1 The Bartholin Institute, Department of Pathology, Rigshospitalet, 2100 Copenhagen, Denmark
Academic Editor: Paola Giussani
Abstract
Particular molecules play pivotal roles in the pathogenesis of many autoimmune diseases. We suggest that the C24:0 sulfatide isoform may influence the development of type 1 diabetes (T1D). C24:0 sulfatide is a sphingolipid with a long carbon-atom chain. A C16:0 sulfatide isoform is also present in the insulin-producing beta cells of the islets of Langerhans. The C16:0 isoform exhibits chaperone activity and plays an important role in insulin production. In contrast, the C24:0 isoform may suppress the autoimmune attacks on beta cells that lead to T1D. Sphingolipid levels are reduced in individuals who later develop T1D but could be increased via dietary supplements or medication.
Keywords
- sulfatide
- C24:0 sulfatide
- type 1 diabetes
- pathogenesis
- fenofibrate
- beta cells
The question has been raised whether during development of type 1 diabetes (T1D), a certain molecule exists which drives the disease. Some molecules play key roles in the development of other autoimmune diseases, such as gluten in coeliac disease, desmosomes in pemphigus, and thyroglobulin in Graves’ disease. These molecules are not virus initiators. In common for autoimmune diseases are involvement of the adaptive immune system and autoreactive T lymphocytes. Therefore, a disease-related molecule that drives the development of T1D may have an impact on immune responses but may not itself be directly attacked.
T1D is widely supposed to be initiated by an enterovirus [1, 2], and antibodies and T cells are directed against beta-cell antigens such as insulin, Glutamic Acid Decarboxylase (GAD), IA-2, and the Zinc-transporter protein [3]. At the very end, nearly all beta cells are destroyed by the adaptive immune system, an outcome that becomes unavoidable after having passed a “point of no return” at a critical juncture, which occurs approximately when the disease is diagnosed. Apparently, the adaptive immune system has no role in the development of type 2 diabetes (T2D), and this disease is driven by the inability of beta cells to produce sufficient quantities of insulin to meet demand.
Inherent physiology suggests that the production of insulin by the beta cells is fraught with danger. One active beta cell in the islets of Langerhans can release one million insulin molecules per minute directly into the blood [4]. The beta cells are surrounded by cells from both the innate and the adaptive immune systems that are ready to react against any misfolded insulin molecules. Beta cells are the only endocrine cells in the human body that secrete highly immunogenic peptides; the adrenal and thyroid glands, as well as the cells that produce the sex hormones, deliver smaller molecules that are less immunogenic. The only other endocrine organ that produces peptide hormones is the pituitary gland, which is situated behind the blood brain barrier that gives a certain immunological protection. Furthermore, the pancreatic beta cells may be particularly vulnerable to cytokine-mediated toxicity and excessive insulin biosynthesis, due to their inability to counteract inflammation and oxidative stress. Plasma levels of oxidative stress markers increase during early onset T1D and are even higher by early adulthood. Oxidative stress occurs when reactive oxygen species levels overwhelm the various antioxidants, and beta cells are more sensitive to oxidative stress than other cells in the islets of Langerhans because they have low levels of antioxidant enzymes such as, e.g., glutathione peroxidase, and catalase [5].
So, how do the beta cells protect themselves? For many years, we studied sphingolipids and found that a particular compound, sulfatide, acts as a chaperone for insulin [6]. Sulfatide facilitates folding of the proinsulin molecule in the Golgi apparatus and preserves insulin crystals in secretory granules at pH 5.5 [7]. When the pH increases to 7.4 during insulin secretion, sulfatide mediates monomerization of the insulin molecules and facilitates exocytosis of insulin granules outside the cell membrane [6, 8]. In addition, sulfatide also has anti-inflammatory properties [9], and it protects the surface of the beta cells against the immune system. The anti-inflammatory properties of sulfatide include decreasing the secretion of proinflammatory cytokines and chemokines [9], suppressing lipopolysaccharide-stimulated inflammation (via toll-like receptor 4) [10, 11], and inducing natural killer T cells [12].
When human islets of Langerhans were analysed, sulfatide was only found in the beta cells [13]. Two isoforms of sulfatide occur in the islets of Langerhans, and these isoforms are distinguished by the lengths of their carbon-atom chains: C16:0 and C24:0 [14]. Studies have shown that the short-chain isoform (C16:0) is responsible for all the physiological aspects of chaperone activity in insulin production [15]. In contrast, the long-chain isoform (C24:0) is responsible for immunogenic suppression [16]. We have shown that the drug fenofibrate increases the level of the C24:0 but not the C16:0 sulfatide isoform [17]. Furthermore, in the non-obese diabetic mouse model, fenofibrate prevented the development of diabetes entirely [13]. Fenofibrate has a good safety profile and has been used for many years to lower cholesterol. Interestingly, sulfatide can bind to angiotensin converting enzyme 2 (ACE2) [18], and patients with T2D exhibit less severe hypertension when sulfatide levels are high [19]. Fenofibrate and sulfatide can counteract severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2), which enters cells via the ACE2 molecule [20]. SARS‑CoV‑2 infection increased the incidence of T1D by approximately 2-fold [21], and the mechanism might be that low sulfatide levels may increase susceptibility to both T1D and coronavirus disease.
In T2D, the body experiences an increased demand for insulin due to excessive weight gain and intake of refined sugars and fatty acids, as well as to insulin resistance. Initially, the beta cells can produce nearly enough insulin; however, they cannot cope with the increased demand for insulin indefinitely. Interestingly, selective depletion of C16:0 but not C24:0 was observed in T2D animal models, such as db/db and ob/ob mice [22]. As described above, the chaperone activity of sulfatide in insulin production is associated with the C16:0, not the C24:0, isoform; in particular, the C16:0 sulfatide isoform preserves insulin crystals as effectively as raw sulfatide, whereas the C24:0 isoform has no effect [15].
Among the various human T1D prevention trials conducted to date, only those that have involved immunosuppression have shown proven efficacy. Such trials have included therapy with antibodies against CD3 [23] and immunoglobulins or medications that reduce the activity of B lymphocytes [24]. These are all anti-inflammatory effects, and the C24:0 sulfatide isoform may have similar effects in the islets of Langerhans [9, 16].
Consequently, we suggest that the presence of a critical level of active C24:0 sulfatide plays a key role in preventing the development of T1D. The onset of T1D may be suppressed by the presence of the anti-inflammatory C24:0 sulfatide isoform. But why might levels of this C24:0 isoform become depleted?
In a previous study, we found that the level of sulfatide in the islets of Langerhans of recently diagnosed patients with T1D was only 23% of that found in control participants [13]. This reduction in sulfatide levels was associated with reduced expression of enzymes involved in the metabolism of sphingolipids. We also identified polymorphisms in eight genes (ORMDL3, SPHK2, B4GALNT1, SLC1A5, GALC, PPARD, PPARG and B4GALT1) involved in sphingolipid metabolism that may contribute to a predisposition towards T1D [13]. Interestingly, sphingolipid levels are reduced in individuals who later develop T1D, even before beta-cell autoantibodies are present [25].
The relative proportions of the different sulfatide isoforms generated probably rely on the substrates that are available; therefore, the synthesis of C24:0 sulfatide will depend on the presence of adequate long-chain fatty-acid substrates (Fig. 1). Dietary constituents that are relatively rich in long-chain fatty acids include peanut oil, oats, fish, and bitter chocolate; in addition, some intestinal bacteria can produce long-chain fatty acids [26]. On the other hand, butter and meat are rich in C16:0 fatty acids. Human breast milk is rich in long-chain fatty acids; and compared with infant formulas, human breast milk has a significantly higher composition of long-chain polyunsaturated fatty acids [27]. Furthermore, breastfeeding is associated with a lower incidence of T1D. For these reasons, we suggest that the C24:0 sulfatide isoform may play a pivotal role in suppressing the autoimmune reactions that lead to T1D.
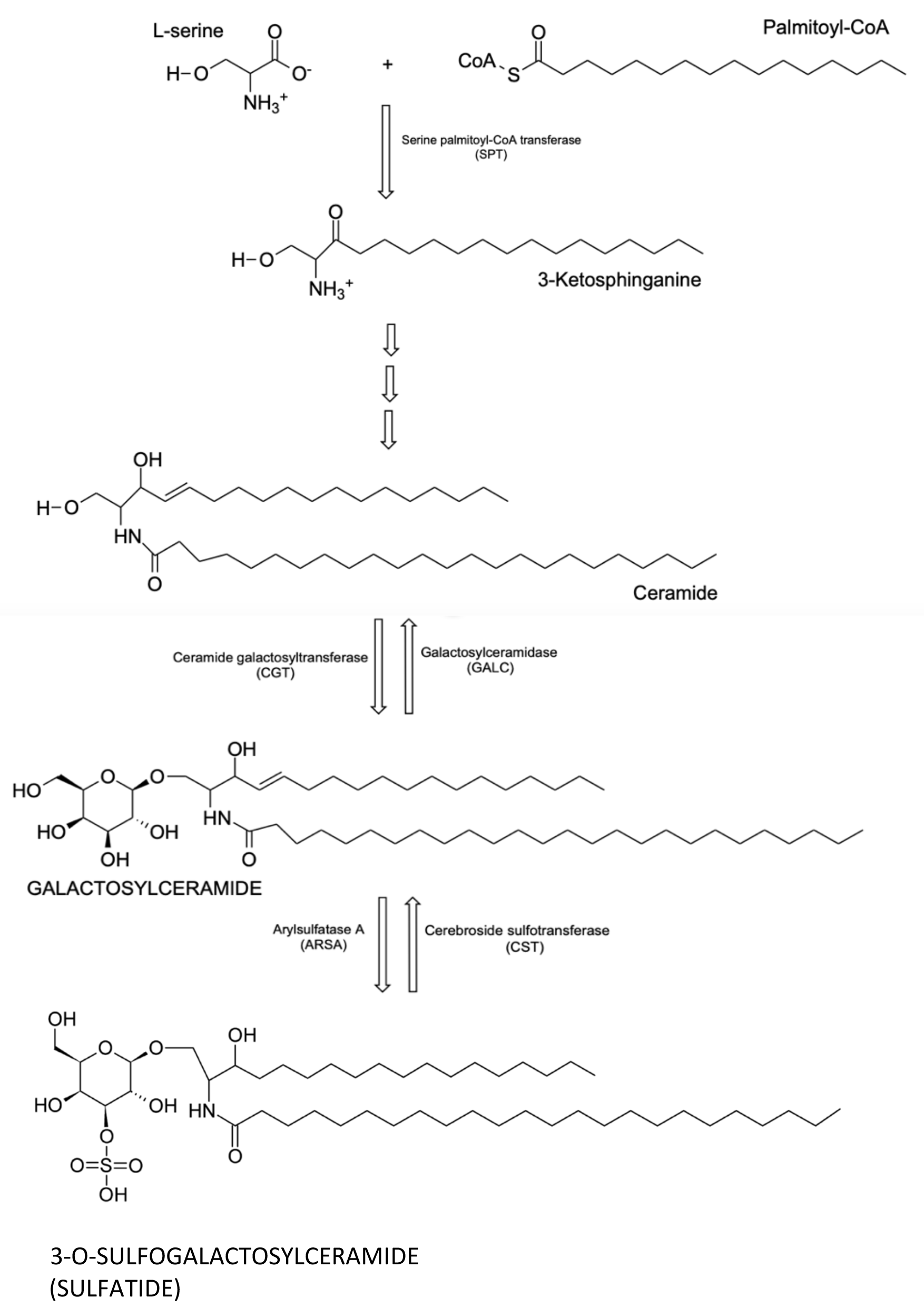 Fig. 1.
Fig. 1.The chemical structure of C24:0 sulfatide and its biosynthetic pathway.
KB conceived the idea and wrote the manuscript together with JCA.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.

