments, such as 3D culture systems. One of the first 3D cultures was prepared in soft agar by Hamburg and Salmon in the 1970s [32]. Since then, promising similarities between the morphology and behavior of cells growing in a tumor mass and cells cultured under 3D conditions have been well described and documented.
With the aim to find more realistic conditions for in vitro study, culturing cells in three dimensions has become a promising method for more physiologically reproducing cellular growth. In fact, spheroids are 3D cell aggregates with cellular electrical activity and intracellular functions. Therefore, they can be regarded as a model to provide a reliable platform for drug screening in vitro [33, 34, 35, 36] as 3D cellular spheroids grow preferentially on non-adhesive coatings or low wettable highly hydrophobic substrates. often in conditions under gravity or shear stress where the expression of molecules like E-cadherin triggers intercellular interaction at the base of cell aggregation. Also, the main focus of spheroids’ studies development is the relationship of physicomechanical signals in cancer formation, where the physical properties of the environment have been discussed [37, 38, 39].
To avoid influences coming from direct contact with heterogeneous surfaces, platforms have been designed to exploit high water repellence for an independent spheroids culture [40, 41]. In these works, the authors carry out the spheroids formation in a kind of hanging drop system, a confined drop in a culture test plate with superhydrophobized walls. Furthermore, the control of the liquid repellence offers a more comprehensive scenario to investigate potential new substrates in modulating cell aggregation processes. In facts not only superhydrophobicity, but also superamphiphobicity or omniphobicity have been recently exploited starting from different materials from aerogels to metallic meshes [42, 43, 44].
The high hydrophobicity of coatings and materials allows the development of more flexible applications by combining surface chemistry and morphology. In this direction, in the literature, some authors have studied superhydrophobic or even superamphiphobic substrates to develop spheroids. Exploiting such surface specificity, some authors investigated and developed a simple, reusable omniphobic surface using metallic mesh as a robust platform with high- performance culture of multicellular and heterogeneous multicell type spheroids. In addition, a hierarchical, textured aluminum mesh was silanized, providing an inert, low wettable surface for the long-term culture of spheroids [43, 44, 45].
Physico-mechanical properties play a key role in growing cell lines and tissue engineering. The effect of alternative materials under mechanical surface stretching has been studied in [46], simulating the response of endothelial cells to similar substrate deformation as in vivo and trying to avoid ligand coating in the substrate by a plasma- treated silicone-based material. Soft materials like deuterated agarose gel have also been investigated in [47] comparing surface roughness as a function of deuteration effect in increasing the modulus consistently with concentration.
Physical parameters like topography, roughness, gradients, and elasticity are critical in the role between the cellular environment and cellular functions in modulating its phenotype and function. Therefore, the exploitation of synthetic substrates structured at the micro-nano scale allows the influence of such physical properties on the cellular function to be studied, also controls substrate characteristics both in 2D and 3D environments for pros and cons of these surfaces as in-vitro application [48, 49, 50].
When an interface is created, the measure of the energy required is defined as interfacial energy or the force per unit length working along with the interfaces at the triple line in equilibrium. In general, the surface of a solid or liquid can be regarded as a thin layer of molecular dimensions with different behaviors concerning the bulk due to the high energy of surface atoms or molecules compared to the inner atoms. This is because molecules or atoms at an interface have less connection with the surrounding molecules or atoms than the atoms or molecules in bulk. Then, the molecules at the interface have the potential and the tendency to create new bonds.
The behavior of a substance at the interface strongly depends on material composition: high energy interfaces imply wetting of the solid surface and liquid spreading on it. In contrast, non-wetting interfaces are characterized by low energy [51].
The molecular composition and the geometry of the atoms often present a partial electrical charge resulting in a polar configuration with two distinct electrical poles. The absence of such charge separation or polarity holds to nonpolar molecules.
London dispersion forces play a role in defining interfacial properties not only
in water-based systems. For example, during the interaction of a polar liquid
like water in contact with a non-polar liquid/solid or air, Gibbs free surface
energy,
The term adhesion describes the tendency of different particles, liquid or solid surfaces, to stick to each other. Different types of attractive forces, such as van der Waals force, electrostatic and capillary force, and chemical bonding, are involved in adhesion also significant for micro- or nano-systems in contact with surfaces. Physical properties like friction, mechanical contact, and tribological performance are strongly influenced by adhesion as well as highly non-adhesive surfaces are required, for example, for antifouling or biofilm formation purposes [54, 55].
Material surface wettability is mainly characterized by the static contact angle
(CA) at vapor–liquid–solid interfaces, a physicochemical quantity depending on
several factors like liquid composition, surface energy, and surface roughness.
Technology advancements have made real-time contact angle measurements available,
allowing spreading dynamics to be carefully followed. Angle ranges have been
assumed to define wettability domains: hydrophilic when the liquid (water) wets
the surface with a static contact angle between 0° and 90°,
hydrophobic with a contact angle between 90° and 180° when
water does not wet the surface. An extreme state of water repellence
(superhydrophobicity) can be observed with
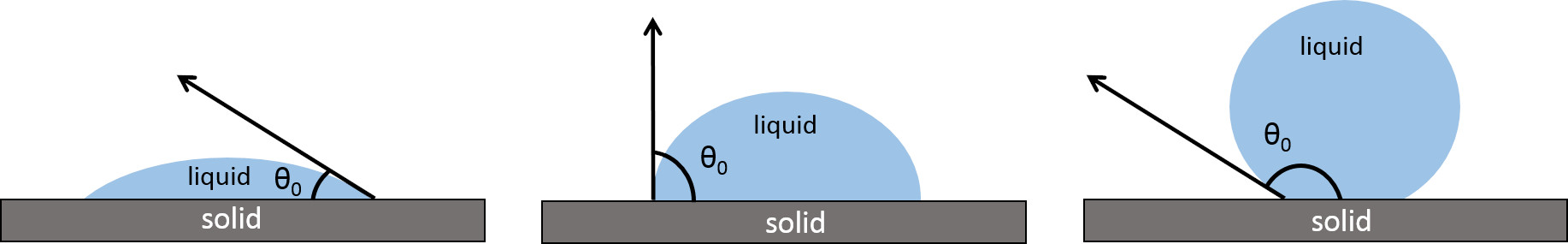 Fig. 1.
Fig. 1.Contact angle of a water drop on a hydrophilic, a hydrophobic, and superhydrophobic surface. Reprinted under the terms of the Creative Commons CC BY license from F. Cirisano, M. Ferrari, Sustainable Materials for Liquid Repellent Coatings, Coatings. 2021; 11 (12): 1508. https://doi.org/10.3390/coatings11121508.
The more recent requirement to control the surface wettability in the presence of non-aqueous liquids and, in particular, non-polar substances such as oils and organic liquids has fostered worth of investigation into the development of oleophobic materials for coatings. Moreover, the behavior of a surface repelling both polar and non-polar liquids is even of greater interest. In particular, for the extreme situation in which a surface has to be highly repellent, it is called superamphiphobicity [56]. This behavior can widely cover important research fields in aqueous and non-aqueous environments like anti- corrosion and antifouling solutions in marine applications where oils or immiscible systems are present, glass protection in solar panels, or biomedical fabrics, to name but a few.
In nature, we can find superhydrophobic or more oleophobic (oil CA
As mentioned above, low surface wettability plays a key role in limiting cell
adhesion and promoting 3D growth phenomena. The low tendency to be wetted by
liquids has been defined as hydro/oleo phobic when CA
A supposed ideal and homogeneous surface can be described by the concept of the
static contact angle. However, in real situations where we deal with
heterogeneous substrates, both chemically and physically, the measure of the
contact angle requires a more complex approach taking into account the effects of
local inhomogeneity. Contact angle hysteresis (CAH) has been introduced to better
describe a real solid–liquid interface considering roughness and physicochemical
interactions between the phases. Measuring CAH provides an evaluation of the
energy dissipated during the droplet spreading on a surface. In cases like
extreme water and/or oil repellence, as reported in the literature, on a surface
with controlled roughness at the nanoscale level, the CAH is lower than
5°, resulting in very low friction between the liquid and the solid
substrate. While the hysteresis is always conceptually
Historically, the Wenzel and the Cassie-Baxter models are the main approaches
proposed to describe the influence of surface structure on contact angle. In the
first model, we find the surface area increased compared to a smooth substrate
and the liquid in contact with the solid surface entering the grooves. Surface
roughness (r) properties rule the wettability: when r is greater than 1, a
hydrophobic surface (CA
The second model proposed by Cassie and Baxter is more general and provides a consistent description of extreme wettability states like superhydrophobicity, with water CA larger than 150°. The surface is composed of air pockets and solid pillars, which distribution and composition make a surface as a fakir carpet structure able to keep water far from entering the cavities. The huge literature in the field may offer a different solution at different technological levels to achieve such behavior with the final aim to avoid contact with water.
Most of the methods, if not all, to obtain a superhydrophobic or, more generally, a super liquid-repellent surface includes the combination of a certain roughness within a micro nanoscale and a low surface energy coating agent on a surface [61]. The dual scale roughness and low surface energy material lower the adhesion of water molecules toward a superhydrophobic state. However, few points are found connecting the preparation of such highly repellent substrate mainly in two ways: increasing the roughness of a low surface energy material or lowering the surface energy of a rough material. These results hold to a wide range of applications from self-cleaning glass to antifouling and anticorrosion solutions to biomedical fields like in this work [33].
One of the main drawbacks affecting the lifetime of super liquid-repellent surfaces is identified as the Cassie–Wenzel transition or destabilization of a Cassie state toward a Wenzel state. In particular conditions where physical or chemical phenomena acting at the surface imply an often-irreversible loss of the air trapped, the Cassie wetting regime is a higher energetic state compared to the Wenzel state, where the droplet is energetically more favorable. Such transitions can be triggered by many events like an external pressure or force on the droplet, vibrational motion, by applying external stimuli at the substrate, or by adsorption of active surface molecules like surfactants or proteins.
Surface roughness (Sa) has been investigated in its modulation action, both in vitro and in vivo, on cellular morphology, proliferation, and phenotype expression as a biological response of cell aggregates in contact with the substrate. In the literature, few authors not only have reported investigations related to microsurface roughness influence on cell differentiation, but also have contributed to discriminate the effect of different scales, from macro to nano, on specific cell lines, despite evidencing the effect of some surface inhomogeneities in inhibiting cell proliferation [62, 63, 64, 65].
Nevertheless, anisotropicity could not always be regarded as a negative feature since disordered systems are often the key to a selective response induced at micronanoscale by chemical modifications at the surface level, as evidenced in some important works by Anselme et al. [66, 67, 68].
On the other hand, analytical tools for spheroid characterization are still limited to the techniques developed and standardized for 2D models: most of the works indeed only indirectly assume a spherical growth of the aggregates used as 3D culture [69, 70]. In this aspect, non-destructive techniques like confocal 3D profilometry can represent an advanced approach to characterize different cell evolution states as reported by the present authors in combination with more assessed techniques [71].
As already previously seen, 3D spheroids represent a new frontier for best mimicking the specificity of the cell microenvironment, and different methods, independently on liquid repellence, are used to form them as follows (Fig. 2) [72, 73]:
Hanging drop method: Cell cultures are suspended in droplets of the desired cell line to force aggregation.
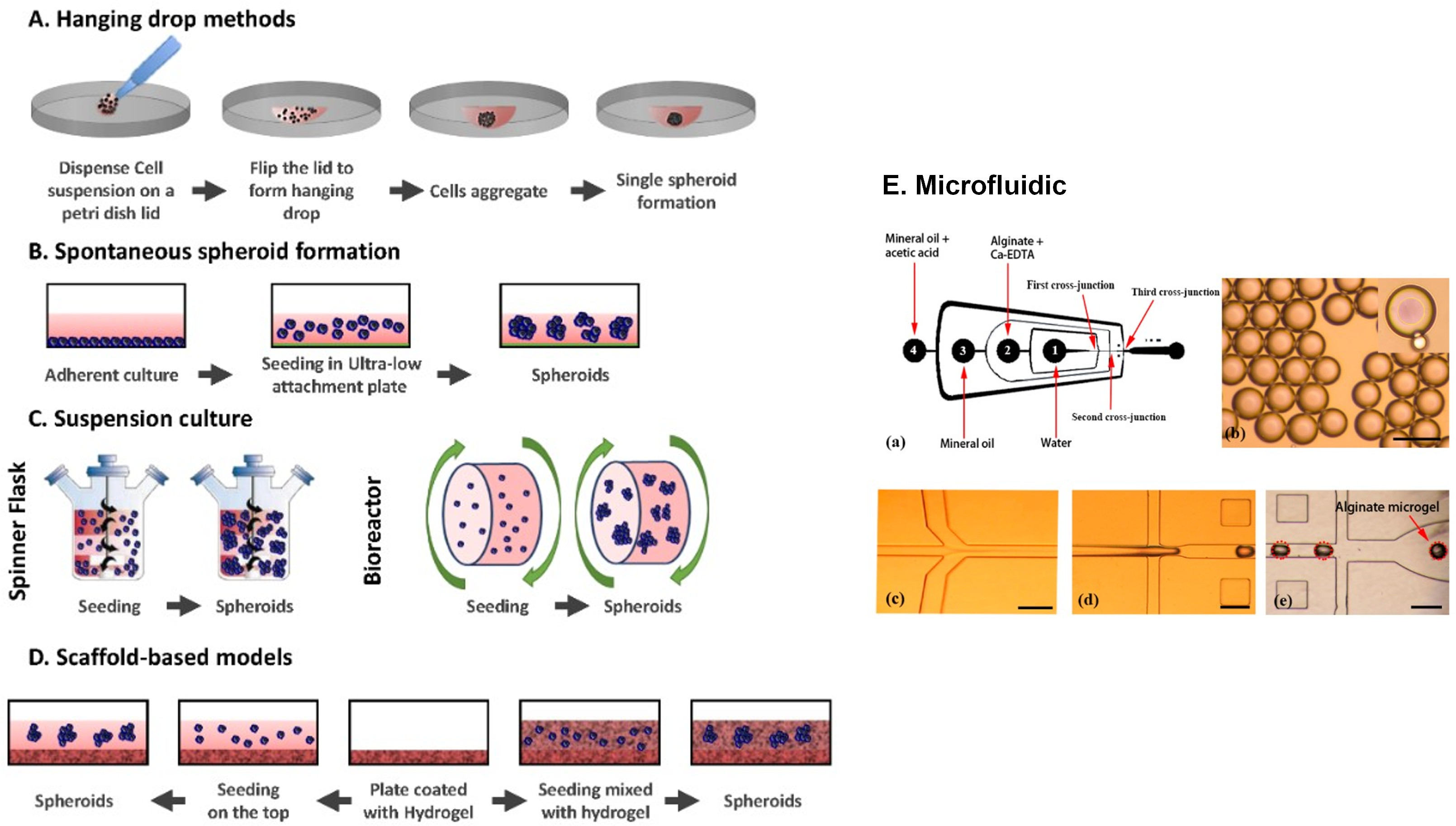 Fig. 2.
Fig. 2.Common 3D techniques used for the creation of spheroids. (A) Hanging drop methods. Cells are deposited on a petri dish lid, which is flipped over a petri dish containing PBS. (B) Ultra-low attachment plates. Cells are seeded in an ultra-low attachment plate which prevents them from adhering. (C) Suspension cultures. Cells are placed in spinner flasks (left) or bioreactors (right) and put under gravitational forces. (D) Scaffold based-models. Cells are either seeded on the top of a hydrogel (left) or embedded in it (right). (E) Schematic diagram of a one-step flow-focusing device for producing core-shell alginate particles. Adapted with permission under the terms of the Creative Commons CC BY license from J. Hoarau-Véchot, A. Rafii, C. Touboul, J. Pasquier, Halfway between 2D and Animal Models: Are 3D Cultures the Ideal Tool to Study Cancer-Microenvironment Interactions? International Journal of Molecular Sciences. 19 (2018) 181 and from Q. Sun, S H. Tan, Q. Chen, R. Ran, Y. Hui, D. Chen, and C-X. Zhao, Microfluidic Formation of Coculture Tumor Spheroids with Stromal Cells As a Novel 3D Tumor Model for Drug Testing, ACS Biomaterials Science & Engineering, 4 (12), 4425–4433, 2018, American Chemical Society.
Usually is possible to obtain one spheroid per drop and control its size but it is not suitable for in situ observation.
• Suspension culture, spinner flask, and bioreactor: Cell culture is suspended in a container under continuous stirring and movement. Cells cannot attach to the substrate and start aggregation and self-assembly in these conditions. However, the techniques do not allow the control of the size of spheroids obtaining multiple cell spheroids with large size and a necessary manual sequential screening. Mechanical damage to cells often occurs. In microgravity conditions, variation of this method reduces hydrodynamic force and cell damage.
• Ultra-low attachment platforms: An inert substrate preventing cells from attaching to the well surface and forcing cells to aggregate forming structures like spheroids was used as a substrate. If the wettability was not controlled, random interactions of cells could occur. High hydrophobicity prevents substrate interactions and the development of multiple spheroids of considerable size. Therefore, manual sequential screening is necessary.
• Microfluidic systems: Devices where cells can be cultured and continuously infused in micrometer-sized chambers have the advantage of developing a miniaturized protocol for culturing and screening 3D tumor spheroids. This technique is not compatible with high-throughput drug screening due to the use of specific and dedicated valves and channels that usually cannot control the drug concentration with accuracy.
• 3D scaffolds: Natural or synthetic scaffolds are used because they can offer the possibility of a correct cell attachment and reorganization into 3D spheroid structures. Cells can either be deposited on the top of the solid matrix or mixed with liquid hydrogel matrix to incorporate the cells during solidification. However, this method makes it challenging to have the same composition from one batch to another. Some hydrogels also have weak mechanical properties may lead to early degradation and can cause immunogenic reactions.
In most papers, the characterization of spheroids’ size and geometry is still to be defined as a standard method. Instead, most authors start by coupling optical and fluorescence microscopy interpreting or assuming the sphericity only indirectly by statistical approach or by reconstruction by image analysis software.
Spheroid’s characterization is fundamental to assess the growth and the development of the structure and to validate the used method. Nowadays, numerous techniques are used to study 3D tumor spheroids characteristics, like morphology, topography, size, and cellular organization [74].
Optical and fluorescent microscopy is the most used method to obtain about size, morphology and internal organization data. In particular, fluorescent microscopy is used to perform fluorescence-based live/dead assays to determine and observe the distribution of dead and live cells in the spheroids. These techniques are the most widely used due to their relative cheapness and ease of use.
Nowadays, confocal laser scanning microscopy (CLSM) is one of the most interesting microscopic fluorescent modalities to characterize spheroids. The obtained images are particularly defined and can allow for highlighting the different molecules present in the preparation with different colors, allowing to appreciate the three-dimensionality. However, CLSM was not able to observe thick specimens due to limited light penetration and the used working distance
Electron microscopy (scanning – SEM or transmission - TEM) is often used to acquire 3D spheroids images with high magnification and resolution to investigate spheroid morphology and visualize cell-cell physical interactions. Usually, high vacuum scanning electron microscopy is the commonly used modality. However, this approach is associated with collapse and modification of spheroid morphology when the sample is coated with gold to enhance the substrate conductivity. To overcome these issues, low vacuum SEM and cryogenic SEM were used. In general, TEM is employed to study the penetration and the distribution of nanoparticles into the spheroid.
Circularity should be a more realistic parameter to be discussed [75] even if, more recently, advanced methods like Optical Coherence Tomography (OCT) have been successfully used for precisely determining the height or diameters of the aggregates.
Nevertheless, the techniques for the growth and development of spheroids only on hydrophobic or superhydrophobic surfaces fall principally into two macro-categories depending on substrate orientation:
• the SH surface is inclined by 180°, and the drop is hung on the surface called a hanging drop method
• the surface is in a neutral position, and the drop is deposited on the surface.
The hanging drop method exploits the gravity to generate in a single drop one or more spheroids. The method involves using a surface on which numerous drops can be placed, remaining attached as it faces down. To reach this goal, on the SH surface, some portions are covered and maintained hydrophilic or subsequently modified to lose hydrophobicity and promote water adhesion. In this way, it is possible growing spheroids at the liquid/air interface from a population of cells avoiding interaction (spreading) on the surface.
Seo et al. in 2014 [76], reported a facile strategy to potentiate the
therapeutic efficacy of 3D stem cell spheroids using bio-inspired
superhydrophobic surfaces with switchable water adhesion properties for the
hanging drop method. This superhydrophobic surface with switchable water adhesion
properties was fabricated by deposition of a gas-sensitive palladium (Pd) layer
onto vertically aligned silicon (Si) nanowires (NWs) and by subsequent coating
with dodecylalkyltrichlorosilane (DTS) to decrease the surface energy of the
material. The obtained Pd-covered Si NWs surface (Pd/Si NWs) shows a large water
contact angle (WCA) (
Neto et al. [41] successfully used HD method on SH platform to mimic in vivo tumor models on the lab-on-chip scale by spheroid formations (CA = 156°). The surface was prepared by physically modifying the polystyrene (PS) sheet by UVO exposure through a photomask to generate the arrays of micro indentations able to fix the droplets to the surface when it was placed face down.
To grow 3D spheroids, Shao et al. [78] used Morpho butterfly wings as bio templates that could provide a natural superhydrophobic surface, CA = 151°, without any modification due to the presence of chitin and protein components. A hydrogel pattern, poly (ethylene glycol) diacrylate, was deposited on the wings to create, after UV polymerization, the hydrophilic pattern to hang droplets (Fig. 3).
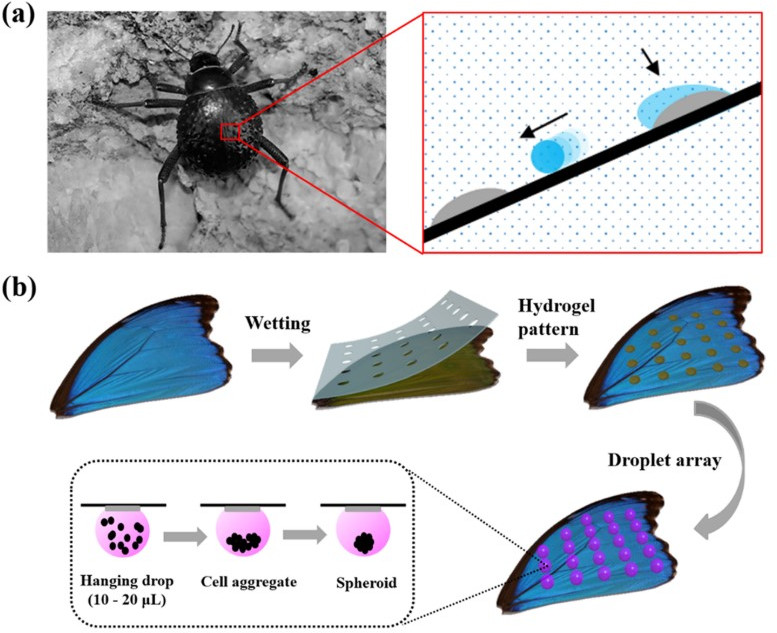 Fig. 3.
Fig. 3.Bioinspired hydrogel arrays. (a) Stenocara beetle with hydrophilic-hydrophobic patterns on the back. (b) Schematic illustration of the fabrication of hydrophilic spots on the superhydrophobic butterfly wing and the formation of cell spheroids. Reprinted with permission from C. Shao, Y. Liu, J. Chi, Z. Chen, J. Wang and Y. Zhao, Droplet Microarray on Patterned Butterfly Wing Surfaces for Cell Spheroid Culture, Langmuir 35 (10), 3832–3839, 2019, American Chemical Society.
Xia et al. [79] prepared a patterned superhydrophobic/superhydrophilic
surface for spheroid formation by the hanging drops method. The SH part of the
surface was made by superhydrophobic TiO
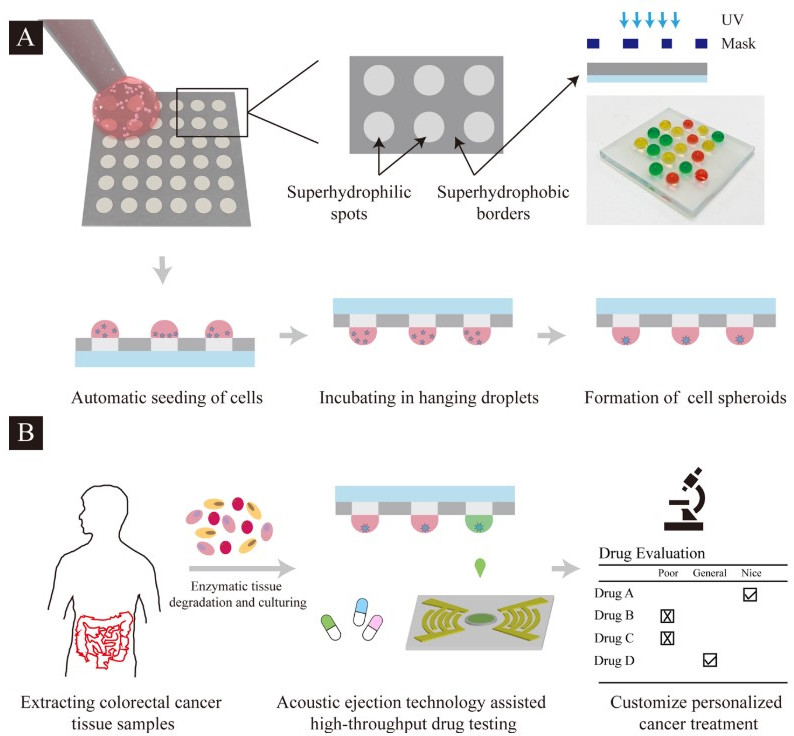 Fig. 4.
Fig. 4.Schematic diagram illustration of the formation and screening of patient-derived tumor spheroids on the droplet microarray platform. (A) Manual cell seeding on superhydrophilic-superhydrophobic microarrays followed by growth of cell spheroids in hanging droplets. (B) Extraction of the patient tissue, followed by drug screening, and evaluation through the droplet microarray platform combined with the acoustic droplet ejection technology. Reprinted with permission from Y. Xia, H. Chen, J. Li, H. Hu, Q. Qian, R.-X. He, Z. Ding and S.-S. Guo, Acoustic Droplet-Assisted Superhydrophilic-Superhydrophobic Microarray Platform for High-Throughput Screening of Patient- Derived Tumor Spheroids, ACS Applied Materials & Interfaces 13 (20), 23489–23501, 2021, American Chemical Society.
Popova et al. [80, 81] and Ueda et al. [82] developed a Droplet Microarray fabricated on a glass slide patterned with square superhydrophilic spots on a superhydrophobic surface (CA = 165°). The method requires that a thin, superhydrophilic layer of nanoporous poly(hydroxyethylmethacrylate-co-ethylene dimethacrylate) (HEMA-EDMA) was photografted with 2,2,3,3,3-pentafluoropropyl methacrylate (PFPMA) through a specific photomask to create superhydrophobic regions, CA = 154° and arrays of superhydrophilic spots with a particular geometry and size.
Oliveira et al. [83] prepared a surface for hanging drop by a simple methodology using polystyrene flakes from commercially available polystyrene plates. PVC stickers were glued on the polystyrene flakes surface in the form of an array of little squares to protect the area, that after modification, will remain wettable. The surfaces were modified according to a phase separation protocol described in [83] in which PS precipitated, forming a rough surface with CA = 154°. To improve the medium exchange and circulation into the hanged drops, the authors have perforated the PS substate and needle were introduced (Fig. 5) [84].
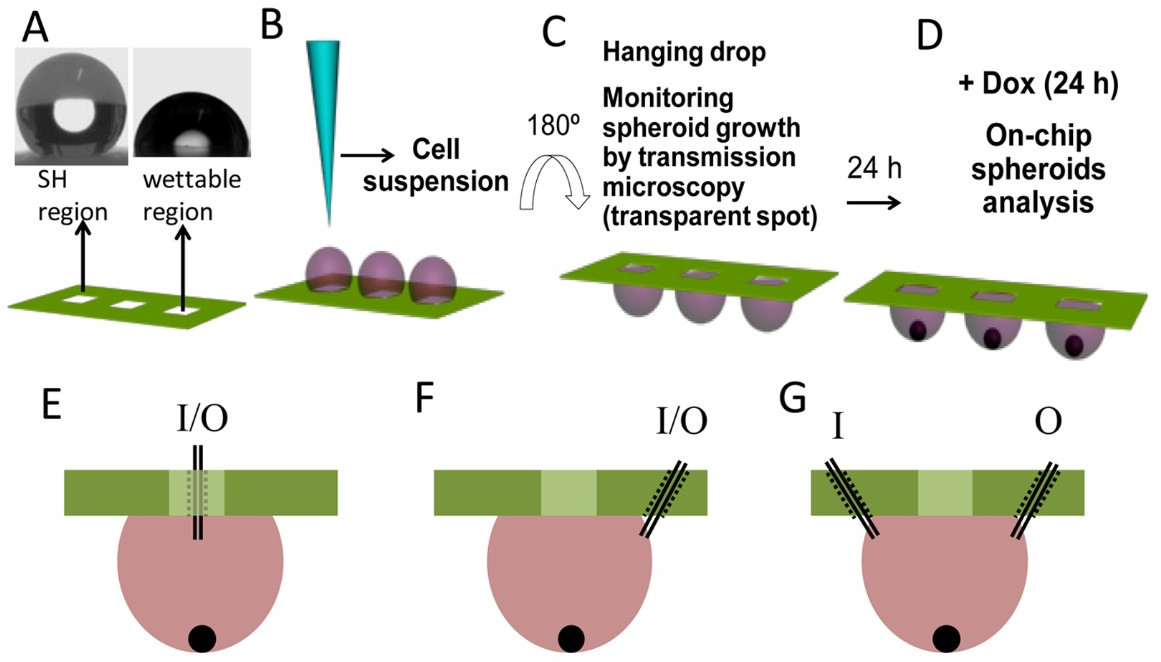 Fig. 5.
Fig. 5.Schematic representation of the procedure for the production of spheroids. (A) superhydrophobic surfaces patterned with wettable transparent spots (water droplet profiles on the superhydrophobic region, left, and wettable region, right). (B) Addition of a cell suspension into the wettable spots of a superhydrophobic patterned chip by pipetting (method 1). (C) Turning of the platform 180° to create a hanging drop setup. The spheroids were let to form for 24 h. (D) Dox was added to each well in combinatorial logic. The addition of Dox to the spots was performed by pipetting after tilting the chips (around 110°). However, the system was also adapted in order to avoid moving the platform, which may disturb the normal formation of the spheroids. We modified the system by making small holes (represented in dashed lines), to achieve multiple configurations with the same platform. The medium was reached by a needle tip (represented in black lines, inside the holes). (E) We perforated the inner part of the wettable regions of the array, in order to add and remove medium directly from the spot. (F) In another configuration, to avoid evaporation and contamination of the medium, we drilled the superhydrophobic region of the chip, 1 mm away from the wettable spot. As such, we accessed the medium laterally. (G) The number of holes in the system could be increased, and their position could be changed. For example, we created a two- entrance system, with an inlet (I) and an outlet (O), so the medium had a dynamic composition over time. Reprinted with permission from M. B. Oliveira, A. I. Neto, C. R. Correia, M. I. Rial-Hermida, C. Alvarez-Lorenzo and J. F. Mano, Superhydrophobic Chips for Cell Spheroids High-Throughput Generation and Drug Screening, ACS Applied Materials & Interfaces 6 (12), 9488–9495, 2014 American Chemical Society.
Different from the works above, Sun et al. [85] proposed a medium-reservoir-integrated superhydrophobic (MRI-SH) substrate to develop a new hanging drop (HD) platform. With this approach, they propose a surface that can grow spheroids over a long period of time. The creation of the HD surface was a combination of PDMS molding and laser etching to obtain a patterned SH surface. The laser- ablated PDMS surface with its micro- and nano-hierarchical roughness combined with the low surface energy of PDMS, shows good superhydrophobic performance (with contact angle up to 156°). On SH surface, a wettable spot array keeps the drops suspended [85].
This section will discuss papers in which the spheroids grow on a hydrophobic/SHS placed in a sitting drop position without any tilt. In this category, there are several methods (microwells, Lab-on-a-bare, mesh, or flat surface), but they all have in common the poor wettability of the substrate.
On a flat SH surface, there is the problem of immobilizing the drops for easy handling. One of the most diffuse approaches is to create on an SHS numerous microwells in which the drop can stick stand, maintaining their sphericity. Sun et al. [86] prepared SH perforated microwells plate for robust and long-term spheroid cultures. To achieve a durable SH coat, a Poly(methyl methacrylate) (PMMA) plate was used as a substrate to produce by CNC hemispherical microwells. Finally, the hydrophobicity was developed by a three-step method coating process-carried out using an airbrush. The hydrophobising suspension was made by mixing silica nanoparticles, epoxy resin, and trichloromethane in different ratios for each spray step. Cell culture medium CA on the SH PMMA plate was about 154° and decreased to 150° after 15 days of immersion in the medium [86]. Because of the spheroids was developed at the bottom of the microwells, due to the gravity, there was a thru-hole at the bottom of each one to ensure oxygen supply to cell aggregate.
Also, quasi-spherical microwells were used to confine and study 3D spheroids by Liu et al. [87]. The authors, in this case, prepared the microwells plate by ice lithography: the water droplets were deposited on hydrophobic/superhydrophobic surfaces, roughened polydimethylsiloxane (PDMS) trichloroperfluorooctylsilan functionalised, and then frozen as a mould for soft lithography by PDMS [87]. The authors produced microwells with different concave shapes and volumes depending on the starting PDMS plate (CA 100°, 120° and 150°). The produced spheroids differ in diameter and number depending on the kind of concave microwells used.
Something similar to the microwell concept was used by Chen et al. [40] by applying the commercial product NeverWet Multi to a standard cell 384-well culture plate by spray coating (Rust Oleum) in two- step process to obtain a superhydrophobic surface with CA = 152° (Fig. 6).
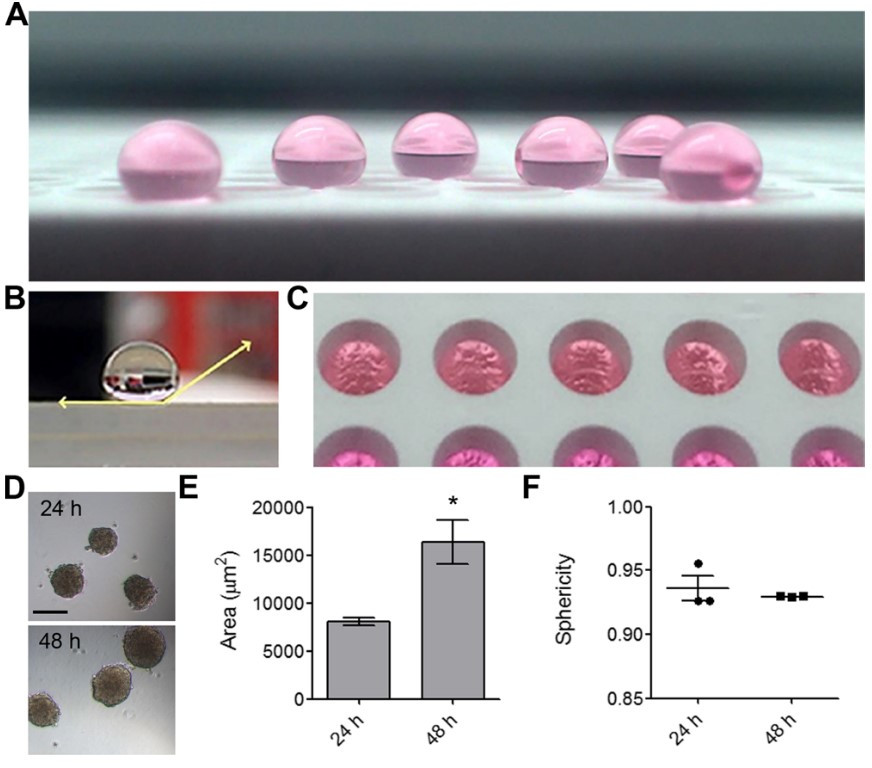 Fig. 6.
Fig. 6.Production of mOEC cell spheroids in NLMs. (A) Shown are NLMs on
a superhydrophobic-coated surface. (B) Horizontal view of a water droplet on a
superhydrophobic-coated surface with water contact angle highlighted. (C) NLM
generation in a 384-well plate coated with a superhydrophobic treatment, viewed
from above. (D) Representative image of mOEC spheroids after 24 h incubation and
48 h of incubation. (E) Area (
High-strength SH macroporous monoliths with the multiwell plate can be obtained by Hayase et al. [88] by the simple mixing of biocompatible substances such as boehmite nanofiber aqueous acetate dispersions with methyltrimethoxysilane. For example, on polymethylsequioxane (PMSQ) monoliths’ superhydrophobic surface (CA = 152°), the authors created sub- millimeter structures by computer numerical control (CNC) milling creating grooves to hold the drop in place (Fig. 7).
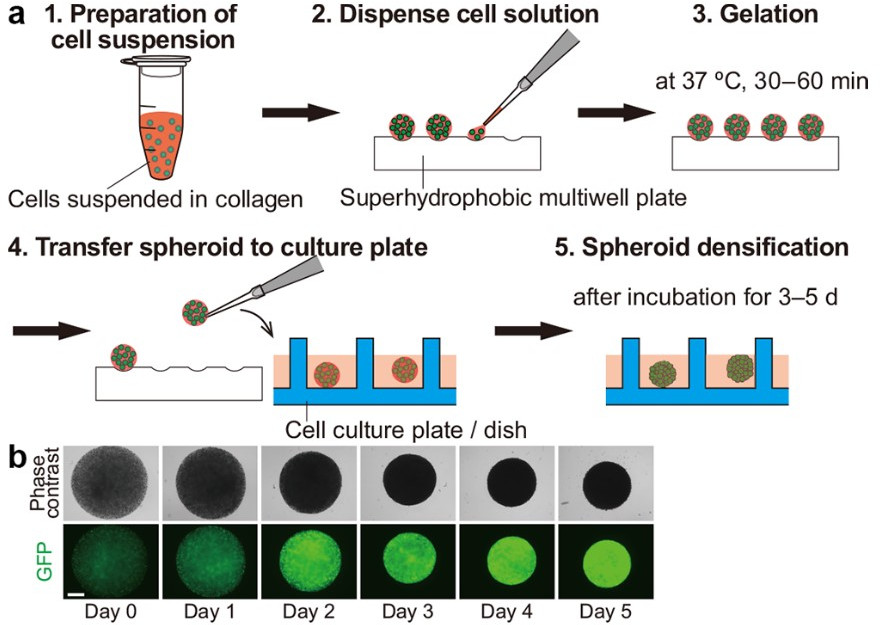 Fig. 7.
Fig. 7.Spheroid formation using the superhydrophobic multiwell plate.
(a) Five-step protocol for cell spheroid formation. (b) Time-lapse images ofan
MDA-MB-231 cancer cell line spheroid formed according to the protocol. The scale
bar indicates 500
Instead of a continuous surface, in some papers, it is possible to find the use
of functionalized mesh as a substrate. If a bottom reservoir is positioned under
the mesh, this design maintains sufficient humidity to prevent droplet
evaporation for long-term spheroid culture. Xu et al. [42] used a
stainless-steel wire mesh (305 mesh) as a conductive substrate to obtain a
durable superamphiphobic surface for 3D spheroids to grow. Metal substrate was
used for the electrodeposition of PEDOT to create a first template, followed by
the CVD of TEOS for the hierarchical structure generation. The superamphiphobic
silica aerogel surface was finally obtained following the fluorination (POTS) by
CVD, obtaining water CA = 174° and for numerous oils, a CA
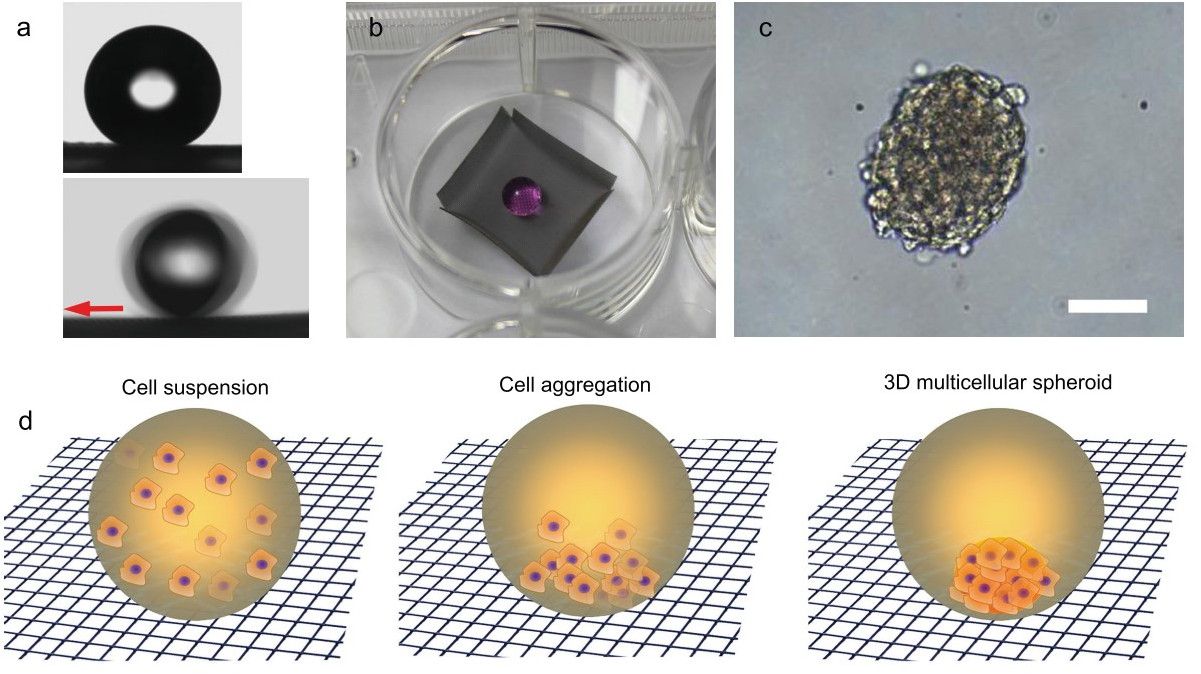 Fig. 8.
Fig. 8.Wettability properties and 3D cell growth in real and simulated conditions. (a) The contact angle (upper) and sliding angle (lower)
measurement for aqueous cell culture medium droplets (
Also, Boban et al. [44], starting from an aluminum mesh, were able to
prepare a hierarchical nano-textured omniphobic surface using a three-step
process. The first step was an acid etching followed by immersion in boiling
water. The last step was the surface treatment with fluorosilane
(1H,1H,2H,2H-heptadecafluorodecyl triethoxysilane) to reduce the surface energy
obtaining a water CA
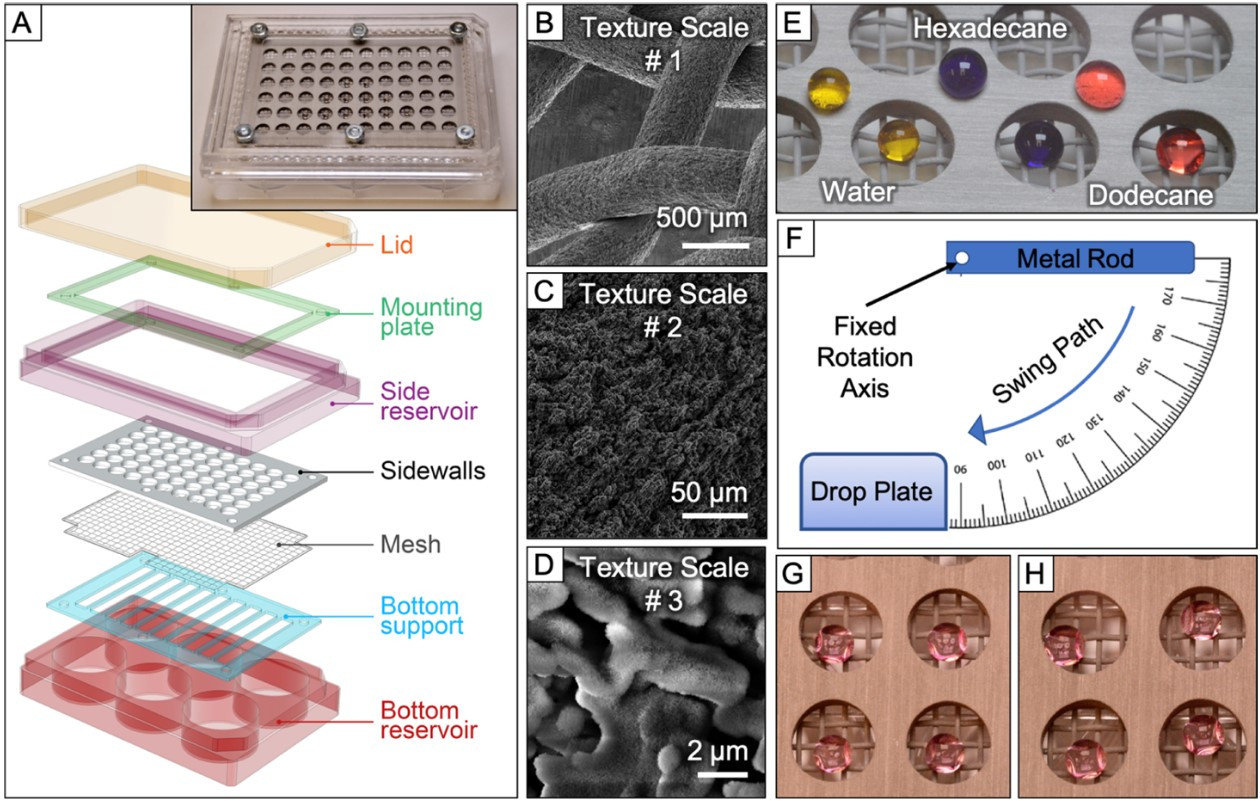 Fig. 9.
Fig. 9.Novel omniphobic platform for multicellular spheroid generation.
(A) Schematic illustration of the developed platform. The inset shows an image of
the complete device with the lid removed. (B-D) Scanning electron microscopy
images for the omniphobic aluminum mesh. The three images highlight the three
hierarchical length scales of texture: (B) mesh geometry, (C) microscale texture
on the wire surface, (D) nanoplatelets of boehmite. (E) 10
Antunes et al. [89] studied the formation of tumor spheroid using 3D microgels deposited on SHS instead of water-based droplets. The SHS was produced from circular polystyrene (PS) petri dish spray- coated with a commercial superhydrophobic product, FluoroThane-MW reagent, that provides a contact angle of about 150°. Microgel 3D spheroids were prepared using photocrosslinkable hyaluronan-methacrylate (HA-MA) and gelatin-methacrylate (GelMa). By this method, it is possible to grow spheroids in solvent-free conditions.
In this study, Lee et al. [43] used different polymer films with controlled hydrophobicity and demonstrated that the cell-to-surface interaction plays a key role in controlling cancer cell morphology, behavior, and tumorigenic characteristics. These features were investigated on polymer such as poly(2- hydroxyethyl methacrylate) (pHEMA), the most hydrophilic polymer with water CA of 39.9°, and on poly(cyclohexyl methacrylate) (pCHMA) , water CA of 82.5°. The change in the amount and conformation of the adsorbed albumin along with the surface hydrophobicity largely affected the subsequent cellular characteristics. Concerning the previously reported works, in this paper the surfaces were only hydrophobic, at the most.
Bianco et al. [90] studied the promotion of 3D spheroid by different
surfaces such as Lab-On-a-Brane (LOB), Lab-On-Chip (LOC), and Thin Membranes
(TMs) with different wettability. Different wettability to reduce water adhesion
was exploited employing PDMS and fluorolink F10 and to create an oleophobic
surface, hydrophobin HFBII was employed. LOC and LOB were fabricated by soft
lithography using PDMS. In particular, by comparing with all these substrates,
they observed that 3D cell aggregation was favored in LOBs, independent of
surface wettability, but mind that the surfaces produced, except those extremely
hydrophilic, have CA in a range between 40° and 102°.
Therefore, we are dealing with slightly hydrophobic surfaces, so the promotion of
cell aggregation in 3D may primarily be a consequence of the membrane flexibility
and its high O
In this work, as defined above, we focused our attention on those techniques for the growth and development of spheroids on hydrophobic or superhydrophobic surfaces that fall into two macro-categories depending on how it is oriented: hanging drop and sitting drop methods. The following sections will summarize the most relevant biological aspects of spheroids formation using these two approaches.
The efficiency in producing multicellular spheroids from dispersed cells results in a function of numerous factors. Among them, the own production approach, the involved cells, and the cell culture conditions are essential features to be considered. There are numerous ways of generating multicellular spheroids, including modified non-adhesive well plates, agitated culture vessels, hanging drops, microgravity, and microfluidic devices. In this work, as defined above, we focus our attention on those techniques for the growth and development of spheroids on super liquid-repellent surfaces that fall into two macro-categories depending on how it is oriented: hanging drop sitting drop methods.
The actual cell microenvironment could be altered by the metabolites produced by the cells, which can also affect the cell behavior. Factors related to the transport phenomena, mainly those concerning mass and momentum transfer, are the most critical influences in producing homogeneous spheroids and their characteristics (Fig. 10). For instance, the mass transport of nutrients and oxygen within the spheroid could be restricted for the larger spheroids resulting in nutrient depletion and hypoxia in the intimate regions. In addition, due to diffusional limitations, the larger spheroids would show lower proliferation capability [91] and accumulate carbon dioxide and metabolic reaction products as lactate in their inner regions [92]. The effects related to mass transfer limitation may conclude in cell necrosis in the center of the spheroid [93, 94].
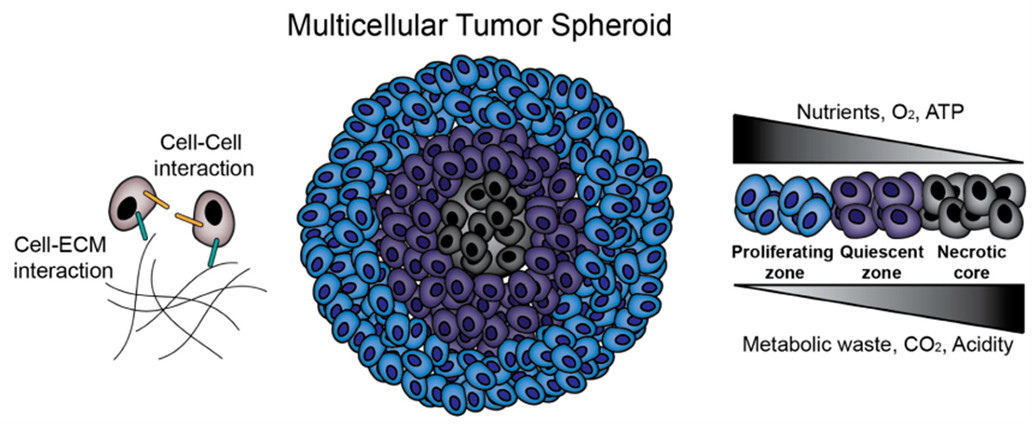 Fig. 10.
Fig. 10.Multicellular tumor spheroids (MCTS) biology. MCTS provide an in vitro platform for the investigation of cell–cell and cell–extracellular matrix (ECM) interactions. Additionally, MCTS mimic in vivo solid tumors in terms of nutrient, oxygen and pH gradients and zone formation. Reprinted under the terms of the Creative Commons CC BY license from. Kamatar, A.; Gunay, G.; Acar, H. Natural and Synthetic Biomaterials for Engineering Multicellular Tumor Spheroids, Polymers, 12, 2506, 2020, MDPI.
The mass transport of nutrients and oxygen within the spheroid structure may be harmful or helpful, depending on the final application of the derived structures. Thus, when 3D models are used as building blocks for tissue engineering, the incidence of such gradients is detrimental due to the presence of the necrotic core that would reduce their assembly of human tissues and the applicability of the derived spheroid. However, for 3D tumor models, this feature is a positive aspect, due to the similarity to the in vivo tumor microenvironment, which makes it more favorable for studying mechanisms of resistance, migration, tumor invasion of malignant cells under chemotherapeutic treatments [95].
The accurate characterization of 3D spheroids involves both the quantitative and qualitative analysis of the derived structure. However, most of the available techniques and protocols were initially designed for 2D culturing, supposing a weakness point. The application of direct methods for counting viable cells, either by manual or automatic methods, requires the dissociation of spheroids in individual cells. The process would be complicated as a function of the involved cells into the spheroid structure. When colorimetric or bioluminescent assays as indirect methods for determining viable cells are considered, these assays would be useless due to the cell-cell and cell-matrix interactions. Such interactions would require the most severe conditions, which may induce potential cell death, affecting the final cell viability determination. For all these reasons, most of the studies included the qualitative live/dead assay to verify the integrity of the cell into the spheroids. Fluorescent probes, such as calcein-AM [96] and fluorescein diacetate (FDA) are frequently used to qualitatively analyze of cell viability. For the apoptotic or necrosis analysis, ethidium bromide (EtBr), ethidium homodimer (EthD-1) [96], and propidium iodide (PI) are used [97].
Even though tumor cell lines are the most involved, in studies including spheroids as a reliable in vitro model, 3D models for non-tumor cells could be of great interest. For example, skin models both investigate the complexity of skin and drug testing or skin regeneration protocols in chronic wounds in clinical trials. Few studies are indeed available addressed to this specific direction, and from the same authors, surfaces at different wettabilities from hydrophilic to superhydrophobic have been exploited for culturing of 3D spheroid-like aggregates for tumor and non-tumor cell lines aimed to compare different surface parameters and analysis techniques [98].
Recent works in the literature have demonstrated the ability to produce 3D spheroids using super liquid repellent surfaces. Cell lines, originating from breast [79, 81, 87, 88], carcinogenic stem-cell like [44, 81], cervical [79, 81, 88], colon [79], hepatocellular [78, 85, 86], ovarian [44] and prostate [89], but also non-tumor cell lines like adipose stem [76, 99, 100], fibroblasts [84, 99], osteoblasts [84, 89, 99] and umbilical vein endothelial [99] in either monoculture and co-culture conditions, demonstrating a homogeneous cellular distribution in 3D through the live/dead assay. Further studies concerning the morphological and phenotypical characterization [87] and immunofluorescence staining on cells growing in the 3D environment [44] have been carried out.
A significant weakness of the hanging drop method is the limited volume of the droplets and the intrinsic difficulty in exchanging medium of them, causing fast nutrient depletion and the accumulation of metabolic substances. Recently, Sun et al. [85] have developed a hanging drop device integrated with medium reservoirs to preserve consistent culture media conditions for long-term spheroid culture. Using numerical models and experimental setups, the nutrient depletion and metabolic waste accumulation rate in the culture medium have been evaluated in both (conventional superhydrophobic) C-SH and medium-reservoir-integrated superhydrophobic (MRI-SH HD) devices. Glucose and lactate were chosen as representative nutrients and metabolic waste, respectively. Results demonstrated that the nutrient source in the integrated reservoirs facilitates an undisturbed and sustainable culture of spheroids derived from human hepatocellular carcinoma MHCC97-H cell line for more than 30 days, compared to 21 days for which spheroids disintegrated in the corresponding devices in the absence of medium reservoirs.
The 3D spheres model creates spheroid structures in which cells are disposed of in various layers. The generated architecture close resembles both the physical and biochemical characteristics of a solid tumor mass. Multicellular tumor spheroids are widely applied in the fields of fundamental cancer research and drug discovery. Recent studies in super liquid-repellent surfaces have demonstrated the applicability of the derived 3D structures on the screening of antitumoral drugs on several cell lines.
Doxorubicin (DOX) is a broad-spectrum antitumor drug whose cytotoxicity
mechanism is embedded in the DNA base pairs of tumor cells and thus inhibiting
the transcription synthesis of nucleic acids. When their effects were evaluated
in 3D human hepatocarcinoma (HepG2) cell spheroids on patterned butterfly wing
surfaces [78], the cell viability showed an expected decrease as a function of
DOX concentration under both 2D and 3D spheroid culture conditions. However, for
DOX concentration equal to 0.5 mM, the cell viability decreased to nearly 59%
relative to the untreated control for 2D cultures, and only to 22% relative to
the control for 3D spheroids. Moreover, strong differences in the half-maximal
inhibitory concentration (IC
DOX has also been used in the cell viability of 3D spheroids derived from L929
fibroblasts cell line and SaOs-2 osteosarcoma cell line on pattered
superhydrophobic chips [84]. Even at a high DOX concentration of DOX, L929 cells
were significantly more resistant than SaOs-2 cells, showing selective toxicity.
DOX, together with Oxaliplatin (OXA) and 5-Fluorouracil (5-FU), were used to
evaluate the antitumoral activity on human cervical adenocarcinoma (HeLa) cells
on 3D spheroids induced by superhydrophobic pattering under hanging drop approach
[81]. HeLa cells grown in 3D spheroids were more resistant to the drug treatment
than in 2D monolayer culture. The corresponding IC
When considering 3D spheroids prepared by sitting drop procedures, 5-FU was used to evaluate the antitumoral activity of hepatocellular carcinoma (MHCC97H) cell-derived tumor spheroid using a sessile drop method, in comparison with 2D culture conditions [86]. As expected, with the increase of the 5-FU concentration, cell viability in 2D and 3D culture conditions decreased, demonstrating a clear 5-FU dose-dependent response of MHCC97H cells. However, at the same concentration, MHCC97H spheroids showed greater resistance to 5-FU than their corresponding 2D monolayer, which could be attributed to the longer time needed for the drug diffusion and penetration into spheroids. In agreement with the studies performed using hanging drop methods, derived 3D tumor spheroids also showed higher resistance to anticancer drug exposure than cells in a 2D monolayer culture.
Similar results were obtained with human breast adenocarcinoma (MCF-7)
cell-derived spheroids prepared in quasi-spherical microwells treated with
paclitaxel (PTX) and in comparison with 2D monolayer and agarose-treated 96 well
plates [87]. When MCF-7 cells were treated with PTX in a 2D environment, the
corresponding IC
Using a novel onmiphobic platform, drug screening using DOX and PTX on serous ovarian adenocarcinoma (OVCAR) cell-derived spheroids was performed [44]. The experiments were conducted to compare U-bottom, hanging drop, and omniphobic plates. Cell viability upon treatment with doxorubicin (DOX) and paclitaxel (PTX) in spheroids generated on the omniphobic and hanging drop platforms demonstrated significant differences compared to those spheroids generated into the commercially available U-bottom plates.
These results clearly indicated that the cytotoxicity could be completely different in the 3D spheroids than in 2D cultures. As a general trend, the response to the antitumoral drug in 3D culture systems reported greater drug resistance than 2D monolayer models, thus better reflecting the conditions found in in vivo tumors [101]. The increased resistance of tumor-derived spheroids to anticancer compounds compared to monolayer culture of the same type of cells has been reported multiple times previously [69, 102, 103].
Although most the antitumoral studies are based on conventional cell lines, these spheroids could also contribute to precision medicine, in which tumor spheroids derived from patient tumor cells represent a promising and more realistic approach for drug sensitivity and resistance testing. Indeed, through the droplet microarray, malignant colorectal tumor was collected from the patient and the patient-derived cell spheroids were treated with commonly used chemotherapeutic drugs such as 5-fluorouracil (5-FU), cetuximab (cmab), and panitumumab (Pmab) [79]. Results showed dose-dependent effects on cell viability for all three compounds. In addition, Pmab and 5-FU showed the most and worst therapeutic effect on patient-derived tumor spheroids, respectively (Fig. 11).
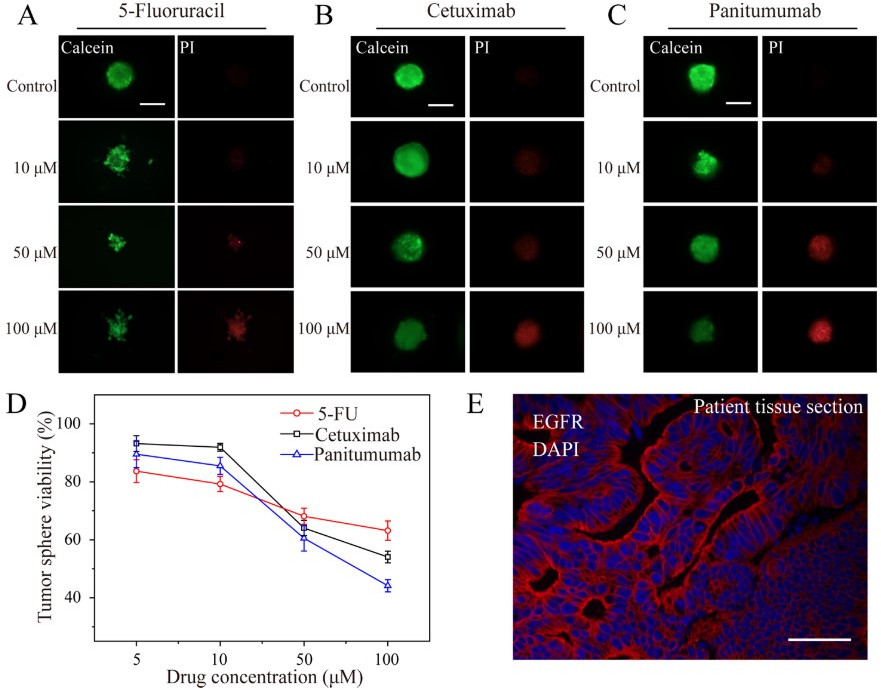 Fig. 11.
Fig. 11.Treatment of patient-derived cell spheroids on the droplet
microarray platform with anticancer drugs. Comparison of the dose- dependent
effect of (a) 5-fluorouracil, (b) cetuximab, and (c) panitumumab on
patient-derived cell spheroids. Spheroids were formed for 48 h, followed by the
addition of drugs using an acoustic droplet ejection device and incubating
spheroids for 48 h before staining and microscopy analysis. Representative
microscopy images of patient-derived cell spheroids treated with different
concentrations of drugs (scale bar = 200
The obtained results agreed with the epidermal growth factor receptor (EGFR) immunofluorescence staining performed on both the 3D spheroids derived from patient tumor cells and the patient’s tumor tissue section. The tissue of the patient showed EGFR++, which was consistent with the therapeutical results obtained with Pamb and cmab, being both EGFR-targeting drugs. The screening drug results further proved that the 3D tumor spheroid is highly similar to the patient actual tumor tissue.
3D spheroids have great potential for cell therapy and tissue engineering due to their therapeutic and regenerative capacity [8]. Furthermore, due to the cell-cell and cell-matrix interactions, spheroids mimic in vivo 3D microenvironments, in which cells exhibit improved proliferation, differentiation, and cellular function compared to cells grown in 2D culture.
Using superhydrophobic palladium-layered silicon nanowires (Pd/Si NWs), Seo et al. [76] demonstrated control on the preparation of human adipose-derived stem cell (hADSC) cell-derived 3D spheroids through a hanging drop culture. The hADSC paracrine activity on the Pd/Si NWs was compared with spheroids produced by suspension culture and hanging drop culture on a petri disc. The vascular endothelial growth factor (VEGF) secretion level was much greater from hADSC spheroids formed on Pd/Si NWs than from conventional methods, suggesting the applicability of Pd/Si NWs to prepare functional human stem cell spheroid cultures with high metabolic activity.
Moreover, the conditioned medium was evaluated in terms of proliferation and capillary formation of human umbilical vein endothelial cells (HUVECs) as a potential angiogenic agent. Both HUVEC proliferation and capillary density were significantly enhanced in the conditioned medium from the Pd/Si NWs group than in those from conventional methods. This study suggests the improved angiogenic efficacy of hADSC spheroids when prepared through hanging drop culture on Pd/Si NWs.
Studies performed by Gettler et al. [100] demonstrated the preparation of spheroids from adipose-derived stromal vascular fraction (SVF) cells. SVF cells derived from rats were firstly encapsulated in collagen I as a gel biomatrix using a 3D bioprinting technology. The spheroid shape was induced by the use of a superhydrophobic surface. The SVF viability in the derived spheroids was maintained in both static and dynamic spinner culture. Studies of stablization over time demonstrated that the spheroids undergo a time-dependent contraction with the retention of angiogenic sprout phenotype over the 14-day culture period, ensuring their transplantation for transplanted for therapeutic applications.
In co-cultures, different cell types are grown together in the same environment, allowing for examining the communication among cells [104, 105]. Such communication includes cell-cell, cell-microenvironment, and paracrine signaling by dissolved factors [106]. These three intercellular interactions allow observing interactions in functional structures that closely resemble in vivo interactions [107].
From a biotechnological point of view, in vitro co-cultures may add value in preparing 3D microtissues as building blocks for tissue repair or in vitro organ models. Furthermore, co-culture spheroid constitutes valuable models for understanding cancer biology and oncological drug development. In this sense, one of the most promising clinical applications of co-culture systems is related to personalized tumor treatment. After collection and analyses of tumor cells of patients, strategies for customized treatment could be developed. Moreover, the induced paracrine signaling could modulate the formation and biological response of the generated structures. Unfortunately, few reports in the literature can be constituted as versatile tools for the co-culture of different cell types in direct and indirect configurations.
Oliveira et al. [99] have recently developed a superhydrophobic platform patterned with wettable regions for the preparation by a hanging drop method of 3D spheroids in direct and indirect co-culture with different cell lines. Spheroids from human stem cells derived from the adipose tissue (hASC) were prepared in indirect contact with fibroblasts (L929 cell line), osteoblast-like cells (Saos-2 cell line), and human umbilical vein endothelial cells (HUVEC). In addition to the integrity studies, this work determined the alkaline phosphatase (ALP) expression as a function of the different co-cultures. The ALP expression in hASC cells was affected by the presence of osteoblasts and endothelial cells. When Saos-2 cells were present in the co-culture, the ALP expression is, on average, twice more ALP activity than that produced by the spheroids generated under monoculture conditions and in co-culture with fibroblast cell line L929. The direct co-culture of hASC with HUVEC cells promoted its osteoblast phenotypic markers, resulting in an increase of ALP expression. The authors highlighted the proposed platform as a versatile tool for the assistance of co-culture of several cells on 3D spheroids, including direct and indirect setups.
Co-culture of both tumor and non-tumor cells on 3D spheroids has been recently performed by Antunes et al. [89] using a sitting drop approach mimicking the compact structure of the tumor-ECM. This work develops the therapeutic screening for prostate to-bone metastasis treatment. The human prostate cancer (PC-3) cells were co-encapsulated with human osteoblast (hOB) cells in 3D microgels containing the tumor ECM-mimicking components, methacrylated hyaluronic acid (HA-MA) and methacrylated Gelatin (GelMA). The antitumoral activity of cisplatin (CDDP) was assayed in 3D microgel co-cultures and compared with single and co-culture 3D multicellular spheroids counterparts. Cytotoxic studies demonstrated that CDDP induce less toxicity in 3D microgels than in the other platforms. The presence of ECM components in the 3D tumor models represents a step forward in the preparation of tumors mimicking the in vivo conditions, allowing cell attachment and proliferation.
The effective retrieval of 3D spheroids from culture systems is crucial for further applications. For closed microfluidic or hanging drop systems, this step is usually challenging. Sun et al. [86] have recently demonstrated that using a superhydrophobic performed microwell plate (SHPMP), retrieving the generated 3D spheroids would be an effortless procedure. The non-wettability of the well surface allowed the spheroid-containing droplets in the wells of the SHPMP to be easily recovered without disrupting the integrity of spheroids under either individual or batch spheroid collections.
Although the two platforms have demonstrated excellent results in the preparation of 3D spheroids derived from both cell lines and patient tumor cells, the sitting drop approaches seem to show additional benefits in assessing the biological properties of the derived structures. Fruit of this revision, the more remarkable concerns are: (i) direct quantitative cytotoxicity assay, such as the classical MTT assay in 2D monocultures, (ii) direct immunoassays, and (iii) high yield and non-disruptive spheroids retrieval.
This work reviews the recent literature on the promotion of cell aggregates formation strongly influenced by surface hydrophobicity. To the best of our knowledge, it can be regarded as the first review work fully dedicated to this emerging topic. However, in spite of the rapid growth of such techniques as a new frontier for in vitro studies, the literature still has to fulfill some points like detection of sphericity, the influence of surface properties, composition, and geometry of preferential promotion of certain cell lines and their growth dynamics.
New insights in preparing spheroids containing endothelial cells or stem cells as building blocks for scaffold-based and -free tissue engineering would benefit from applying superhydrophobic-based surfaces. Angiogenesis represents an essential target for developing new therapeutic strategies. Different issues include blood vessel formation and testing the efficiency of novel pro-and anti-angiogenic compounds in a reproducible and cost-efficient preclinical setting. Related to this issue is the development of organoid cultures. Organoids should satisfy several criteria, such as (i) possess a 3D structure containing cells that establish or retain the identity of the organ from which they were derived, (ii) include the presence of multiple cell types, as in the organ itself, (iii) exhibit some aspects of the specialized function of the organ and (iv) display self-organization according to the organizing principles as in the organ itself. The starting point for developing organoid cultures can be tissue-specific, adult stem cells, cancer stem cells derived from patient biopsies, or pluripotent stem cells, either embryonic or induced. When established with 3D extracellular matrices, the cultures can recapitulate the in vivo architecture, spatial organization, and genetic diversity of the cell populations found in the original organ with remarkable fidelity. Superhydrophobic-based surfaces would contribute to the development of these 3D organotypic structures.
MF, FC and MCM preparation of the manuscript; MF coordination of the manuscript; MF, FC and MCM final review of the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicable.
This work was conceived in the framework of the Cooperation Agreement between Faculty of Pharmacy and Food Science (UB) -Institute for Chemistry of Condensed Matter and Technologies for Energy (ICMATE-CNR) (Codi GREC 18407, 2018-2022) and under the umbrella of the Topical Team: Biofilms from an interdisciplinary perspective from the European Spatial Agency (ESA).
This research received no external funding.
The authors declare no conflict of interest.











