1 Department of Medical, Surgical and Advanced Technologies “G.F. Ingrassia”, University of Catania, 95121 Catania, Italy
2 Department of Law, Criminology, Magna Graecia University of Catanzaro, 88100 Catanzaro, Italy
3 Department of History, Society and Studies on Humanity, University of Salento, 73100 Lecce, Italy
4 Department of Clinical and Experimental Medicine, University of Foggia, 71122 Foggia, Italy
† These authors contributed equally.
Abstract
Introduction: Smart drugs are among the most common drugs used by students. It is estimated that they are second in incidence after cannabis. Although they are usually used for diseases such as attention deficit hyperactivity disorder (ADHD) and dementia, in most cases the use of smart drugs is illegal and without a prescription. Methodological issues: A systematic review was conducted according to PRISMA guidelines. SCOPUS, Medline (using PubMed as a search engine), Embase, Web of Sciences, and Google Scholar were used as search engines from January 1, 1980 to June 1, 2021 to evaluate the association between smart drugs and neuro-enhancement. A total of 4715 articles were collected. Of these, 295 duplicates were removed. A total of 4380 articles did not meet the inclusion criteria. In conclusion, 48 articles were included in the present systematic review. Results: Most of the studies were survey studies, 1 was a prospective longitudinal study, 1 was a cross-over study, and 1 was an experimental study in an animal model (rats). The largest group of consumers was school or university students. The most frequent reasons for using smart drugs were: better concentration, neuro enhancement, stress reduction, time optimization, increased wake time, increased free time, and curiosity. There are conflicting opinions, in fact, regarding their actual functioning and benefit, it is not known whether the benefits reported by consumers are due to the drugs, the placebo effect or a combination of these. The real prevalence is underestimated: it is important that the scientific community focus on this issue with further studies on animal models to validate their efficacy.
Keywords
- Smart drugs
- Neuroenhancement
- Cognitive enhancement
- Brain effect
- Incidence
- Nootropics
The use of illicit smart drugs among college students is second only to cannabis [1]. Smart drugs are generally prescribed for subjects with attention deficit hyperactivity disorder (ADHD), Alzheimer disease, Parkinson disease, and dementia [2]. However, the use of smart drugs among healthy people has been increasing in recent years [3, 4, 5, 6]. In the 2010s, the production of smart drugs tripled [7]. The current prevalence of use in the American student population has been estimated to be between 5–35% [8]. Prevalence rates in Europe are unclear, with less than 10% reported in the UK, which was likely due to low availability [9]. In 2015, 6/8% of German university students used smart drugs [10]. Despite the increase in the prevalence of smart drug users, the use of these substances appears to be underestimated [11]. The most frequently used smart drugs and nootropics are caffeine, modafinil, methylphenidate, and amphetamines. One explanation could be that they are the ones that are more easily obtained even on the black market or through a friend, for example, at school or university. In addition, the easy administration (oral or inhalation) also contributes to ease of consumption [1, 6, 8, 12]. The main reasons for the increase of smart drug use/abuse were: improvement of cognitive functions, reduction of stress, increase in free time, and increase in school and work performance [2, 3, 4, 5, 6, 7, 8]. Some studies have shown moderate cognitive improvements in attention and reduced sleep, with increased confidence [12]. Instead, other studies showed that smart drugs only had a placebo effect. The main data about commonly used smart drugs are summarized in Table 1 (Ref. [13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36]). However, even if the focus on cognitive enhancers is high, the information about their actual functioning is little [4, 5, 6, 7, 8, 9]. Smart drugs are a public safety problem, because they are often taken in combination with other drugs with an increased risk of interaction. Furthermore, they still have partially unknown side effects (increased risk of suicide, psychiatric disorders, increased cardiovascular risk). This aspect plays a crucial role in public health, considering the increase in the use of smart drugs in young and worker populations [13, 14, 15, 16, 17, 37, 38]. To date, there are few longitudinal and experimental studies on the effects of smart drugs, certainly also for ethical reasons [8, 9, 10, 11, 12]. However, their use is increasing, even if the benefits and side effects of these drugs are not well known. Therefore, it is essential that studies are also implemented on animal models to know the real effects. The risk of addiction and abuse is still debated. However, psychosis, insomnia, and irritability are common [37, 38].
| Drug | Main route of administration | Cognitive effects | Approved use | Off label or investigational use |
| Methylphenidate [13, 14] | Oral administration | Increased memory, attention, concentration and wakefulness | ADHD, narcolepsy | Opiate withdrawal syndrome, chronic fatigue syndrome, major depressive disorder |
| Modafinil [15, 16, 17] | Oral administration | Increased memory and wakefulness | Narcolepsy | Doping, ADHD, multiple sclerosis, depression syndrome, opiate withdrawal syndrome |
| Amphetamine [18, 19, 20] | Oral administration, snorting, intravenous injection | Mood enhancement, increased attention and wakefulness | ADHD, narcolepsy, obesity | Doping |
| Armodafinil [21] | Oral administration | Increased wakefulness | Excessive daytime sleepiness associated with obstructive sleep apnea, narcolepsy, shift-work disorder | ADHD, chronic fatigue syndrome, major depressive disorder |
| MDMA (Ecstasy) [22, 23, 24, 25, 26, 27, 28, 29] | Oral administration | Mood enhancement, general wellbeing, increased empathy | Post-traumatic stress disorder, alcohol addiction | |
| Cocaine [30, 31, 32, 33] | Inhalation, chewing of coca leaves, intramuscular injection, subcutaneous injection, snorting, intravenous injection | Mood enhancement, euphoria, sexual arousal, loss of contact with reality, agitation | Local anesthetic, vasoconstrictor | |
| Ketamine [34, 35, 36] | Intravenous injection, intramuscular injection, oral administration, snorting | Dissociation, analgesia, dysphoria, delirium, euphoria, difficulty concentrating, visual and auditory hallucinations, amnesia, sedation | Dissociative anesthesia, resistant depression, acute pain treatment | Bipolar disorder, post-traumatic stress disorder, anxiety disorders, mood disorders |
A systematic review was conducted according to the PRISMA guidelines [36].
SCOPUS, Medline (using PubMed as the search engine), Embase, Web of Sciences, and Google Scholar were used as search engines from 1 January 1980 to 1 June 2021 to evaluate the association between smart drugs and neuroenhancement. Medical subject headings (meSH) was used with the following words: (smart drugs) AND (neuroenhancement); (smart drugs) AND (brain); (smart drugs) AND (cognitive); (smart drugs) AND (enhancement).
The following exclusion criteria were: (1) review, (2) articles not in English, (3) abstract, (4) editorial, (5) poster, and (6) communications at conferences. The inclusion criteria were: (1) Original Article, (2) Case Report, (3) articles in English, and (4) Animal Studies.
M.E. and G.C. initially evaluated all the articles, evaluating the title, the abstract, and the whole text. F.M. and M.E. reanalyzed the chosen articles independently. In cases of conflicting opinions concerning the articles, they were submitted to M.S.
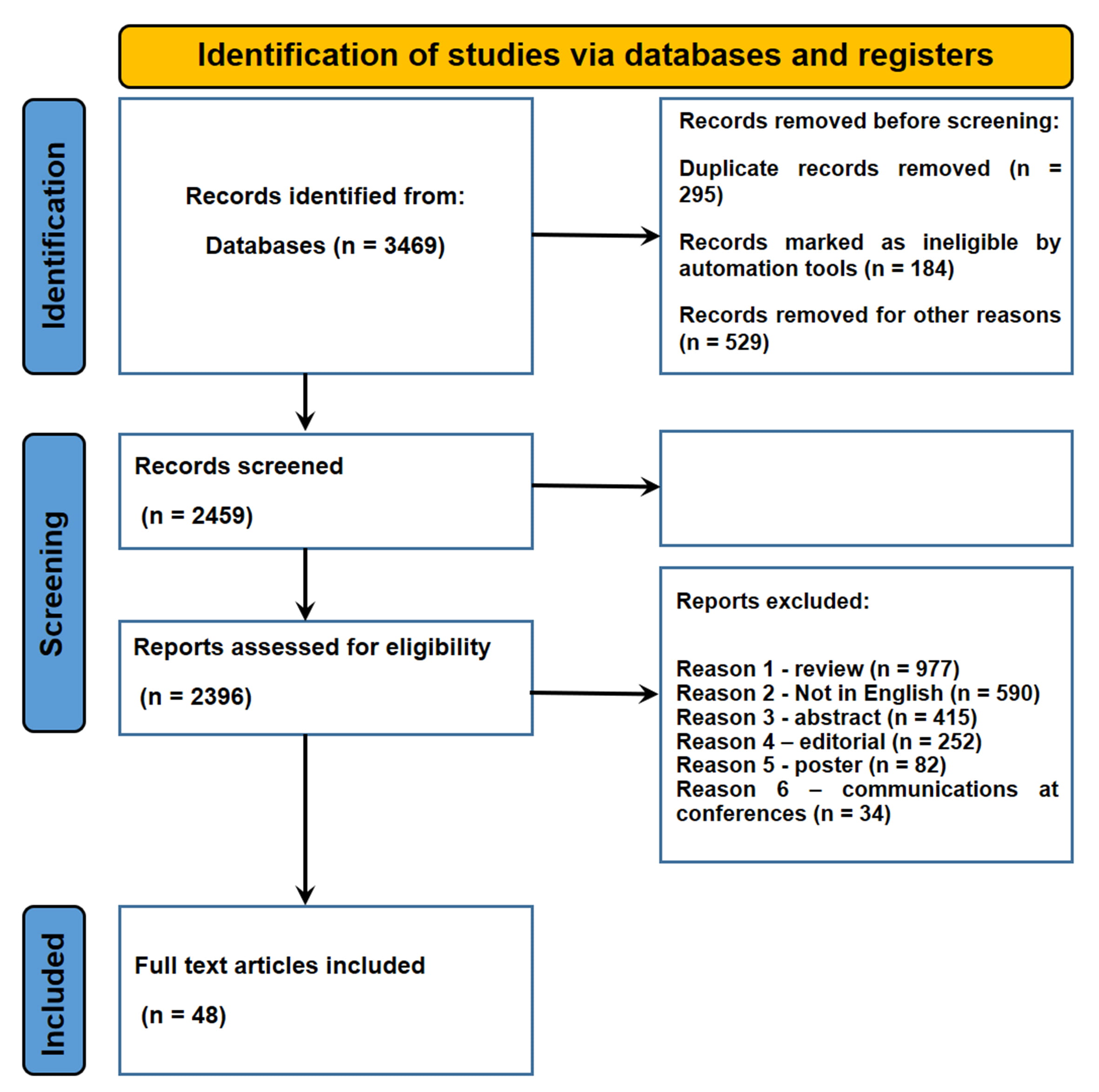
A total of 4715 articles were collected. Of these, 295 duplicates were removed. A total of 4380 articles did not meet the inclusion criteria. In conclusion, 48 articles were included in the present systematic review (Fig. 1).
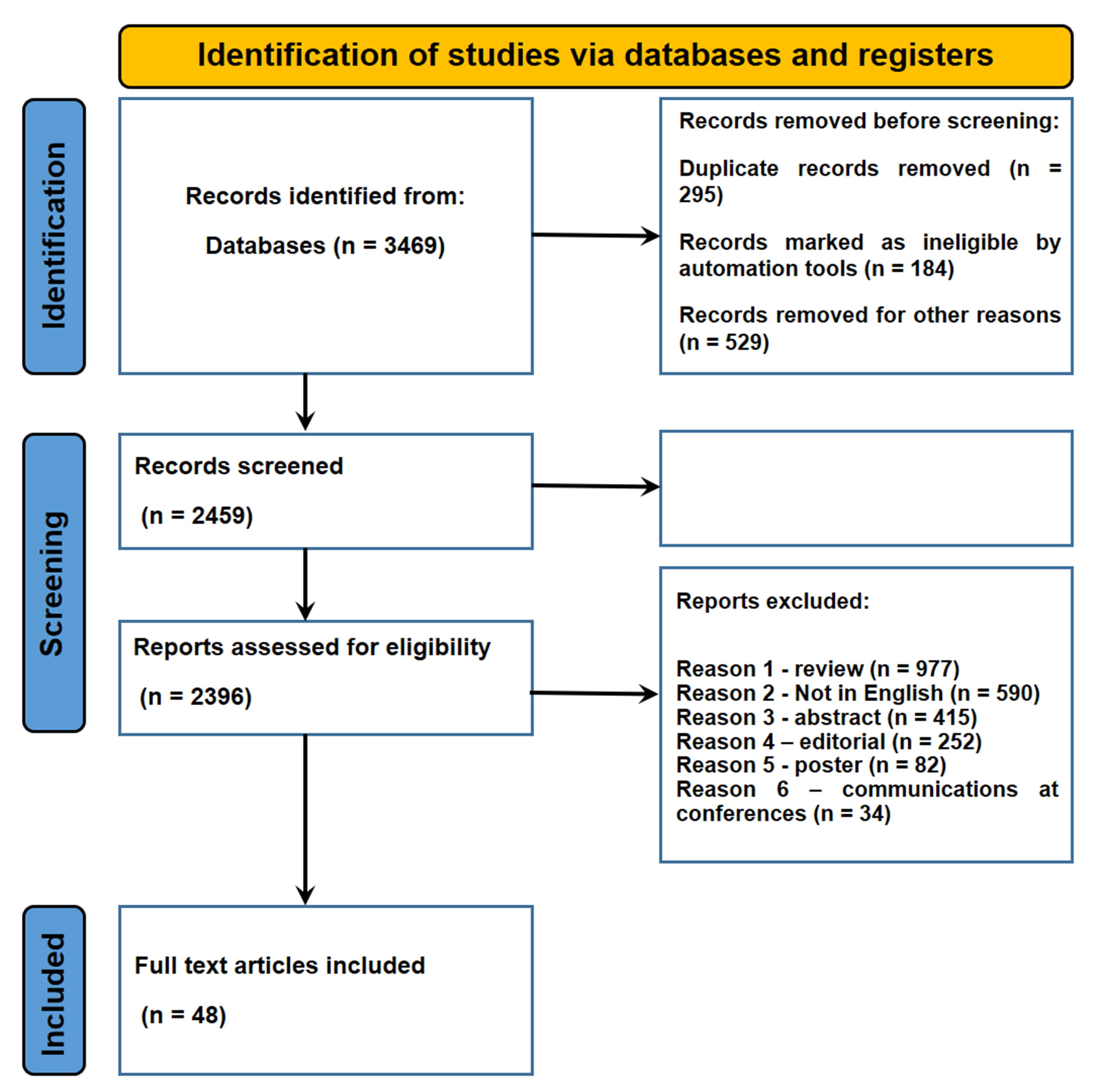 Fig. 1.
Fig. 1.Flow diagram illustrating included and excluded studies in this systematic review.
Most of the studies were surveys. Only 1 was a longitudinal prospective study, 1
was a cross-over study, and 1 was an experimental study in an animal model
(rats). Most of the studies involved students. In the majority of cases, the most
used smart drugs were modafinil, methylphenidate, methamphetamine, and
amphetamines. This systematic review showed that the most frequent reasons for
using smart drugs were: better concentration, neuroenhancement, stress reduction,
time optimization, increase in time awake, increase in free time, and curiosity.
There are conflicting data on the prevalence of smart drug use among students,
ranging from 2% to 80% [39, 40, 41]. In the population, especially among students,
the average prevalence of use of smart drugs was 22.81%, with a median of 12.65
The prevalence of the use of smart drugs is increasing [42, 43, 44]. Male sex is a risk factor for their use [39, 40, 45, 46, 47]. According to some studies, most students used smart drugs sporadically and without a doctor’s prescription [42, 48]. Legal or illegal smart drugs were bought from friends, websites, or pharmacies. Some authors showed an increase in concentration from their studies and better memory [10, 42, 43, 49]. In some studies, students either did not believe in the neuroenhancement effect or had no effects from their use [50]. A strong individual variability in smart drug users has been hypothesized [51]. A lack of knowledge of the side effects and the possibility of abuse has emerged in many studies [39]. Many authors argue that there should be greater awareness about “smart drugs” through information campaigns [45]. In fact, Fond G. et al. [52], showed that the increased use of steroids as smart drugs raised a new public health problem, as corticoids can have serious side effects.
Table 2 (Ref. [10, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 11, 53, 12, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81]) highlights the main aspects of this systematic review.
| Reference | Study design | Target | Type of smart drugs | Main findings |
| Maier, L.J. [42] | Survey | 6275 students | Methylphenidate (4.1%), sedatives (2.7%), beta-blockers (1.2%), alcohol (5.6%), cannabis (2.5%), amphetamines (0.4%), cocaine (0.2%) | The reasons for smart drugs use was: increased learning (66.2%), relaxation (51.2%), stress reduction (39.1%), performance improvement (32.2%), and curiosity (20%). A significant number of students experienced a learning improvement after smart drug use. Most students used them sporadically before exams and not daily. |
| Hupli, A. [43] | Survey | 113 students | Not specified: legal and illegal drugs, and nutritional supplements | Of the 24/113 students interviewed admitted the use of cognitive enhancement drugs; only 5 suffered from attention deficit hyperactivity disorder (ADHD) and used smart drugs with a medical prescription, the others did not have a prescription. The reasons for using smart drugs were: improved grades in school, increased creativity, increased concentration, streamlined study, relaxing with friends, and better time management. 18 found positive effects, the others were disappointed because they did not have these results. |
| Micoulaud-Franchi, J. A [44] | Survey | 206 students | Caffeine tablets, Methylphenidate, Amphetamines, Modafinil, Piracetam | 5.8% of the students used illicit smart drugs to: improve academic performance, improve concentration, and increase wakefulness in the sleep/wake ratio. 16.7% of all smart drugs users employed smart drugs to cause euphoria or improve their mood. |
| Colucci, P. [49] | Experimental study | Rats | Methylenedioxypyrovalerone | A group of rats were given amphetamine and MDPV (individually or together) to evaluate memory improvement. These two drugs act on the noradrenergic and dopaminergic systems. These two molecules showed a memory improvement. |
| (MDPV), Amphetamine | ||||
| Dubljević, V. [11] | Survey | 11,000 university students | Not specified: legal and illegal drugs, and nutritional supplements | Most college students who used smart drugs copied/falsified exams or otherwise cheated on exams. |
| Arria, A. M. [53] | Longitudinal prospective study | 1253 university students | Methylphenidate hydrochloride, Methylphenidate, Dextroamphetamine, Methamphetamine, Amphetamine, cannabis and alcohol | Abuse of smart drugs in college students showed a positive association with the skipping of classes and school commitments. |
| Brand, R. [12] | Survey | 1438 students | Not specified: legal and illegal drugs, and nutritional supplements | The reason for the use of smart drugs in students was divided into 2 types: “neuroenhancers” or “fatigue-fighters”. |
| Pacifici, R. [45] | Survey | 2621 young adults (14–35 years old) | Not specified: legal and illegal drugs, anabolic steroids, and nutritional supplements | The main risk factors for using smart drugs were: male sex, bad family relationships, negative influence of friends. The information and awareness campaign on these types of drugs should be improved. |
| Vargo, E.J. [10] | Survey | 13 students | Modafinil, Methylphenidate hydrochloride, Methylphenidate | Modafinil was bought through friends or internet sites and the reason for its use was to catch up on late exams and be in good standing with the university path. Most of the students reported an improvement in work performance. |
| Hildt, E. [46] | Survey | 18 university students | Modafinil, Methylphenidate hydrochloride, Methylphenidate, Methamphetamine, Amphetamine | From the analysis of the students’ responses, smart drugs were not only used to improve school performance, but also to create a balance between study and free time. The students wanted to maximize their time and improve their memorization in order to have more free time. |
| Champagne, J. [50] | Survey | 420 university students | Not specified: legal and illegal drugs, anabolic steroids, and nutritional supplements | The risk of using smart drugs was in students who believed in these drugs and their effects. Those who did not believe in using smart drugs or thought it was wrong to use them had a lower risk of abuse. |
| Franke, A.G. [48] | Survey | 1035 university students | Not specified: legal and illegal drugs, anabolic steroids, and nutritional supplements | The prevalence of smart drug abuse was higher than people thought. Most consumers did not have a prescription. |
| Stoeber, J [54] | Survey | 272 university students | Not specified: legal and illegal drugs, anabolic steroids, and nutritional supplements | There was an increase in the prevalence of the use of smart drugs. |
| Dietz, P. [55] | Survey | 1021 university students | Not specified: legal and illegal drugs, anabolic steroids, and nutritional supplements | One-time use of smart drugs was found in 88% of students, the reasons were: improved mood, improved cognitive performance, curiosity, and decreased stress. Only 19% had a prescription for their use. |
| Franke, A.G. [39] | Survey | 255 teachers of schools | Not specified: legal and illegal drugs, anabolic steroids, and nutritional supplements | 73.3% of teachers knew about smart drugs, mainly thanks to TV, Internet, and through students. Half of the teachers thought that smart drugs were useless and ineffective. 40% thought smart drugs were addictive. Opinions on the side effects of smart drugs were different and the teachers did not know them in depth. |
| Franke, A.G. [40] | Survey | 1145 surgeons | Not specified: legal and illegal drugs, anabolic steroids, and nutritional supplements | 9% of surgeons used smart drugs, even illicit ones, at least once in their life. The reason for taking these drugs was: increased concentration, trying to reduce stress, high workload, and reducing fatigue. Coping strategies should be part of physicians’ training programs. |
| Deline, S. [47] | Survey | 5967 people | Not specified: legal and illegal drugs, anabolic steroids, and nutritional supplements | The most frequent effect was the reduction of anxiety or stress (80%). Most people thought they had an increase in memory, concentration, and increased wakefulness. |
| Van Der Schaaf, M.E. [51] | Cross-over study | 19 students | Methylphenidate | Methylphenidate improved learning in students with high working memory. However, the effect was found to have strong individual variability on cognitive enhancement. |
| McDermott, H. [56] | Survey | 506 university students | Not specified: legal and illegal drugs, anabolic steroids, and nutritional supplements | Half of the students reported using smart drugs for recreational purposes, 20% said they used them as cognitive enhancers. Use was more common among males than females. The most frequent reasons for the use of smart drugs were: improving concentration, staying awake, and meeting the demands of the courses. |
| Steward, A [57] | Survey | 15 university students | Modafinil | The reason was to improve academic performance, improve concentration, increase wakefulness in the sleep/wake ratio. |
| Mousavi, F. [58] | Survey | 579 students | Modafinil, Methylphenidate, Amphetamine | The use of smart drugs improved cognitive performance. |
| Javed, N. [59] | Survey | 400 students | Methylphenidate | The reason was to improve academic performance, improve concentration, and increase wakefulness in the sleep/wake ratio. |
| De Bruyn, S. [60] | Survey | 3159 university students | Modafinil, Methylphenidate, Amphetamine | 8.7% of students used smart drugs to improve concentration. |
| London-Nadeau, K. [61] | Survey | 433 university students | Modafinil, Methylphenidate, Amphetamine, Caffeine | 74.7% of students used smarts drugs to improve academic performance. |
| Pighi, M. [62] | Survey | 33–77 university students | Modafinil, Methylphenidate, Amphetamine | 5/30% of students used smart drugs to improve concentration. |
| Fallah, G. [63] | Survey | 560 university students | Methylphenidate, Amphetamine | 79.3% of students used smart drugs to improve academic performance. |
| Maier, L.J. [64] | Survey | 109,398 students | Not specified: legal and illegal drugs, anabolic steroids, and nutritional supplements | The reason was to improve academic performance, improve concentration, and increase wakefulness in the sleep/wake ratio. |
| Lucke, J. [65] | Survey | 1136 students | Not specified: legal and illegal drugs, anabolic steroids, and nutritional supplements | 6.5% of students used smart drugs to improve academic performance. |
| Riddell, C. [66] | Survey | 642 university students | Modafinil, Methylphenidate, Amphetamine | 6.32% of students used smart drugs to improve academic performance. |
| Papazisis, G. [67] | Survey | 591 university students | Not specified: legal and illegal drugs, anabolic steroids, and nutritional supplements | 10/25% of students used smart drugs to improve academic performance. |
| Ram, S. [68] | Survey | 449 university students | Modafinil, Methylphenidate, Amphetamine | The reason was to improve academic performance, improve concentration, and increase wakefulness in the sleep/wake ratio. |
| Lazuras, L. [69] | Survey | 450 university students | Modafinil, Methylphenidate | The reason was to improve academic performance, improve concentration, and increase wakefulness in the sleep/wake ratio. |
| Jain, R. [70] | Survey | 541 university students | Methylphenidate | 11% of students used smart drugs to improve academic performance. |
| Vagwala, M.K. [71] | Survey | 66 university students | Modafinil, Methylphenidate, Amphetamine | The reason was to improve academic performance, improve concentration, and increase wakefulness in the sleep/wake ratio. |
| Jensen, C. [72] | Survey | 38 university students | Modafinil, Methylphenidate, Amphetamine | The reason was to improve academic performance, improve concentration, and increase wakefulness in the sleep/wake ratio. |
| Gudmundsdottir, B.G. [73] | Survey | 521 university students | Not specified: legal and illegal drugs, anabolic steroids, and nutritional supplements | 11% of students used smart drugs to improve academic performance. |
| Fond, G. [52] | Survey | 1718 university students | Modafinil, Methylphenidate, Amphetamine | Of students used smart drugs to improve academic performance. |
| Lengvenyt˙ e [74] | Survey | 579 university students | Modafinil, Methylphenidate, Amphetamine | 33% of students used smart drugs to improve academic performance. |
| Emanuel, R.M. [75] | Survey | 18 university students | Modafinil, Methylphenidate, Amphetamine, Piracetam | 5.8% of students used smart drugs to improve academic performance. |
| de Oliveira C. P. B. [76] | Survey | 1865 university students | Modafinil, Methylphenidate, Piracetam | The most frequent reason for consumption of smart drugs was due to the increase cognitive performance. Most of the students obtained these substances from a friend. The prevalence of users was 22%. |
| Maier L. J. [77] | Survey | 3056 university students | Modafinil, Methylphenidate | The most frequent reason for consumption of smart drugs was due to the increase cognitive performance. The prevalence of users was 14.3%. |
| Singh I. [41] | Survey | 877 university students | Not specified: legal and illegal drugs, anabolic steroids, and nutritional supplements | The reason was to improve academic performance, improve concentration, and increase wakefulness in the sleep/wake ratio. The prevalence of users was 2%. |
| Cândido R. C. F. [78] | Survey | 438 university students | Methylphenidate | The reason was to improve academic performance, improve concentration, and increase wakefulness in the sleep/wake ratio. The prevalence of users was 5.8%. |
| Dietz P. [79] | Survey | 2284 students | Caffeine pills | The reason was to improve academic performance, improve concentration, and increase wakefulness in the sleep/wake ratio. The prevalence of users was 15%. |
| Schelle K. J. [80] | Survey | 1572 students | Not specified: legal and illegal drugs, anabolic steroids, and nutritional supplements | The reason was to improve academic performance, improve concentration, and increase wakefulness in the sleep/wake ratio. |
| Rubin-Kahana D.S. [81] | Survey | 1453 students | Amphetamines, and Modafinil | Almost half of the users (47.1%) acquired the drug with a prescription, but without a diagnosis of a related medical disorder. Factors found to impact include: fear of failing the exam, self-reports of being a competitive person. |
| Fond G. [52] | Survey | 1718 students and physicians | Methylphenidate, Modafinil, and Steroids | Lifetime prevalence of psychostimulant use was 33%. The consumption mainly aimed at increasing academic performance and wakefulness during competitive exams preparation. Corticoids were the most frequently consumed before methylphenidate and modafinil. |
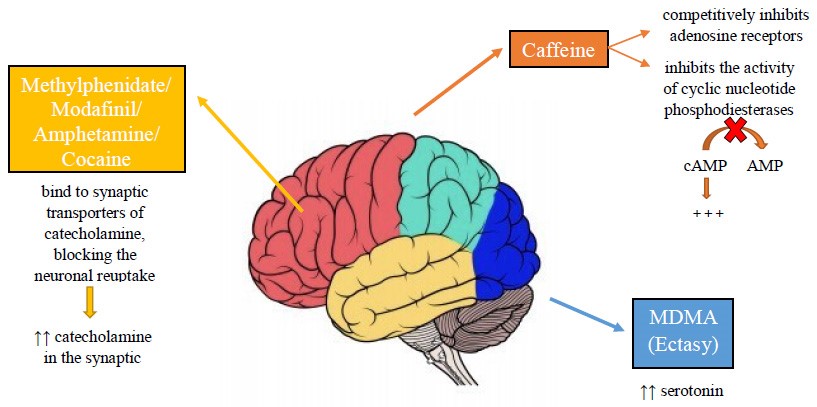
Smart drugs act on the cerebral cortex by modifying the concentrations of catecholamines, in particular, noradrenaline and dopamine. However, the pharmacodynamics are different for each individual drug (Fig. 2).
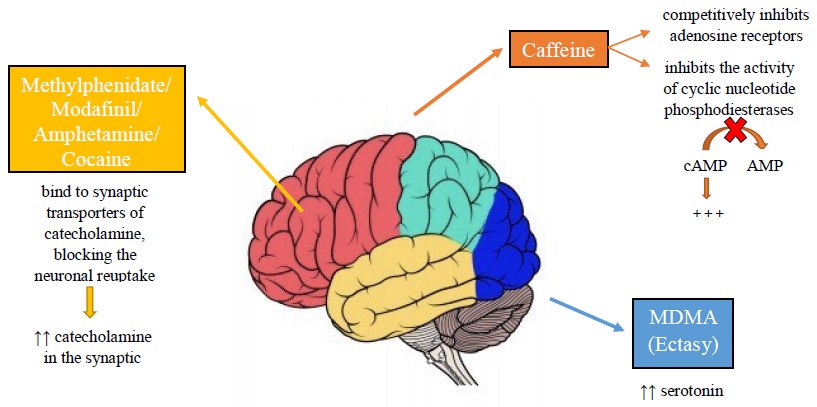 Fig. 2.
Fig. 2.Pharmacodynamics of the main smart drugs.
Caffeine is a common substance for cognitive improvement. Caffeine can be consumed through caffeinated drinks, energy drinks, or tablets [82]. Caffeine has three main mechanisms of action through which it improves alertness, attention and concentration: (i) inhibition of the activity of cyclic nucleotide phosphodiesterases and increase of Cyclic adenosine monophosphate (cAMP); (ii) blockade of adenosine receptors; (iii) mobilization of intracellular calcium [83, 84, 85, 86, 87, 88, 89, 90, 91].
Modafinil stimulates the noradrenergic system of the thalamus, of the frontal cortex, and of the hypothalamus. Modafinil stimulates the dopaminergic receptors of the accumbens, of the striatum, of the frontal cortex, and of the locus coeruleus [92]. This leads to an increase in the concentration of synaptic norepinephrine and dopamine [93, 94]. Amphetamine leads to an increased activation of central b-adrenoceptors [49]. Amphetamine is believed to act primarily through the dopaminergic system at the striatum and nucleus accumbens [95]. The risk of addiction to smart drugs is still debated [96]. Although many smart drugs work by increasing dopamine, there is no evidence of real addiction by abusers [97]. According to Swanson et al. [98] many students who used smart drugs were well aware of a possible addiction.
In fact, this systematic review of the literature shows that most people using smart drugs are unaware of potential addiction [39, 40, 44, 45, 46, 47, 48, 49, 50, 11, 53, 12, 54, 55]. In particular, a survey conducted among the teachers of a school included in this review showed that half of the teachers thought that smart drugs are useless and ineffective, while 40% thought smart drugs were addictive. Opinions on the side effects of smart drugs were different, and the teachers did not know them in depth [62]. Recent studies have reported an increase in the use of these drugs without a prescription in the last few years. Most of the students used performance-time enhancing drugs, and the main reasons were the following: improvement of cognitive functions, reduction of stress, increased free time, and increased school performance [97, 98, 99, 100, 101, 102, 103].
These results are consistent with the present systematic review that highlights that there was a dramatic increase in the use of smart drugs not only among students but also among workers (surgeons, teachers); most of the people taking smart drugs did not have a prescription. Finally, this study reiterates that the main reasons for the use of smart drugs were: improvement of cognitive functions, reduction of stress, increase in free time, and increase in school performance. Research on smart drugs is increasing. However, the real effectiveness of these drugs has not yet been fully investigated [104].
Several reasons lead to use of psychotropics. Sakakibara et al. [105] claimed that the effects of psychiatric drugs affect human sensitivity. Thus, certain moods can influence the effects of smart drugs. In addition, employees and students who confessed to using smart drugs to improve cognitive abilities often had a mental disorder, even if mild [106]. This would provide further evidence that neuroenhancement is used with self-medication for therapeutic and pain-relieving purposes [105, 106].
According to Lynch G et al. [92], methylphenidate (Ritalin) increased alertness. However, this seemed to occur more on simple tasks than on difficult ones. The same authors claimed that methylphenidate also increased working memory; however, the real effects were not fully investigated.
Cognitive enhancement of modafinil was also debated in the literature [86, 87, 88, 89, 90, 91]. Randall et al. [107] claimed a marked improvement in attention in healthy human subjects, while Turner et al. [108] did not find these effects. As a result of these findings, cognitive enhancement was thought to be subjective, and modafinil did not result in overall cognitive enhancement. Beracochea et al. [109], however, showed that it increased concentration on the development of simple rules.
A meta-analysis [86] showed that for methylphenidate there was no evidence for
neurological enhancement, although there was evidence for increased working
memory. Modafinil had moderate effects on attention, although repeated doses only
served to maintain wakefulness. Nicholson et al. [37] claimed that
modafinil has a greater effect in people with a lower IQ. The same authors also
argued that smart drugs increased self-confidence. Side effects were dizziness,
insomnia and nervousness, as well as tachycardia. At high intraperitoneal doses
(5–10 mg/kg) of methylphenidate in healthy rats there was an increase in
locomotor activity and a decrease in cognitive activity. At lower doses (0.25–1
mg/kg) of methylphenidate in animal models, motor activity was not affected but
there was an increase in cognitive activity [2, 110]. This effect was due to an
increase in dopamine and norepinephrine levels in the prefrontal cortex: at high
doses dopamine binds to dopamine 2 (D2) receptors, while norepinephrine binds to
These results are consistent with those shown in this systematic review. Although the use/abuse of smart drugs is increasing, their real functioning is not yet fully understood [110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123]. One reason is that there are few experimental studies both on humans, which are limited to simple surveys, and on animal models. There are evident ethical problems with the experimentation of smart drugs on humans. However, it is crucial to increase experimental studies on animal models on the use of smart drugs to understand the real functioning of these molecules [124, 125, 126, 127, 128, 129, 130, 131].
However, peripheral and central mechanisms are involved in the development of memory for an event. Although these mechanisms are not yet known, hormones are known to affect memory primarily through plasma levels of steroids, adrenaline, and glucose. Piracetam seems to work by affecting peripheral steroid levels. Instead, amphetamines, methylphenidate (Ritalin), antidepressants, and anxiolytics seem to act directly centrally by reducing anxiety, improving attention [132, 133, 134]. Instead, methylphenidate acts by increasing the levels of extracellular catecholamines (adrenaline and noradrenaline), contributing to a strengthening of memory [51]. Caffeine also appears to affect memory and ability to learn. In fact, a study conducted on 48 individuals, moderate consumers of caffeine-based beverages, showed, both at a behavioral and neuroanatomical level, a correlation between caffeine intake and greater short-term memory efficiency [135].
It is of crucial importance to regulate the use of smart drugs, to inform the community about safety, efficacy and social consequences, mainly when they are not prescribed. In addition, the community should be informed about alternative cognitive enhancement pathways such as regular rest, meditation and physical activity. Finally, the development of a legal market for smart drugs should be encouraged in order to counteract the black market that trades drugs of poor quality and purity [136, 90].
Some authors report ethical problems regarding the consumption of smart drugs: is it ethical to use them to improve cognitive functions? Is it ethical to take these drugs to gain an advantage? The answer is debated from an ethical and social point of view. They should be banned because they create an “unnatural” advantage (such as doping in sport). However, according to other authors, their increased use among students is inevitable, as school and university are competitive, and the ban implies the use of illegal ways to obtain these products. Finally, their consumption is expected to be even higher in the future [137, 138]. Adverse events, especially long-term, are only partially known: methylphenidate is associated with drug addiction and suicide attempts. Modafinil, on the other hand, is related to psychiatric disorders, increased cardiovascular risk, and severe allergic reactions [37, 139]. These aspects are of great interest to the population and to public safety/health considering the high use/abuse of smart drugs. The increase in competitiveness among school and university students, the increasingly demanding work, will inevitably lead to an increase in the abuse of smart drugs without in-depth knowledge.
A recent systematic review [140] of the literature was based solely on the use of drugs among school and university students. Although most of the people who use smart drugs are students, the present study is not limited to university students alone but also to the knowledge/use among teachers; surgeons were also evaluated [46, 50].
This systematic review focused on and showed an important general picture of the prevalence of smart drug use, and on potential academic or work performance. Future research on this topic needs to be encouraged, establishing more precisely why cognitive enhancement is subjective and the effective dosage to achieve it. Furthermore, this systematic review revealed an imbalance between the use of smart drugs among students and the ignorance of educators on the subject (teachers, family). Thus, it is important that information and awareness campaigns aimed at both young adults and parents begin.
The knowledge of the effects of smart drugs is still not fully understood; this systematic review highlights how the beneficial and collateral effects are still unclear. There are conflicting opinions; in fact, as regards their actual functioning and benefit, it is not known whether the benefits reported by consumers are due to drugs, the placebo effect or a combination of these. Literature studies have shown a growing prevalence among university and school students. The reasons were: improvement in concentration, performance, time optimization, and increase of free time. The real prevalence is underestimated: it is important for the scientific community to focus on this issue with more studies on animal models to validate their effectiveness. In fact, most of the studies analyzed were surveys with few experimental studies, even if this was due to ethical problems. Television campaigns to raise awareness among families and children are also important.
Conceptualization, ME, GC, FM, FS and MS; methodology, ME, GC, FM, GLR, NDN, GM, FS and MS; validation, ME, GC, FM, GLR, NDN, GM, FS and MS; formal analysis, ME, GC, FM, FS and MS; writing—original draft preparation, ME, GC, FM, FS and MS; writing—review and editing, ME, GC, FM, GLR, NDN, GM, FS and MS. All authors have read and agreed to the published version of the manuscript.
Not applicable.
We wish to thank the Scientific Bureau of the University of Catania for language support.
This research received no external funding.
The authors declare no conflict of interest.
ADHD, attention deficit hyperactivity disorder; IQ, intelligence quotient; cAMP, Cyclic adenosine monophosphate.