1 School of Life Sciences, University of Sussex, BN1 9QG Falmer, Brighton, UK
2 College of Medicine and Health, University of Exeter, EX1 2LU Exeter, Devon, UK
3 Royal Devon and Exeter NHS Foundation Trust, EX2 7JU Exeter, Devon, UK
Abstract
Tumour metastasis to the brain is a complex process involving crosstalk between
the circulating tumour cells and the blood brain barrier (BBB). Astrocytes, which
reside in the abluminal surface of the microvasculature of the BBB, are now known
to play an essential role in tumour cell migration and invasion into the brain
parenchyma. For instance, pro-inflammatory astrocyte secretions, including
TNF-
Keywords
- Astrocytes
- Blood brain barrier
- Metastasis
- Endothelium
It is estimated that cancer will affect 4 million people in the UK by 2030, an increase from 2.1 million today [1]. Metastasis to the brain involves tumour cells detaching from the primary tumour site by degrading the extracellular matrix (ECM) and entering circulation, followed by arrest and extravasation into a distant organ with the subsequent formation of secondary metastatic lesions [2]. One of the most common type of primary cancer that gives rise to brain metastases is the lung, with nearly 65% of patients estimated to develop this complication [3]. Breast carcinoma and melanoma are other known predominant cases of brain metastasis [4]. Despite brain metastasis being the most common of intracranial tumours, treatment options are limited, with chemotherapy proving clinically ineffective in patients with secondary metastatic brain lesions due to the inability of the drugs to penetrate the BBB [5]. Therefore, brain metastasis has a poor prognosis and is a significant contributor to mortality in patients [6], with the median survival rate ranging from 9 to 15 months [7]. This highlights the urgency to understand the mechanisms underlying tumour cell entry through the BBB and the subsequent rise of secondary lesions. Although rare (3%–5% incidence), brain tumours can metastasise from the brain to other organs, known as extraneural metastases. This can occur through lymphatic drainage, invasion through the dura or bone or invasion through the venous system [8]. However, extraneural metastases is resisted through the lack of ECM proteins in the central nervous system as well as the capillary basement membrane being a physical barrier against metastases migrating into the blood stream [9]. As the severity remains higher for cancer cells metastasising to the brain, this emphasises the need to explore the mechanisms underlying brain metastasis. Although numerous studies have reported the overall extravasation of circulating tumour cells (CTCs) into the brain, the molecular and cellular basis of this mechanism remains largely unknown. Therefore, in this review, we aim to present the current understanding of the molecular interactions and crosstalk between the components of the BBB including endothelial cells, astrocytes and pericytes along with CTCs in the blood-tumour barrier (BTB) that may contribute to the potential development of treatments for this deadly disease.
The BBB is a selective, partially permeable barrier formed of endothelial cells that tightly regulate the transport of ionic and fluid molecules into or from the cerebral microvasculature and brain parenchyma [10]. With tight-junctions and the compact structure of the endothelium, the BBB provides optimal conditions for neuronal function through the regulation of ionic traffic and protection from ionic fluctuations [11]. The fundamental structure of the BBB consists of endothelial cells, astrocytes and pericytes. These form the neurovascular unit (NVU), with endothelial cells being the primary cells of the BBB that interact with the CTCs (Fig. 1) [12].
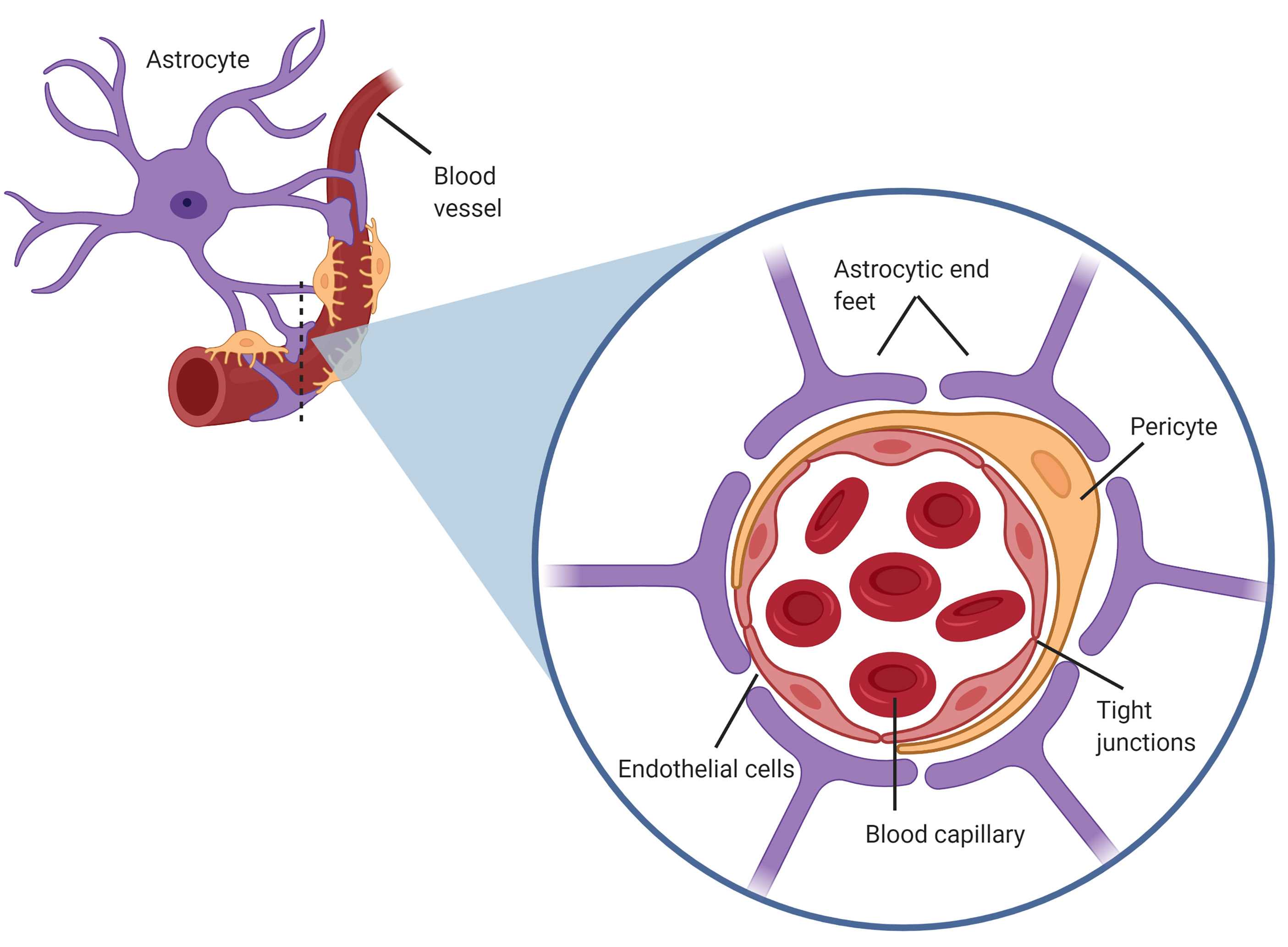 Fig. 1.
Fig. 1.Structure of the blood brain barrier (BBB), also known as the neurovascular unit, consisting of astrocytic end feet on the abluminal side of the capillary endothelium in close proximity with pericytes also coating the endothelium.
Astrocytes are glial cells in the central nervous system that are essential components of the blood brain barrier [10]. These cells maintain healthy BBB integrity by secreting factors that form and maintain strong tight junctional complexes between endothelial cells [11]. The astrocytes, reside adjacent to the endothelium, with their end feet almost entirely covering the abluminal membrane of the cerebral capillaries [11]. Astrocyte processes at the endothelium form an additional barrier, the glia limitans, by the formation of gap junctions and tight junctions between neighbouring astrocytic projections [13]. The astrocytic end feet tightly connect to the endothelium through specific interactions between the basal lamina and ECM [14]. Pericytes also lie in close contact with the abluminal side and are intimately associated with astrocytic end feet [13]. During development, pericytes coat the endothelial vasculature to produce a basal lamina that promotes accumulation of astrocytic end feet around the endothelium [13]. Due to this intimate relationship, both astrocytes and pericytes in the BBB play vital roles in maintaining barrier integrity and regulating permeability [11].
Successful cerebral secondary colonisation requires CTCs to adhere to and transmigrate through the cerebral vascular endothelium, invade the brain parenchyma, proliferate and form secondary lesions [15]. However, not all cancers have the propensity to enter the brain. There is evidence that certain types of cancers such as breast, lung and melanoma are indeed more prone to metastasising to the brain. For instance, patients with Her-2 positive metastatic breast cancer treated with trastuzumab are two-folds more likely to have brain metastasis than other breast cancer types [16]. A similar observation was also found in melanoma patients treated with EGFR kinase inhibitor gefitinib. Interestingly, overexpression of Stat3 in resected human melanoma brain metastases suggested a preferential migratory effect of cancer cells into the brain parenchyma [17]. Stat3 expression also increased angiogenesis and invasion [17]. Interestingly, elevated levels of activated leukocyte cell adhesion molecule (ALCAM) in non-small-cell lung cancer (NSCLC) resulted in an enhanced brain metastasis formation by increased tumour cell dissemination and interaction with BBB endothelium [18].
Additionally, breast cancer cells expressing genes including prostaglandin
synthesising enzyme cyclooxygenase-2, MMP1, angiopoietin-like 4 (ANGPTL4), latent
TGF-beta1 binding protein (LTBP1), epidermal growth factor receptor (EGFR) ligand
HBEGF and fascin-1 were shown to enhance brain metastasis via producing
pro-metastatic phenomenon such as inflammation, invasion and cancer cell motility
[19]. Moreover, expression of
In a study investigating the role of truncated glioma-associated oncogene homolog 1 (TGLI1) revealed that its high expression results in increased breast cancer metastasis to the brain [20]. TGLI1 resulted in an activation of stemness genes CD44, Nanog, Sox2 and OCT4 in radioresistant cancer stem and metastasis-initiating cells, and through activation of astrocytes, this TGLT1 enhanced brain metastasis [20]. Interestingly, T-cell-induction of Guanylate-Binding Protein 1 expression in oestrogen receptor negative breast cancer cells resulted in preferential seeding to the brain, which could be a novel targeting strategy for immunotherapy [20].
Nevertheless, once at the interface of the BTB, through key interactions, CTCs attach to the endothelium lining, alter the normal physiology of the cerebral vasculature to a pro-tumorigenic state and migrate through the BBB. This metastatic cascade is believed to mirror leukocyte transmigration through the BBB during inflammation [21]. Initial leukocyte rolling and adhesion at the cerebral endothelium is mediated via E-selectin adhesion molecules, followed by activation, arrest, crawling and subsequent migration mediated via intercellular adhesion molecule (ICAM-1) and vascular cell adhesion molecule (VCAM-1) [22]. This raised the possibility that the same adhesion molecules also facilitate and mediate extravasation of CTCs metastasizing to the brain. Previous studies illustrate that direct interactions between tumour cell secretions and the cerebral endothelium facilitates the initial adhesion and arrest of metastatic tumour cells. For instance, Soto et al. [23] demonstrated that the same endothelial adhesion molecules E-selectin, ICAM-1 and VCAM-1 associated with leukocyte trafficking are upregulated on the cerebral endothelium after injection of metastatic breast cancer cells, with the cells overexpressing the natural ligands to the adhesion molecules.
Rai et al. [24] showed that non-small-cell lung carcinoma (NSCLC) cell
lines, A549 and SK-MES-1, secrete specific pro-metastatic proteins that directly
alter the integrity of the cerebral endothelial glycocalyx. The endothelial
glycocalyx is a dense intra-luminal layer lining the healthy cerebral vascular
endothelium connected via proteoglycans and glycoproteins forming a mesh network
of soluble plasma- and endothelium- derived molecules [25]. The glycocalyx
contributes to the maintenance of vascular homeostasis by forming an electrical
barrier and regulating vascular permeability [26]. This limits the leakage of
macromolecules into the capillaries and regulates adhesion of circulating
leukocytes to the cerebral vascular endothelium [27]. Embedded within the
glycocalyx are the same adhesion molecules ICAM1, VCAM1 and E-selectin observed
in leukocyte extravasation [24]. The A549 and SK-MES-1 cell line secretome
consists of inflammatory cytokine tumour necrosis factor-alpha (TNF-
Due to this complex structure and crosstalk within the NVU in the tumour microenvironment, with endothelial cells playing a direct role in metastasis, it is crucial to further understand the specific contributory factors of astrocytic end feet and the astrocyte secretome on BBB physiology and their role in the BTB.
During early cerebral colonisation, reactive astrocytes secrete plasminogen
activator, an anti-metastatic factor, to inhibit the invading cancer cells [30, 31]). Plasmin induces conversion of membrane-bound astrocytic FasL into an
apoptotic signal for cancer cells and inactivation of axon pathfinding molecule
L1CAM1 that cancer cells utilise to spread along the brain capillary [31].
Interestingly, these invading cancer cells in the perivascular space secrete
serpins to shield this astrocyte-induced defence. However, later on, within the
developing cancer microenvironment, astrocytes instead play a pro-tumourigenic
role. For instance, earlier studies demonstrate a direct pro-metastatic influence
of astrocytes in co-culture with PC14-PE6 lung tumour cells [32], and more
recently it has been reported that astrocytes produce and secrete matrix
metalloproteinase enzymes -2 and -9 (MMP-2/-9) in the presence of tumour cells
that degrade and remodel local ECM, allowing tumour invasion. Moreover, these
cells were also shown to induce cancer cell proliferation and chemoprotection by
producing pro-inflammatory cytokines IL-6 and TNF-
More specifically, Wang et al. [34] investigated the hypothesis that astrocyte secretions play a direct role in tumour cell metastasis to the brain. MDA-MB-231 breast cancer cells, sarcoma 180 (S180) cell lines and lung adenocarcinoma (H2030) cells were incubated in astrocytic conditioned media from neonatal rats [34]. The Boyden chamber migration assay showed increased tumour cell migration in response to astrocytic conditioned media (CM), with approximately 200 invading H2030 cells with astrocyte CM compared to 0 for the control without CM, suggesting that factors within the astrocytic secretome play a role in cancer metastasis [34] (Table 1, Ref. [34, 35, 36, 37, 38, 39, 40]). These findings were directly supported by more recent data demonstrating that direct application of astrocytic conditioned medium to MDA-MB-231 metastatic breast cancer cells and MCF10A healthy breast epithelial cells significantly increased invasiveness and velocity of migration of both cell types [41]. Additionally, it was observed that the astrocyte secretome also directly alters morphology of both cell types, forming an elongated and enlarged cellular structure in comparison to control cancer cells without astrocytic conditioned medium [41]. It has been shown that breast cancer cells migrate through the BBB with the adoption of an elongated morphology [4]. This provides a direct link between astrocytes and metastatic cancer cell migration, with the presence of astrocytic secretome facilitating and enhancing tumour cell and healthy epithelial cell migration [41], suggesting this potential is present in the healthy brain. Therefore, astrocytes play a direct role in tumour cell metastasis, perhaps before any potential signalling from tumour cells by increasing transmigration and altering tumour cell morphology of both tumour cells and healthy epithelial cells to a highly favourable phenotype for extravasation and subsequent secondary cerebral colonisation of the brain.
| Astrocyte secretion | Cell line | Function | Pathology | Reference |
| MMP-2/-9 and TIMPs | NSCLC, H2030, S180, MDA-MB-231 | Enzymatic degradation of ECM components, inflammation | Cancer, Amyotrophic Lateral Sclerosis (ALS) | [34] |
| CXCL10 | sBT-RMS | Inflammation and invasion | Cancer | [35] |
| TNF- |
PBMC, NSCLC, HARA-B | Inflammation and proliferation | Cancer, Diabetes | [36] |
| Tenascin-C | Inflammation and metastasis | Cancer, haemorrhage, trauma | [37] | |
| Multiple Sclerosis | ||||
| IFN- |
MDA231-BrM2, ErbB2-BrM, H2030-BrM3 | Inflammation and proliferation | Cancer | [38] |
| IL-6 | HARA-B | Inflammation and proliferation | Cancer | [36] |
| IL-1 |
HARA-B | Inflammation and proliferation | Cancer | [36] |
| Polyunsaturated fatty acids | BrM (WM4265.2, WM4265.2-BrM1) | Pro-metastatic and proliferation | Cancer | [39] |
| AEG-1 | NSCLC | Upregulates release of MMPs | Cancer | [40] |
To investigate the composition of the astrocytic secretome that contributes to the invasiveness of tumour cells, the astrocytic conditioned media was treated with a range of MMP inhibitors [34]. MMP’s are a group of enzymes known to be implicated in cancer metastasis and tumour invasiveness, particularly MMP-2 and MMP-9 that directly degrade collagen and protein components of the ECM and are secreted by both astrocytes and tumour cells [42]. Astrocytes induce the cleavage of inactive pro-MMP-2 and pro-MMP-9 secreted by tumour cells to the active form which degrades collagen IV present in the basement membrane of the BBB, subsequently remodelling the ECM and increasing permeability and enhancing glioma, breast and lung tumour invasiveness to the brain [34]. This is supported by recent studies observing reduced collagen IV in the basement membrane of the BTB within the metastatic cancer microenvironment [43]. Astrocytes indirectly increase the activation of plasmin, a protease, from plasminogen that cleaves and activates tumour cell derived MMPs via interaction with the urokinase plasminogen activator (uPA) [44] (Fig. 2). It has also been shown that reactive astrocytes upregulate MMP secretions specifically within the tumour microenvironment, suggesting a link whereby astrocyte secretions of MMPs in particular, are increased in response to extravasating tumour cell secretions [45], including interleukin-8 (IL-8), plasminogen activator inhibitor-1 (PAI-1) and macrophage migration inhibitory factor (MIF) that activate the astrocytes and induce upregulation of MMPs. In the pathological state, astrocytes are activated and categorised by the upregulated presence of intermediate filament glial fibrillary acidic protein (GFAP) and are termed “reactive astrocytes” [46]. This response to brain insult is known as astrogliosis with astrocytes upregulating GFAP and pro-inflammatory cytokines and chemokines to induce neuroinflammation [37]. The astrocyte culture medium with MMP inhibitors was added to an invasion assay with S180 and H2030 cell lines that resulted in a significant reduction in cancer cell invasion. This was further tested in vivo via the injection of astrocyte-conditioned tumour cells into the left ventricle of the heart in immunodeficient mice to directly enter the circulation to the brain and access the cerebral microenvironment. Astrocyte-conditioned tumour cells demonstrated a more severe tumour metastasis and invaded the brain earlier than astrocyte conditioned tumour cells treated with an MMP inhibitor suggesting a critical role of astrocytes and MMP secretions in the induction of tumour cell transmigration [34]. These findings were supported by recent data demonstrating that inhibition of MMPs within astrocyte culture medium reduced the migratory effects of MDA-MB-231 breast cancer cells in a dose dependent manner [41]. Therefore, astrocytes in the tumour microenvironment not only directly alter morphology and metastatic potential of tumour cells and healthy epithelial cells prior to tumour cell signalling, but also simultaneously secrete MMPs and interact with tumour cell MMP secretions to facilitate extravasation. This suggests that MMP’s are an essential component of astrocytic and tumour cell secretions in the cancer microenvironment that facilitate transmigration.
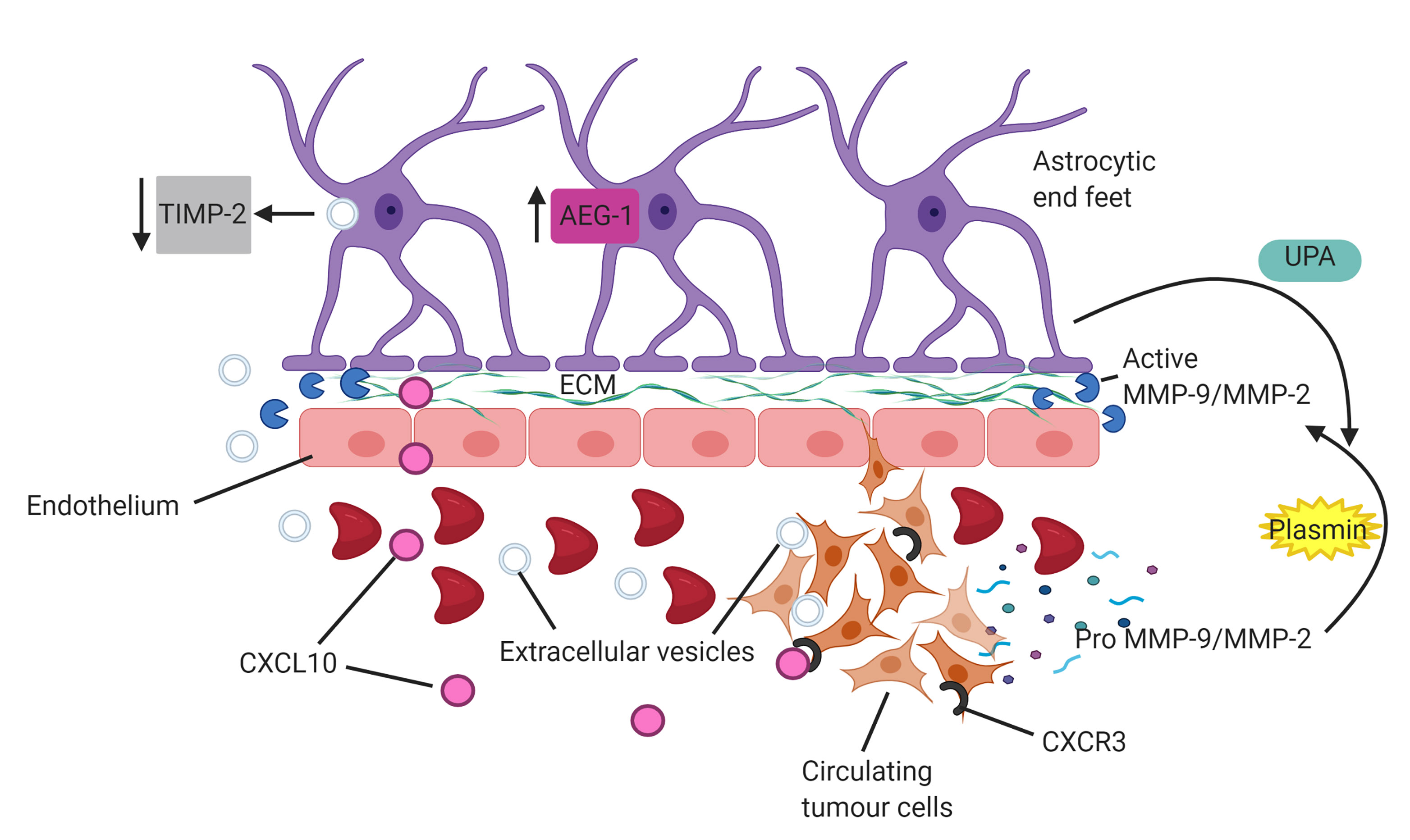 Fig. 2.
Fig. 2.Astrocytes facilitate invasion of circulating tumour cells (CTCs). Astrocytes produce matrix metalloproteinases (MMPs) and induce cleavage of tumour derived MMP-2/MMP-9 via activation of plasmin through interactions with urokinase-type plasminogen activator (UPA). Upregulation of astrocyte elevated gene 1 (AEG-1) enhances release of MMP’s into tumour microenvironment. Uptake of tumour cell derived extracellular vesicles into astrocytes downregulates expression of tissue inhibitors of MMPs (TIMP-2), subsequently diminishing modulation of MMP expression. Astrocyte derived CXCL10 chemokine upregulates the receptor, CXCR3, on CTCs to enhance chemoattraction and migration of tumour cells.
Sun et al. [47] demonstrated that overexpression of astrocyte elevated gene-1 (AEG-1) correlates with high expression and activation of MMP-9. This subsequently enhances tumour invasiveness and metastasis, emphasising the role that astrocytes play in facilitating tumour cell migration. Notably, NSCLC cells engineered to express AEG-1 displayed high levels of MMP-9 correlating to increased invasiveness compared with control [47]. Furthermore, Ding et al. [48] reported that overexpression of AEG-1 also increases lung tumour cell angiogenesis and growth. More recently, Li et al. [40] showed that knockout of AEG-1 inhibited proliferation, migration and invasion of A549 lung cancer cell lines, with overexpression of AEG-1 rescuing this inhibition. This suggests that astrocytes not only secrete MMP’s and cleave pro-MMP’s in the cancer cell secretome, facilitating the degradation of the BBB, but also overexpress AEG-1 which subsequently upregulates MMP-9 expression in the tumour microenvironment and promotes tumour angiogenesis and migration (Fig. 2). Beyond this, Morad et al. [49] demonstrated that extracellular vesicles (EV) derived from metastatic breast cancer cells, following transcytosis across the BBB, can be internalized by surrounding astrocytes via endocytosis. This internalization modulates expression of astrocyte derived MMPs and tissue inhibitors of MMPS (TIMPs) to favour a pro-metastatic tumour microenvironment. Interestingly, metastatic breast cancer EV’s, once internalized by astrocytes, downregulate astrocytic TIMP-2 and in turn reduce inhibition of MMP-2 function on the degradation of the basement membrane of the BBB [49] (Fig. 2). This further highlights a significant role of astrocytes in the facilitation of metastasis, with breast cancer cell EV’s reprogramming astrocytes to reduce modulation of MMP activity through TIMPs. This mechanism along with a strong upregulation of reactive astrocyte derived MMP’s specifically within the cancer microenvironment in response to cancer cell secretions subsequently enhances MMP remodelling of the ECM to create a highly favourable metastatic niche for extravasation. An additional mechanism that promotes brain metastasis through astrocyte-CTC EV crosstalk is the transfer of exosomal microRNAs from astrocytes to CTCs specifically in the cerebral tumour microenvironment. This transfer downregulates PTEN, a vital tumour suppressor, and ultimately promotes tumour cell survival in the brain [50]. This demonstrates both astrocytes and CTC derived EVs enhancing migration as well as diminishing tumour suppression in the brain.
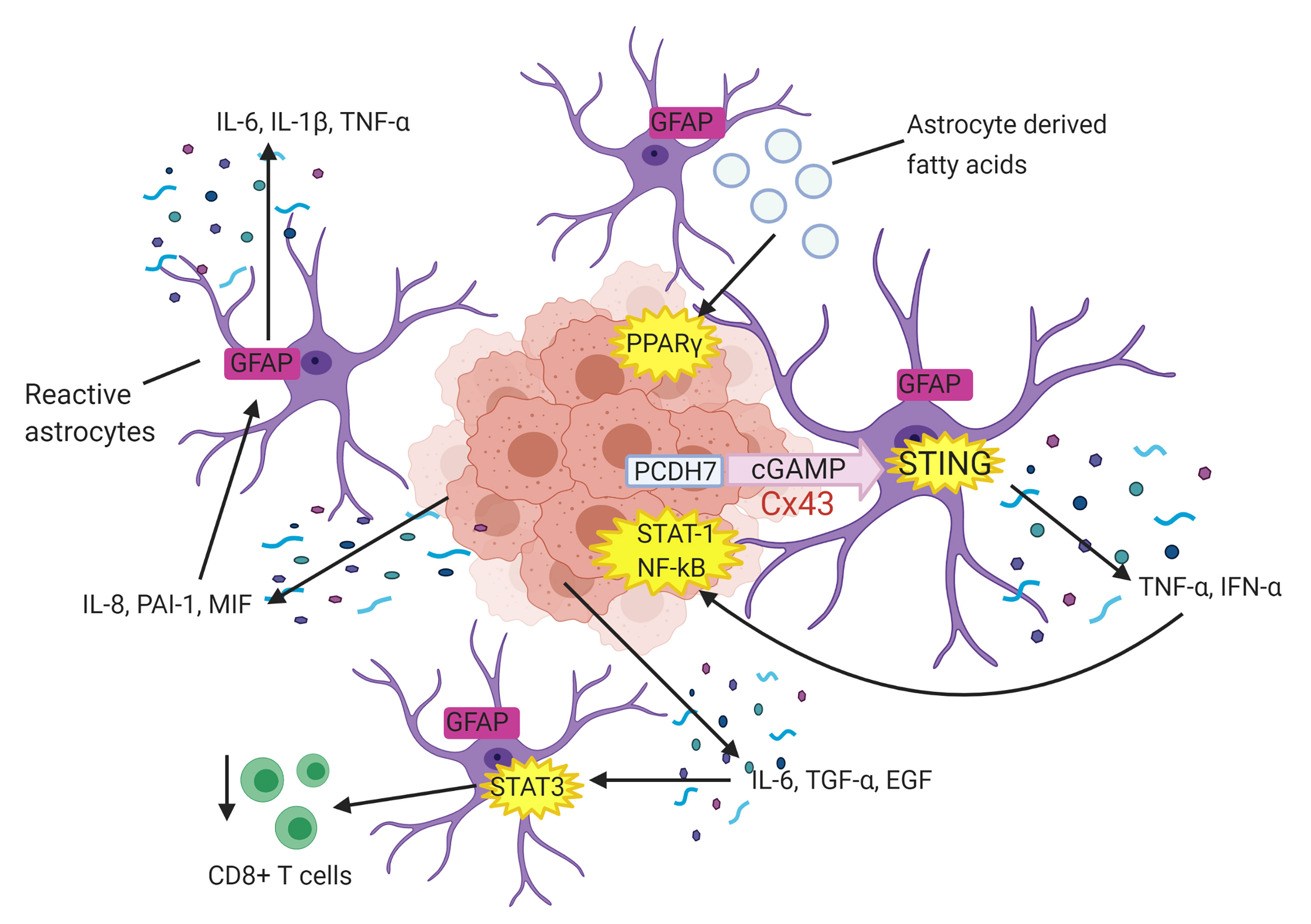 Fig. 3.
Fig. 3.Astrocytes facilitate tumour cell proliferation. Tumour cell
derived interleukin 8 (IL-8), plasminogen activator inhibitor 1 (PAI-1) and
migration inhibitory factor (MIF) activate surrounding astrocytes which
subsequently secrete pro-proliferative factors interleukin 6 (IL-6), interleukin
1
Therefore, astrocytes play a pivotal role in promoting transmigration of tumour cells across the BBB as well as angiogenesis, growth and survival of tumour cells once they invaded the brain parenchyma. However, Wang et al. [34] observed only a partial inhibition of cancer cell migration with MMP antibodies, suggesting MMP-2/-9 are not the sole factors within the astrocyte secretome aiding cancer cell migration. Despite this, these authors provide evidence for astrocytes facilitating cancer cell migration in metastasis, reacting to and cleaving MMPs from cancer cell secretions and permitting extravasation.
In addition, it is well established that inflammation fosters proliferation and migration of tumour cells, with the tumour microenvironment being predominantly orchestrated by astrocyte-derived inflammatory cytokines [51], which induces the metastatic process. As leukocytes use parallel trans-endothelial migratory mechanisms to metastatic cancer cells, this drives the proposal that astrocytic pro-inflammatory cytokine secretions in response to CTCs may also support and facilitate trans-endothelial migration and ultimate secondary colonisation of tumour cells in the brain [52]. For instance, Doron et al. [35], provided evidence of circulating metastatic melanoma cells hijacking the astrocyte protective inflammatory response to aid in metastasis. CXCL10 is a chemokine secreted by astrocytes in the tumour microenvironment as a protective mechanism during metastasis. However, CTCs exploit this mechanism to their advantage, with upregulation of astrocyte derived CXCL10 in the metastatic environment enhancing tumour cell migration towards astrocytes [35]. The authors observed an upregulation of CXCR3, the CXCL10 receptor, in brain-tropic melanoma cells, with overexpression of CXCR3 in vivo enhancing chemoattraction of melanoma cells and formation of metastases [35] (Fig. 2). This provides further evidence of CTCs directly manipulating astrocytes in the tumour microenvironment to favour and facilitate metastasis.
Migration, proliferation and invasion are key steps in tumour growth and
metastasis which are dependent on crosstalk between CTCs and the brain
microenvironment. Invading tumour cells induce both mechanical (contact
inhibition by cadherin and
Seike et al. [36] went further to investigate whether the mutual
relationship between astrocytes and lung cancer cells was specific to the HARA-B
cell line. Primary cultured astrocytes were co-cultured with 3 additional cell
lines derived from human squamous cell carcinoma (QG56, EBC-1) and NSCLC (PC-9).
Proliferation of the additional lung cancer cell lines also significantly
increased in the presence of astrocytes, with 350% cell proliferation for HARA-B
cells, 200% proliferation for QG56 cells, almost 500% proliferation for EBC-1
cells and 150% proliferation for PC9 cells compared to basal proliferation
(100%) without astrocytes [36]. This further supports the notion that astrocytes
and tumour cells have a mutual relationship whereby reciprocal stimulation and
cross talk promotes proliferation and survival of lung tumour cells in the brain
[36]. Furthermore, different concentrations of recombinant mouse cytokines (m),
such as mIL-1
In addition, Chen et al. [38] demonstrated that lung carcinoma cells
express protocadherin 7 (PCDH7) to engage astrocytes in functional connexin 43
(Cx43) gap junctions. These gap junctions allow lung tumour cells to transfer the
second messenger cGAMP to astrocytes which activates the astrocytic immune
cGAS-STING pathway. This subsequently results in the release of inflammatory
cytokines from reactive astrocytes, including TNF-
Zou et al. [39], showed that metastatic melanoma and breast cancer cell
lines in astrocyte culture medium also promoted cancer cell growth. Astrocytes
surrounding the metastatic foci in the brain naturally release polyunsaturated
fatty acids into the microenvironment to aid in neuronal synaptic formation and
function [39]. However, these astrocyte-derived fatty acids are pro-metastatic
and activate the peroxisome proliferator-activated receptor
Within the tumour microenvironment, the blood brain barrier is disrupted and undergoes conformational changes dependent on the progression of metastasis [43]. These physiological changes occur once metastases have been established in the brain and characterise the blood tumour barrier which is highly permeable compared to the healthy BBB [13]. Such changes have been shown to facilitate and aid in the metastatic process by increasing BBB permeability and the ability of cancer cells to extravasate and invade the brain parenchyma [43]. The formation of the BTB involves the accumulation of reactive astrocytes that secrete pro-inflammatory cytokines [13]. Due to the complex interactions within the NVU, astrocytes have the potential to play a key role in cancer extravasation and metastasis within the BTB. For example, aquaporin-4 (AQP-4) is a water channel within the astrocytic end feet that covers the BBB basement membrane and aids in supplying nutrients to surrounding neurons and maintaining vascular homeostasis and ionic regulation in the healthy brain [56]. Additionally, AQP-4 is coexpressed with agrin, a proteoglycan present on the basal lamina, which accumulates around cerebral capillaries for BBB tightening [11]. However, a significant loss of AQP-4 at cerebral vessels at the tumour interface at week 3 post-injection of A549 NSCLC line was observed which persisted throughout the development of mid- and late-stage metastasis, with a 12.18-fold decrease in AQP-4 expression at week 6 [43]. This correlates with the loss of agrin in human brain tumours which could result in an increased permeability of the BBB [57]. Loss of aquaporin-4 has also been observed in breast cancer metastasis to the brain and relates to a loss of polarization of the astrocytic end feet at the BTB contributing to the loss of BBB integrity caused by metastasising cells and favouring their migration [43]. Aquaporins are key regulators of BBB integrity, with studies demonstrating that upregulation of AQP-4 in vivo in gliomas compensates for reduced astrocytic end feet [58]. Furthermore, these findings support observations of decreased levels of AQP-4 in astrocytic end feet in the BTB compared with control, with a reduced and distal distribution of AQP-4 from the end feet at the BTB not localized at the cerebral capillaries [59]. This suggests that not only is AQP-4 expression within astrocytic end feet significantly reduced in the metastatic tumour microenvironment, but that the localization of the end feet is altered and no longer polarized at cerebral blood vessels. AQP-4 reduction is also associated with inflammation [43], potentially induced by pro-inflammatory cytokines in the tumour microenvironment. This suggests that altered astrocyte physiology within the BTB is linked to specific factors released by tumour cells at the metastatic interface and contributes to increased BBB permeability, ultimately aiding the metastatic cascade of cancer cells to the brain.
Furthermore, the absence of astrocytes at the BTB is correlated with down-regulated expression of Mfsd2a, a fatty acid transporter in the cerebral endothelium [60]. In the healthy brain, Mfsd2a mediates transcytosis within endothelial cells, with murine Mfsd2a knockout studies disrupting the cerebral vascular endothelium and increasing transcellular transport and permeability [60]. These findings highlight that astrocyte interactions with the cerebral endothelium are altered in the tumour microenvironment, subsequently degrading BBB integrity and increasing the permeability for cancer cell invasion to the brain parenchyma. Therefore, astrocytes have a significant role in the facilitation of cancer metastasis to the brain, with the tumour microenvironment directly altering astrocyte physiology and displacing astrocytic end feet to enhance entry into the brain.
To provide a complete picture of the tumour microenvironment at the BTB
interface for metastasis, it is essential to include contributions and potential
metastatic facilitation from pericytes. Pericytes are multi-functional mural
cells that abluminally partially line endothelial cells, coating capillaries and
venules throughout the body, with the highest density of pericytes found at
cerebral capillaries within the NVU of the BBB [61]. Pericytes are vital in
regulating expression of tight junction components and maintaining BBB integrity
and vascular tone [62]. Platelet-derived growth factor receptor-
Uzunalli et al. [43] visualised the BTB in immunodeficient mice over 6
weeks using immunofluorescence microscopy after intracardial injection of NSCLC
cell lines. At late-stage NSCLC metastasis in week 6, there was a 2.78-fold
decrease in PDGFR-
It is known that in the cancer microenvironment there is an upregulation of
endothelial adhesion molecules for the arrest and subsequent migration of tumour
cells across the BBB [24]. However, it is not known whether the astrocyte
secretome directly interacts with the endothelium of the BBB during metastasis
and upregulates expression of the same adhesion molecules. These events are vital
for aiding CTC migration at the BTB interface, and such adhesion molecules
provide a potential therapeutic target outside the brain parenchyma. Recent
studies provide evidence of potential contributions from astrocytic secretome
interacting with the cerebral endothelium and upregulating adhesion molecules for
transmigration. For instance, Spampinato et al. [65] demonstrated that
the inflammatory cytokine secretions, including TNF-
Furthermore, patient-derived cancer models are being developed with researchers recently establishing a patient-derived xenograft model system of NSCLC brain metastases [7]. This is being developed with the aim to improve therapeutic approaches to brain metastases. Moreover, further studies are currently being undertaken to investigate potential differential molecular crosstalk in enhancing brain metastasis and therapeutic targets. For instance, in vitro 3D models mimicking the complex BBB microenvironment is currently being developed to closely study the crosstalk between different cell types, barrier function and drug design [70, 71, 72]. Lee et al. [73] have recently developed a microfluidic 3D in vitro system of the BBB by adopting the CNS angiogenesis model. This model successfully showed in vivo-like interactions between pericytes, human brain microvascular endothelial cells and astrocytes, including expressions of tight junctions. Additionally, the authors reported drug penetration via the efflux system that exists in the BBB, a promising step to studying mechanisms of drug delivery in vitro in a CNS model [73]. Moving forward, this model could be developed further to study pathologies by mimicking a damaged BBB to study extravasation of tumour cells into the brain, generating physiological shear pressure which has been shown to regulate vascular permeability and by measuring TEER as another metric of BBB function [74, 75].
Within the healthy brain, the neurovascular unit is essential in maintaining vascular endothelial homeostasis, permeability and integrity. However, in the cancer microenvironment, this interface is disrupted, with alterations in astrocyte, pericytes and endothelial properties characterizing the BTB. Circulating tumour cells secrete a complex mix of factors that directly interact with surrounding astrocytes, activating these astrocytes and inducing pro-metastatic secretions that aid in cancer cell adhesion, migration and proliferation. Metastatic tumour cells exploit surrounding astrocytes to facilitate extravasation and survival in the brain, forming a mutual relationship, with reciprocal stimulation being pivotal for tumour growth and survival. Understanding this complex interface of astrocytes, pericytes and cerebral endothelial cells and its manipulation by tumour cells in the tumour microenvironment will provide additional targets for metastatic treatment.
LB wrote the majority of the paper. NG and JW partially wrote and critically appraised the paper. GG and MZIP critically revised the paper and supervised the project.
Not applicable.
Figures were created in BioRender.com.
This research received no external funding.
The authors declare no conflict of interest.



