1 School of Postgraduate Studies, International Medical University, 57000 Kuala Lumpur, Malaysia
2 School of Health Sciences, International Medical University, 57000 Kuala Lumpur, Malaysia
3 Faculty of Medicine, Universiti Malaya, 57000 Kuala Lumpur, Malaysia
4 Faculty of Medicine and Health Sciences, Universiti Tunku Abdul Rahman, 43000 Selangor, Malaysia
5 School of Medicine, International Medical University, 57000 Kuala Lumpur, Malaysia
†These authors contributed equally.
Academic Editor: Graham Pawelec
Abstract
Background: Oncolytic properties had been demonstrated in Mammalian Orthoreovirus (MRV) and Avian Orthorevirus (ARV). Besides MRV and ARV, Pteropine Orthoreovirus (PRV) is also categorized under the genus Orthoreovirus. PRV7S (Sikamat virus) is an orthoreovirus isolated in Malaysia. Present study aims to investigate the oncolytic effects of PRV7S on ranges of nasopharyngeal carcinoma (NPC) cells through apoptosis in comparison to MRV3. Methods: Non-cancerous nasopharyngeal (NCNP) and NPC cells were infected by PRV7S and MRV3. The effects of PRV7S on the proliferation inhibition and apoptotic activity of NPC cells was examined using MTT assay and flow cytometry. Additionally, western blot assay was performed to analyze the expression of RAS and apoptotic protein. Lastly, qPCR assay was performed to demonstrate that PRV7S and MRV3 replicated in infected-NPC and infected-NCNP cells. Results: The proliferation of NPC cells were significantly inhibited after PRV7S infection in a time dependent manner in comparison to infected-NCPC cells. Flow cytometry analysis showed that PRV7S infection was able to induce apoptosis on NPC cells at 48 hpi. Western blot results showed that upon PRV7S infection, N/H/K RAS protein expression was reduced, whereas caspase-3 protein expression increased in NPC cells. qPCR assay showed higher viral load of PRV7S found in infected-NPC compared to infected-NCNP cells. Conclusions: PRV7S inhibits the proliferation and induces apoptosis of NPC cells similar to MRV3. Therefore, PRV7S is a potential oncolytic virus.
Keywords
- pteropine orthoreovirus
- nasopharyngeal carcinoma
- mammalian orthoreovirus
- oncolytic virus
- apoptosis
- bat reovirus
Nasopharyngeal carcinoma (NPC) is a specific type of cancer which occurs between the head and neck region. NPC is also known to be one of the most common cancer among the South-East Asian and Southern China population [1, 2]. According to World Health Organization (WHO), in the year of 2020, NPC is the 4th most prevalent cancer among both females and male at all ages, accounting for 4.6% and 7.4% respectively of the overall cancer cases in Malaysia. NPC is often characterized as poorly differentiated or undifferentiated carcinoma, making the prognosis of the disease difficult for physicians. Thus, majority of the NPC patients are only diagnosed at advanced stages such as stage III/ IV of the disease [1, 3]. Presently, common treatments for NPC include local tumor surgery, chemotherapy, and radiotherapy. Chemotherapy and radiotherapy are known to be unselective cancer treatments, by killing both cancerous and healthy cells which lead to major post-treatment side effects to the patient. Therefore, oncolytic virus and monoclonal antibody therapies are being extensively studied as alternative cancer treatments.
Reovirus is a member under the family Reoviridae, a double stranded RNA, non-envelope, unmodified wild-type virus which are commonly isolated from the respiratory or enteric tract [4, 5]. Evidence from previous studies have shown reovirus display selective oncolytic effects on human carcinoma cells, and hence, reovirus become a potential subject in oncolytic virus therapy research and development [3, 4, 5]. Mammalian Orthoreovirus 3 (MRV3) also known as reovirus Type 3 Dearing (T3D), is an oncolytic reovirus that has been extensively investigated in preclinical studies and clinical trials. Pelareorep (REOLYSIN®, Oncolytic Biotech Inc) is a proprietary isolate of the human MRV3, it contains live, replication-competent reovirus in its purified form [6, 7, 8]. MRV3 has been studied in clinical trial I and II with combination treatment of chemo-drugs, cisplatin and carboplatin on head and neck cancer. The results demonstrated promising results on the combination treatment of MRV3 and chemo-drug [7, 8, 9, 10].
Pteropine Orthoreovirus (PRV), which is the fusogenic subgroup of the orthoreovirus family, it was first isolated in Australia and now being discovered and isolated in Malaysia, Hong Kong, China, Japan, and other parts of the world. Chronologically, in Malaysia, PRVs were isolated from fruit bats in Pulau Tioman, and from patients in Kampar, Melaka and Sikamat [11, 12, 13, 14, 15]. Generally, PRV infected patients display broad disease spectrum ranging from acute respiratory distress to influenza-like illness. Most common symptoms reported are sore throat and cough. Furthermore, there was no report of death due to PRV and long-term study of PRV infection [16]. As PRV share similar genetic relations to MRV3, in this current study we examine the potential of PRV as oncolytic virus by investigating the cytopathic effect of PRV7S (Sikamat virus) in between NPC cells and non-cancerous nasopharyngeal (NCNP) cells and its oncolytic ability through apoptosis.
CNE1, TWO1, HONNE1, SUNE1 are nasopharyngeal carcinoma (NPC) cells utilized this
study, while NP69 and NP640 are non-carcinoma nasopharyngeal (NCNP) cells. CNE1,
TWO1, HONNE1, SUNE1 and NP69 cells were cultured in Roswell Park Memorial
Institute (RPMI) 1640 medium with 10% fetal bovine serum (FBS) with 1%
penicillin/streptomycin antibiotics. NP460 cells were cultured in keratinocyte
serum-free medium supplemented with bovine pituitary extract (Gibco, USA). The
cultured cells were incubated in humidified atmosphere with 5% CO2 at 37
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was
performed to access the cell viability of NPC and NCNP cells after infected by
PRVs at different multiplicity of infection (MOI) 10, 1 and 0.1. MOI was
calculated based on TCID50 [3]. MTT assay was performed in a time point manner
with cell density of 1
A total of 1
PRV7S induced cell apoptosis on CNE1, TWO1 and NP69 cells (total of 18 samples
including biological replicates) were assessed by using flow cytometry analysis
at 48 h post infection. All flow cytometry analysis were conducted by using FACS
Calibur (Becton Dickson [BD]) and analyzed by Cell Quest Pro software. Annexin V
apoptosis detection kit (BD Biosciences) were utilized in this study according to
manufacturer’s protocol. Prior 48-hours-PRV7S-post-infection, all cells were
harvested and then dual stained by Annexin V FITC antibody and propidium iodine
(PI) for 30 minutes at 37
After infection with MOI 0.1 and 10 of PRV7S for 6, 24 and 48 h, respectively,
NPC (CNE1 and TWO1) and NCNP (NP69) cells were collected for Western blot
analysis. The cells were lysed by using ice-cold RIPA buffer with protease and
phosphatase inhibitors. The total protein concentrations were measured with the
Bradford method. Protein lysates were separated by sodium dodecyl sulfate
polyacrylamide gel electrophoresis (SDS-PAGE) with 12% polyacrylamide gels.
Next, the gels were transferred to PVDF membrane via wet transfer. After wet
transfer, the membrane was washed with TBST and 5% bovine serum albumin to
prevent from unspecific bindings. Furthermore, the membranes were probed with
primary antibodies (1:1000 dilution) in Hikari solution (Nacalai Tesque, Japan).
The primary antibodies against N/H/K-Ras (Elabscience, USA) and Caspase-3
(FineTest, China) were used, while
Real-time polymerase chain reaction (qPCR) assay was performed to detect viral
RNA in the infected cell culture. In brief, cells were harvested, and RNA was
isolated by using Trizol (Life Technologies, USA) according to manufacturer’s
protocol. Equal molar of total RNA (0.5ug) was utilised to generate complementary
DNA (cDNA) using using random primers in Tetro cDNA synthesis kit protocol
(Bioline, United Kingdom) as per manufacturer’s protocol. Real-time PCR was
performed using SensiFAST SYBR kit (Bioline, United Kingdom). Primers sequences
of PRVSig1F2 5′-TGC TGA TTG GAA YGC TGA CT-3′ and PRVSig1R2 5′-CKG AAA AGG YTT
GAK ACG CC-3′ were used for quantification of PRV by targeting PRV sigmaA/1 gene
[17]. The qPCR protocol started with 95
The cytopathic effects of PRV7S were assessed by using MTT assay in NPC cells
(CNE1, TWO1, HONNE1, and SUNE1) over a time interval. According to Fig. 1,
infected NPC cells viability decreased significantly (
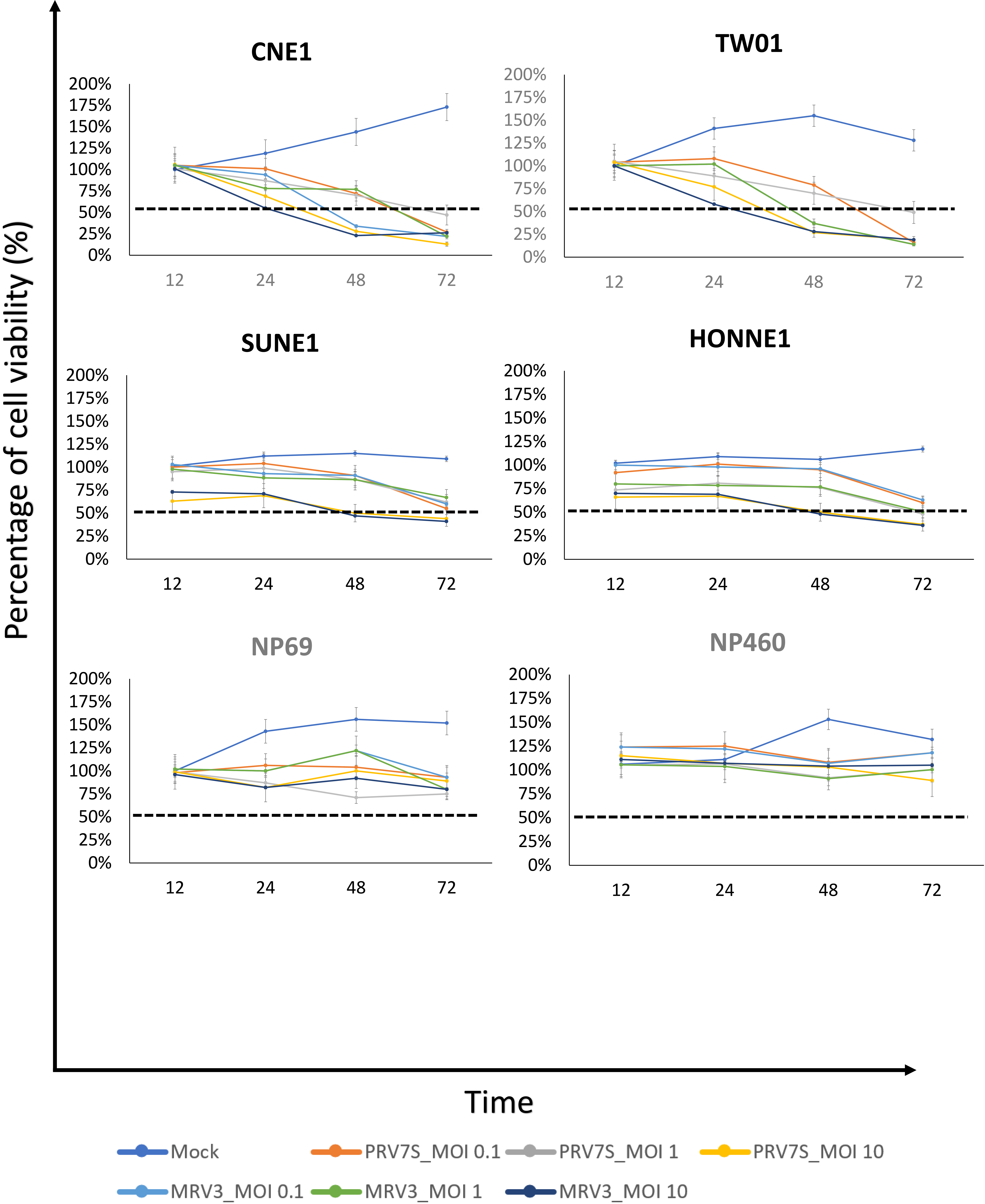 Fig. 1.
Fig. 1.Cell viability of nasopharyngeal carcinoma (NPC) cells, TW01, CNE1, SUNE1 and HONNE1 and non-carcinoma nasopharyngeal (NCNP) cells, NP69 and NP460 infected with PRV7S (Sikamat virus). MTT assay was performed at different MOI (10, 1 and 0.1) for 12, 24, 48 and 72 hrs. Black dotted line as 50% cell viability indication and different line colors represent PRV7S (Sikamat virus), MRV3 (Mammalian Orthoreovirus Type 3), and mock infection at respective MOIs.
The cytopathic effects of PRV7S on cellular morphology of infected CNE1, TW01 and NP69 were observed under light microscope over 24, 48 and 72 hpi respectively (Fig. 2). The infected NPC cells demonstrated gradual changes over time such as cell shrinkage and cell detachment. These changes further indicate cellular death upon infected by PRV7S. In comparison, the mock infected-CNE1, and mock infected-TW01 displayed define squamous cell morphology, greater confluency, and compact attachment to adjacent cells. On the contrary NP69 cells shown no morphology differences between the PRV7S and mock infection upon observation until 72 hpi. Similar observations were recorded for MRV3- infected cells (Supplementary Fig. 1). Moreover, multinucleated cells were observed on PRV7S infected NPC cells at approximately 12 hpi (Supplementary Fig. 2). This observation indicated besides inhibits cell proliferation, PRV7S infection induced cell death to NPC (TWO1 and CNE1) cells.
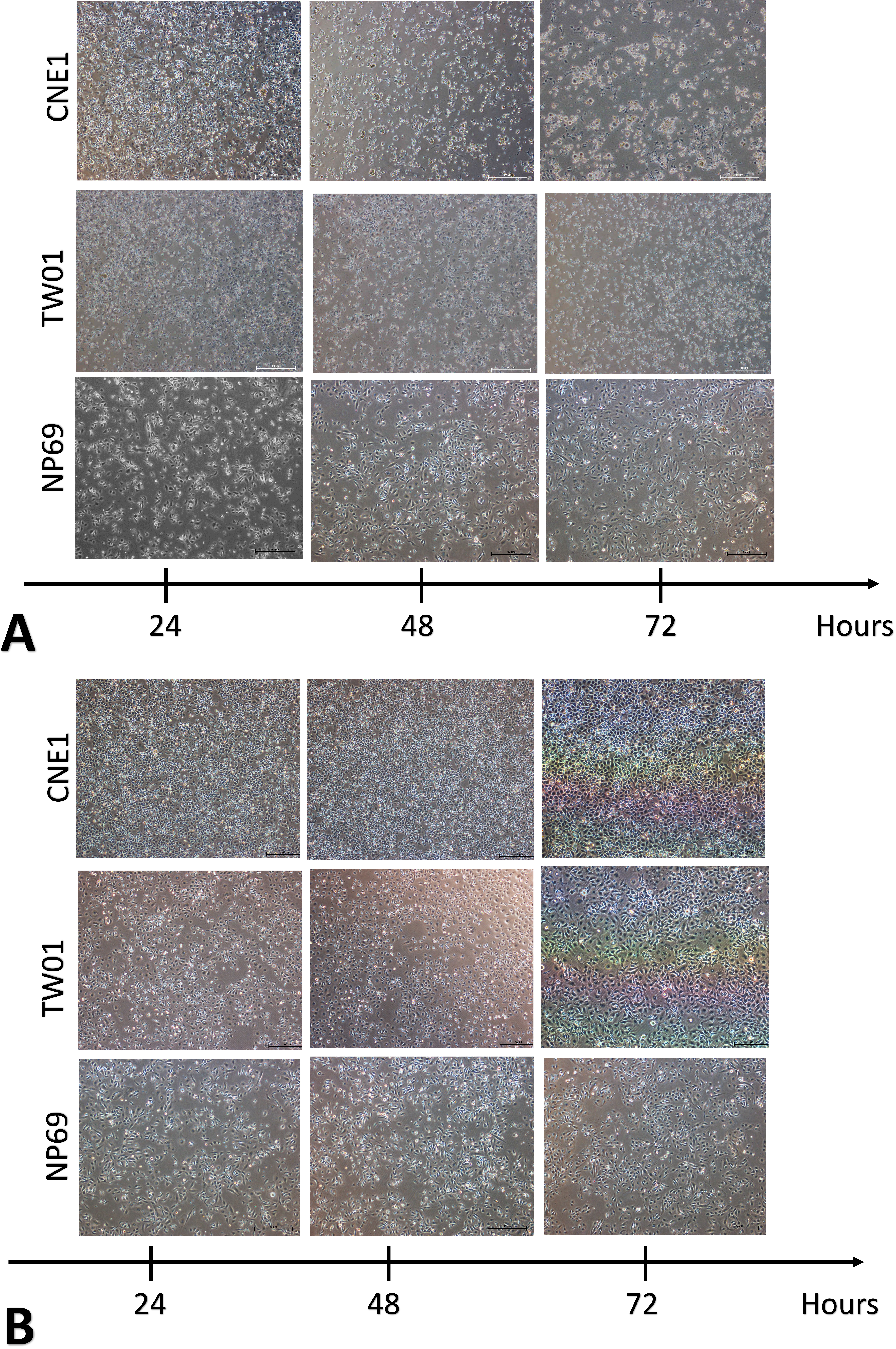 Fig. 2.
Fig. 2.Microscopy observation of (A) PRV7S and (B) mock infected CNE1, TW01 and NP69 at MOI 0.1 for 24 h, 48 h and 72 h.
By using Annexin V-FITC/PI double staining, we were able to determine the percentage of live, early apoptotic, late apoptotic, and necrotic cells distinctively and quantitatively. MOI 0.1 of PRV7S and MRV3 was selected as the working concentration. The early apoptotic rate among PRV7S infected CNE1, TWO1 and NP69 was 22.80%, 67.55% and 16.09% respectively; while late apoptotic rate among PRV7S infected CNE1, TWO1 and NP69 was 44.58%, 30.59% and 17.74%, respectively (Fig. 3A). According to the flow cytometry scatter plot (Fig. 3A), apoptotic cell distribution varies between cell lines. CNE1 infected with PRV7S shown a majority in late apoptotic population, showing cell distribution towards the upper right quadrant of the scatter plot. On the other hand, PRV7S infected TW01 cells shown a majority in early apoptotic population, cell distribution towards the lower right quadrant of the scatter plot was observed. Similar apoptotic rates and populations distribution were also observed from the MRV3-infected CNE1, TW01 and NP69 cells. Besides, PRV7S-infected NP69 cells shown slightly lower apoptosis population, with average of 16.09% early apoptosis population and 17.74% late apoptosis population compared to MRV3-infected NP69 with average of 22.86% early apoptosis population and 20.31% late apoptosis population. Moreover, the results showed that NPC cells infected with PRV7S and MRV3 exhibited a significant increase in total apoptotic cell populations (early + late) compared to mock infection (control), p = 0.0003. Total apoptosis percentage of PRV7S-infected CNE1, TW01 and NP69 were 67.38%, 98.14% and 33.83%, respectively; MRV3-infected CNE1, TW01 and NP69 were 66.53%, 78.71% and 43.17% respectively, versus the mock-infected CNE1, TWO1 and NP69 cells with total apoptosis percentage of 21.9%, 23.96% and 25.81% respectively (Fig. 3B). The results also showed significant increase of early apoptosis and late apoptosis rate between the PRV7S and MRV3 infected group and control group (mock). p-value of early apoptosis and late apoptosis PRV7S and MRV3 infected cells versus mock-infected cells were p = 0.008 and p = 0.006 respectively (Fig. 3B). These findings demonstrated that PRV7S induced cell apoptosis in NPC cells and perhaps in a similar pathway as MRV3.
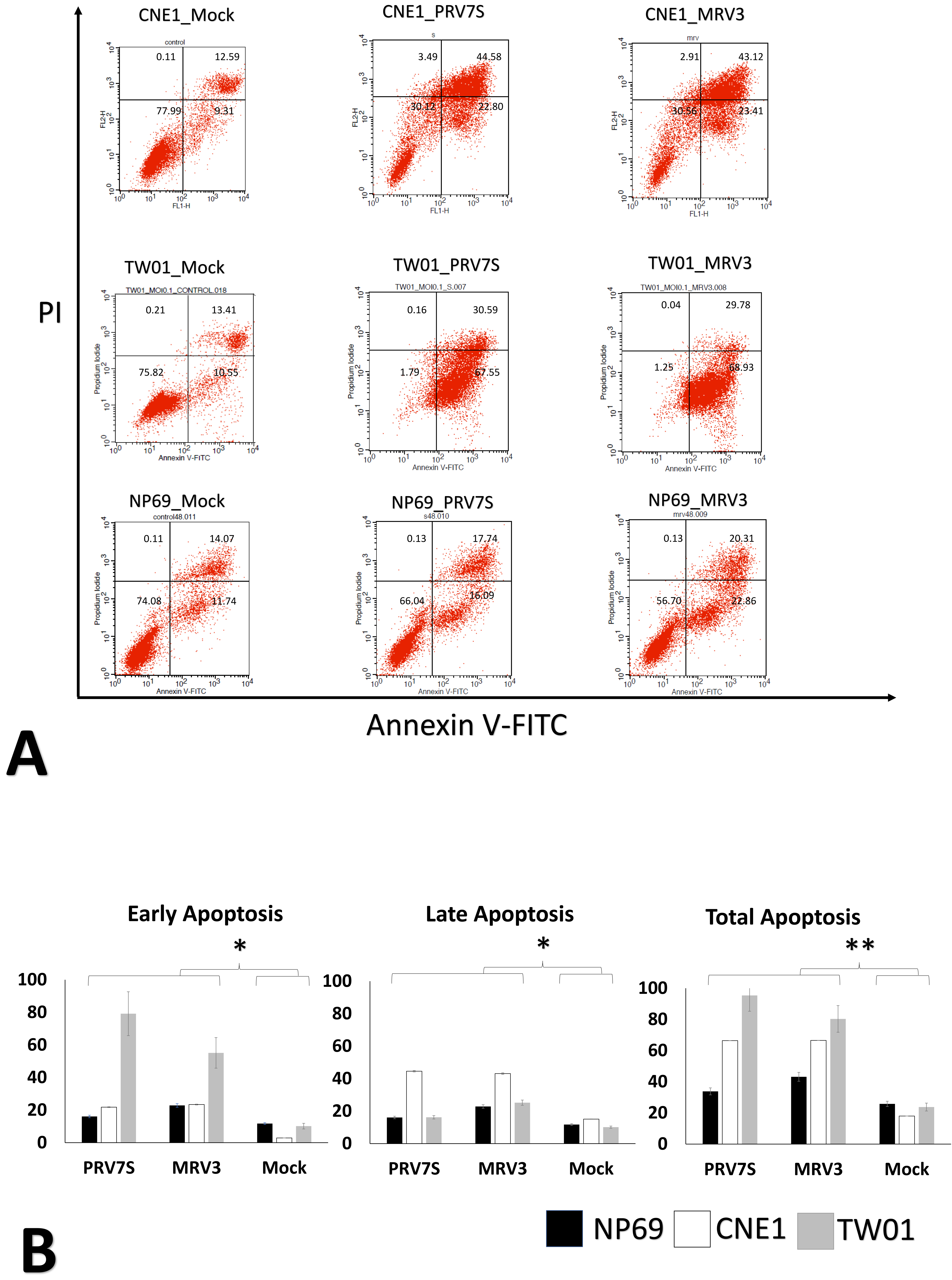 Fig. 3.
Fig. 3.Flow cytometry of apoptotic CNE1, TWO1 and NP69 cells analyzed
by Annexin-V FITC/PI detection kit at 48 hpi. (A) Flow cytometry scatter plot,
proportion of live cells (Lower left quadrant), early apoptosis (Lower right
quadrant), late apoptosis, (Upper right quadrant), necrosis (Upper left
quadrant). (B) Column diagram of late/early/total apoptosis population of NPC
infected by PRV7S (Sikamat virus) and MRV3 at MOI 0.1 versus mock infection
cells. Total sample size, n = 18, statistically significant differences
represented by asterisk, * p
To examine whether RAS signaling pathway is associated with apoptosis, we investigated the protein expression of N/H/K RAS protein and caspase-3 in NPC and NCNP cells infected by PRV7S (Fig. 4). Western blot results showed that caspase-3 expression was not observed in mock-infected TW01 and CNE1 cells. Upon PRV7S infection, caspase-3 expression was upregulated in TW01 and CNE1 at 6, 24 and 48 hpi. Specifically, highest signal of caspase-3 expression was observed at 48 hpi for both PRV7S-infected CNE1 and TW01 cells. Expression of caspase-3 proteins were not observed from the mock-infected NP96. On the other hand, PRV7S-infected NP69 shown faint expression of caspase-3 protein. Both western blots and flow cytometry analysis are supporting that PRV7S infection induced higher apoptosis events in CNE1 and TWO1 but less in NP69 cells.
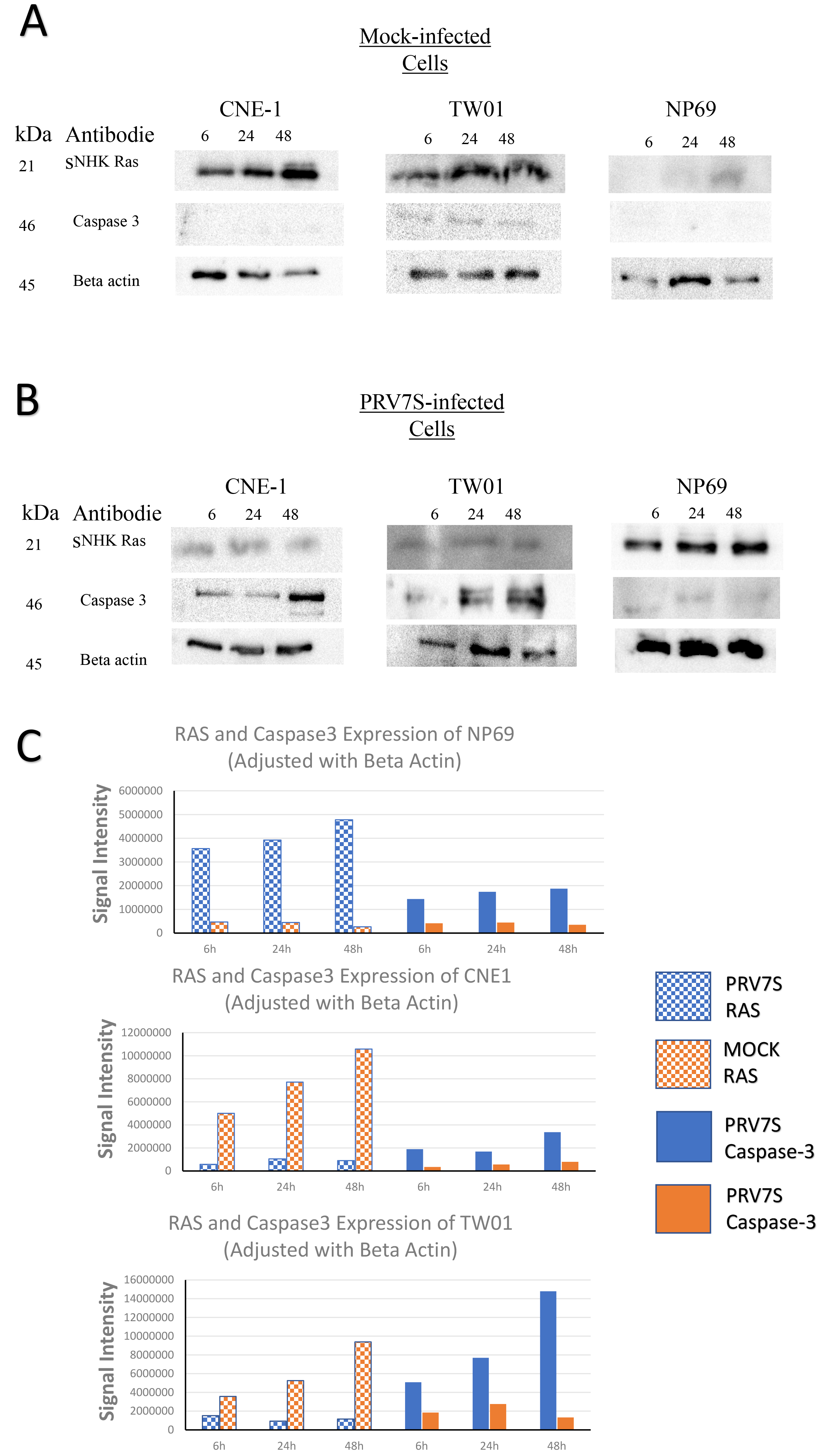 Fig. 4.
Fig. 4.Western blot analysis of nasopharyngeal carcinoma (NPC)
cells, TW01 and CNE1 and non-carcinoma nasopharyngeal (NCNP) cells, NP69. (A)
Expression of NHK Ras and caspase-3 in mock-infected NPC and NCNP,
Expression of N/H/K RAS proteins were downregulated after infection of PRV7S in NPC (CNE1 and TWO1) cells. PRV7S-infected NP69 cells shown expression on N/H/K RAS protein suggesting that RAS transformation occurs despite no observation of cell deaths within 48 hpi. Nevertheless, we observed that PRV7S-infected-NP69 cells experienced senescence and unable to multiply after 14 days pi.
The qPCR assay showed that both PRV7S and MRV3 are able to infected both NPC (CNE1 and TWO1) cells and NP69 cells (Fig. 5). It was observed that PRV7S- and MVR3-infected CNE1 and TW01 cells had higher viral RNA load than infected NP69 cell.
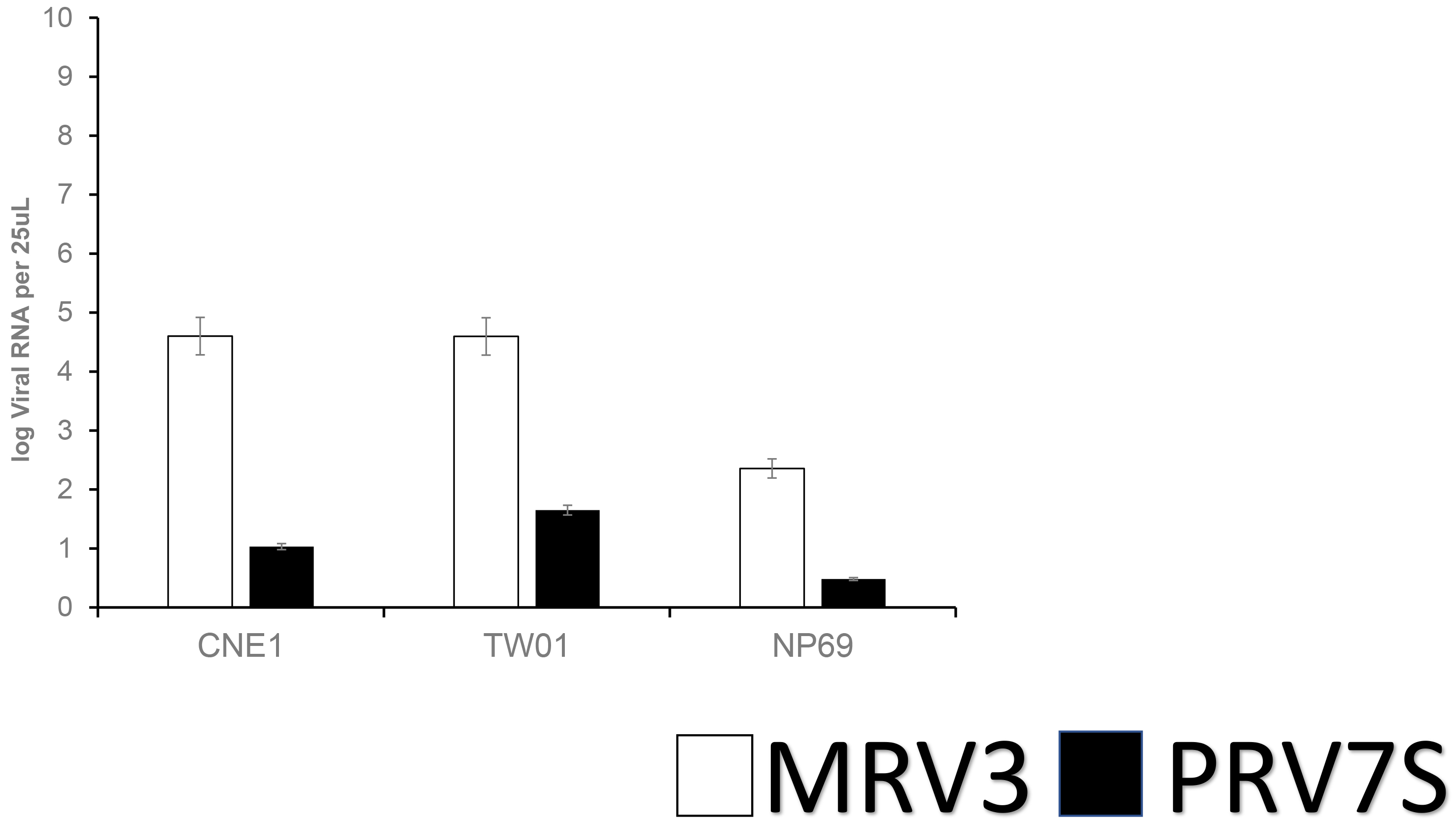 Fig. 5.
Fig. 5.Detection of RNA viral load PRV7S versus MRV3 at MOI 5 at 48 hpi; error bars indicate the standard deviation.
In vitro cell viability reduction upon PRV infection via MTT assay on ranges of NPC cell lines have revealed that nasopharyngeal carcinoma cell lines are susceptible to PRV infection by MOI 0.1, 1 and 10. Reduced cells viability on NPC cells (CNE1, TW01, SUNE1 and HONNE1) were observed at 12 h post-infection and it achieved maximum inhibition at 72 h post-infection regardless of the viral titer. Among the NPC cells tested, CNE1 and TW01 were found highly susceptible towards PRV7S infection among the NPC cells compared to HONE1 and SUNE1. The susceptibility might due to the different characteristic and origin of the cell lines. In comparison, CNE1 was sourced from a well differentiated squamous carcinoma cell biopsy of a patient from China [19]; TW01 was estatablished from keratinizing squamous cell carcinoma of a patient in Taiwan [20]; HONE1 was from a China patient with poorly differentiated squamous cell carcinoma [21]; SUNE1 was derived from a Chian patient with Epstein-bar virus positive poorly differentiated squamous cell carcinoma [22]. According to the MTT assay results, HONE1 and SUNE1, which has poorly differentiated cells characteristics showed lower sentitivity towards PRV7S infection in comparison to CNE1 and TW01. On the other hand, non-carcinoma nasopharyngeal cell lines (NP69 and NP460) viability remained stable after PRV7S post-infection. This study demonstrated that PRV7S able to significantly inhibit cell proliferation in nasopharyngeal carcinoma cell lines in a time dependent manner, regardless of MOI, while causing least proliferation inhibition on non-carcinoma nasopharyngeal cell lines.
Cytopathic effect was observed in the PRV7S- and MRV3-infected NPC cells. Besides that, multinucleated cell formation was observed from the PRV7S-infected NPC cells as hallmark of fusogenic virus infection. However, syncytia formation was not found. Hence, it is hypothesized that despite reducing cell proliferation, PRV7S could analogously induced apoptosis towards NPC cells as per MRV3 [23, 24]. Flow cytometry results revealed that both PRV7S-infected and MRV3-infected NPC cells (CNE1, TWO1) showed increased positive signals for late and early apoptosis compared to the mock infection group. Significantly lower percentage of apoptotic cells population were observed in PRV7S-infected and MRV3-infected NP69 cells. Taken together, PRV7S was able to induce apoptosis in infected NPC cells. Besides, both PRV7S-infected and MRV3-infected NPC cells, CNE-1 and TW01 respectively displayed difference in apoptosis proportion with CNE1 having more late apoptosis population, while TW01 having more early apoptosis population. Necrotic populations are not included as the study focus on investigating the late and early apoptotic populations of NPC and NCNP. These findings further emphasize that PRV7S demonstrated direct oncolytic features similar to MRV3, albeit that the MRV3 strain utilized in this study is not Reolysin strain.
Studies had shown that oncolytic reoviruses are able to selectively replicate in RAS-activated cancer cells and induce apoptosis [6, 17, 24]. Furthermore, published studies demonstrated that MRV3 (Reolysin strain) selectively replicate and induced apoptosis in Ras activated tumor cells via induction of endoplasmic reticular stress induction and caspase-3 processing [17, 24]. MRV3 is shown to induce direct oncolytic in head and neck cancerous cells [3, 9], which differs from the cell lines utilized in this study. Hence, expression of caspase-3 and N/H/K RAS proteins were evaluated by using Western blotting to investigate in the relationship between PRV7S induced apoptosis and activation and/or overexpression of regulatory elements in RAS signaling pathway in NPC. Result shown PRV7S-infected NPC has reduced N/H/K RAS protein expression and increased caspase-3 protein expression compared to the mock-infected NPC cells. RAS proteins are responsible for cell growth and differentiation, while caspase-3 is one of the key executional proteins in apoptosis pathway. Result suggested that PRV7S infection caused inhibitory effect on the expression of N/H/KRAS in NPC leading reduction in cell proliferation and lead to induction of apoptosis in NPC, and thus, upregulation of caspase-3 expression. Therefore, we deduced PRV7S inhibit RAS activated NPC and induced apoptosis. Interestingly, PRV7S infected non-carcinoma nasopharyngeal cell, NP69 N/H/K RAS protein expression was found upregulated. However, NP69 has neither morphological changes nor significant cell death reported in this study despite the increased N/H/K RAS protein expression upon PRV7S infection. The effects of PRV7S on non-carcinoma cell will be further investigated in future study as current report focus on the potential of PRV7S oncolytic effects.
Lastly, PRV7S and MRV3 viral RNA were detected in NPC and NCNP cells. Generally, PRV7S-infected, and MRV3-infected CNE1 and TWO1 cells show higher viral load as compared to NP69 cells at 48 hpi. This result aligns with the suggestion that PRV7S able to replicate inside the cells and has direct oncolytic effect which selectively cause cytopathic effect to RAS activated carcinoma [6, 14, 17, 24]. Moreover, lower viral RNA load of PRV7S was found in the infected NPC and NCNP cells compared to MRV3 viral load. The result suggesting MRV3 has higher replication kinetic in these cells, and suggesting higher viral load being released in supernatant in comparison to PRV7S. Fig. 1 demonstrated rapid reduction in NPC cells viability by MRV3 suggesting that increased cytotoxic effect of the MRV3 may be correlated to viral load by viral replication. Taken all these findings, it appears that MRV3 is superior to PRV7S in term of oncolysis effect in NPC cells.
As PRVs are emerging zoonotic infectious agent that most probably harbored by bats, PRVs posed a safety concern as oncolytic virus in human. Our experimental results shown, PRV7S-infected non-carcinoma cells, NP69 and NP460 had above 50% viability in both MTT and flow cytometry assay, no morphological change was observed in PRV7S infected non-carcinoma nasopharyngeal NP69 cells from 24 h until 72 h post-infection, low protein expression of caspase-3 and lower PRV7S viral load detected upon 48 hpi as compared to NPC. This showed a preliminary safety utilization of PRV7S on non-carcinoma human cells.
The usage of oncolytic reoviruses as an alternative to conventional chemotherapy and radiotherapy are investigated due to its capability to selectively replicate and trigger anti-cancer responses in cancer cells while having minimal pathogenicity in normal cells [23, 24, 25]. Recent clinical trials had been done on Reolysin, also known as pelareorep because of its oncolysis impact against different malignancies [7, 8, 9, 10, 17, 24]. Besides, Avian Orthoreovirus (ARV) also demonstrated to exert oncolytic effects on human hepatocellular carcinoma cells [18, 26]. Above mentioned reovirus are derived from the genus Orthoreovirus, whereby Reolysin belongs to the non-fusogenic group, while ARV belongs to the fusogenic group [15]. It is hypothesized that Pteropine Othoreovirus (PRV), another species of othoreovirus under the fusogenic group would display potential oncolytic effect on carcinoma cells. Our results showed significant oncolysis effect of PRV against Ras-activated NPC cells in time dependent manner and causing least pathogenicity towards NCPC.
The oncolytic mechanism of MRV3 had been much investigated but the potential of PRV as oncolytic virus is less elucidated. Even though the MRV3 utilized in this study is not Reolysin strain, the findings indicated oncolytic activity via apoptosis exhibited by PRV may be of similar mechanisms to MRV3. Further study should be undertaken to improve the understanding on the aspect of PRV oncolytic properties, such as the virulence factor, immune checkpoint blockade activity, apoptosis signaling pathway and delivery method especially on the protein expression of the apoptosis cascade and involvement of RAS signaling pathway of NPC induced by PRV.
PRV, Pteropine orthoreovirus; PRV7S, Sikamat-virus; NPC, nasopharyngeal carcinoma; NCNP, non-carcinoma nasopharyngeal; MRV3, Mammalian Orthoreovirus 3; T3D, reovirus Type 3 Dearing; Hpi, hours post-infection; qPCR, real-time polymerase chain reaction.
AL, SS and KV perfomed and analyzed MTT. AL performed flow cytometry. NAS and PQC performed the real-time PCR. KV, PPL, BKT and STW designed the experiment. KV, RYK, and SMC supervised the experiment. AL, PPL, and KV wrote and edited the manuscript.
Not applicable.
This work was supported by International Medical University Malaysia for the research facilities.
This research was funded by the Fundamental Research Grant Scheme (FRGS) 2019-1, from the Ministry of Higher Education, Malaysia and International Medical University Research Grant.
The authors declare no conflict of interest.
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.31083/j.fbl2704138.





