1 Department of Interdisciplinary Medicine, Microbiology and Virology Unit, University of Bari “Aldo Moro”, 70100 Bari, Italy
2 Department of Microbiology, George Emil Palade University of Medicine, Pharmacy, Science, and Technology of Târgu Mureș, 540003 Târgu Mureș, Romania
3 Emergency/Urgent Department - National Poisoning Center, Riuniti University Hospital of Foggia, 85025 Foggia, Italy
4 Medical Faculty, Clinical Hospital of Tetovo, University of Tetovo, 1230 Tetovo, North Macedonia
5 School of Technical Medical Sciences, “Alexander Xhuvani” University of Elbasan, 3001-3006 Elbasan, Albania
Abstract
The human body is colonized from the birth by a large number of microorganisms. This will constitute a real “functional microbial organ” that is fundamental for homeostasis and therefore for health in humans. Those microorganisms. The microbial populations that colonize humans creating a specific ecosystem they have been collectively referred to as “human microbiota” or “human normal microflora”. The microbiota play an important pathophysiological role in the various locations of the human body. This article focuses on one of the most important, that is the enteric microbiota. The composition (quantitative and qualitative) of microbes is analyzed in relation to age and environment during the course of human life. It also highlights eubiosis and dysbiosis as key terms for its role in health and disease. Finally, it analyzes its bi-directional relationship with the microbiota of the lungs, skin and that of the brain, and consequently for the whole central and peripheral nervous system for the maintenance of health in the human body.
Keywords
- Human microbiota
- Microbiology
- Gut microbiota
- Dysbiosis
- Immune modulation
- Gut-lung axis
- Gut-brain axis
- Gut-skin axis
- Irritable bowel syndrome (IBS)
- Idiopathic inflammatory bowel disease (IBD)
- Colon cancer
- Metabolic diseases
- SARS-CoV-2
The gastrointestinal tract is undoubtedly the main place for the growth of
microorganisms in the human body and according to more recent estimates about
3.8
The amount of germs can be different along the digestive system, from low concentrations for the stomach, duodenum, jejunum and ileum to high concentrations in the colon. In fact, in the colon stand 1011–1013 microorganisms that for the most part belong to various genes of bacteria. About 90% of germs are found in the colon, the last part of the digestive tract that serves as an anaerobic bioreactor. There are various reasons that can help great development for bacterial quantity and variability in the colon. The main factors then are (a) the pH around the neutrality, (b) the decrease of bile, (c) the absence of pancreatic juice and (d) the slow transit in the intestinal lumen of the colon that helps the multiplication and the metabolism through the fermentation of the existing nutrient substrates, and that come by the diet and/or exudates produced by the intestinal epithelium (Fig. 1) [1, 3, 4, 5].
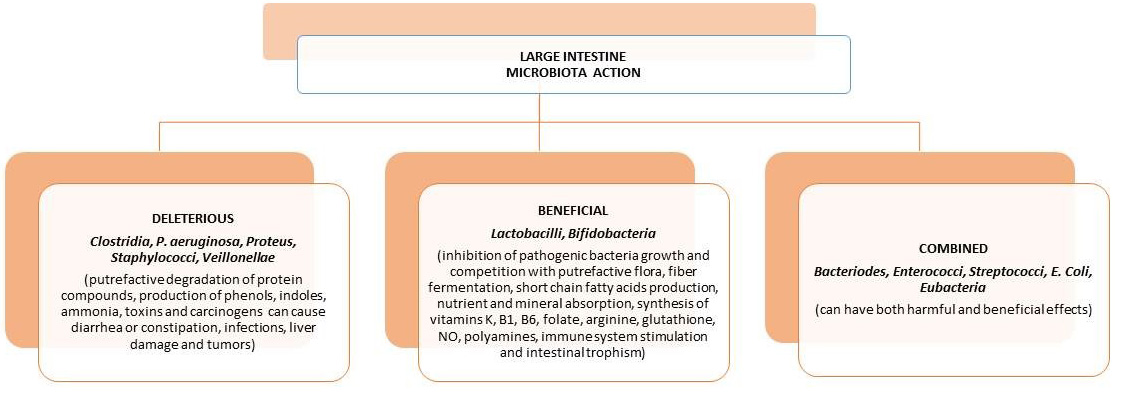 Fig. 1.
Fig. 1.The various actions/effects of the human gastrointestinal microbiota in the large intestine.
The microbiota with its components (mainly bacterial) plays a specific role in normal development in the structures of the immune system network to perform its functions efficiently. To achieve this, the organism with its immune system tolerates at the intestinal level all antigens that can help it (such as food, Simbiotic bacteria) and recognize its action against pathogenic microorganisms. However, the organism benefits from the microbiota through some of the main functions such as: (a) mucus-protective and trophic effects in the epithelium, (b) formation of organic compounds such as the naphthoquinone (Vit. K), energy source by producing SCFAs from unabsorbed food residues, inhibition of pathogen growth, (c) preservation of the integrity of the intestinal lumen epithelium barrier, (d) help in metabolism during the presence of xenobiotics, and (e) the proper function of the immune response [1, 5].
In the human gastrointestinal tract, the composition of each microbiota for each person constitutes an organic “fingerprint”. Thus, the number and location along the lumen of bacterial components would be similar in subjects without specific pathologies. The genera Bacteroides, Firmicutes (genus Clostridium, Eubacterium) are predominant (represent at least ¾ of the microbiota), and after them Verrucomicrobia and Actinobacteria. The colon being a mostly microaerophilic or oxygen-free environment, most microbes are anaerobic. Within this microbiota are mostly overrepresented the Bacteroides, Gram-positive sporigens (such as Ruminococcus, Eubacterium, Bifidobacterium, Peptostreptococcus, and others) and Gram-positive bacilli are mainly represented by the genus Clostridium. Instead, in the large intestine enviromental anaerobic or aerotoxic conditions are present. Gram-positive bacilli are mainly represented by the genus Clostridium. To a lesser extent, anaerobic bacteria such as Enterobacteriaceae, Enterococci, Lactobacilli and Streptococci, necessary for microbial homeostasis, appear in the large intestine. But there are other species that have been cultivated (it is estimated that only 30% of the species can be cultivated with the available techniques), other species whose presence was only indicated by their characteristic DNA sequences and therefore the complexity of the gastrointestinal microbiota was revealed (Fig. 2) [1, 3, 4, 6].
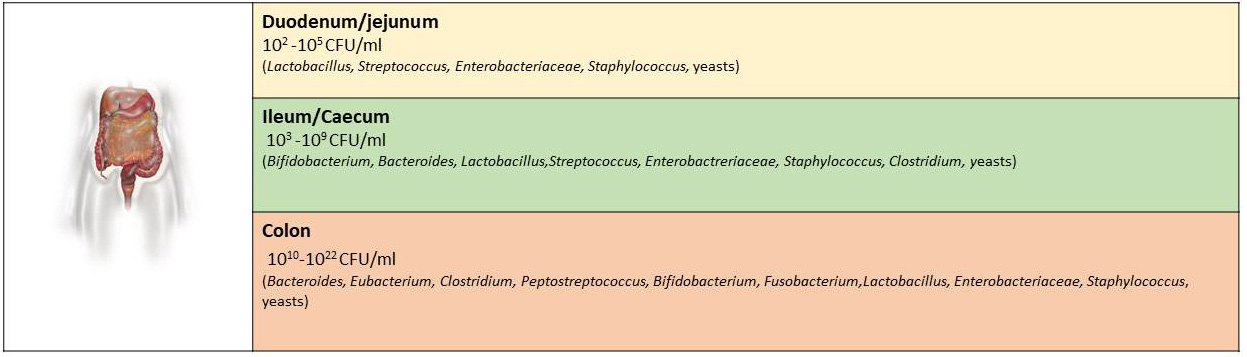 Fig. 2.
Fig. 2.The main genera of bacteria that have been identified in specific locations of the gastrointestinal tract and their corresponding number.
Among the populations of the enteric microbiota we can also distinguish the category of ancient (methanogens), various eukaryotic species, viruses and especially bacteriophages, fungi (mainly yeasts). The composition between the lumen of the intestinal tract associated to the mucous membranes and that of the lumen have differences not only in the microbial composition (Fig. 3) [1, 7, 8, 9].
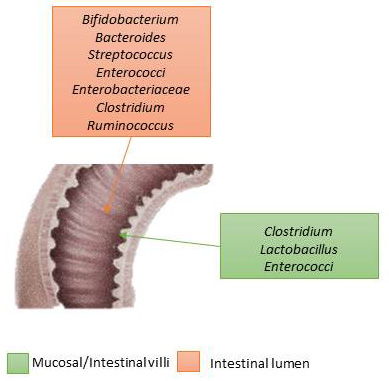 Fig. 3.
Fig. 3.Intestinal microbiota’s differences between the mucosa (where bacteria are attached) and the intestinal lumen (where bacteria lean on).
The germ-free model (GF) concept, is based on the fact that during the perinatal life the fetus lives in a sterile context and subsequently with the procedure of a non-vaginal but surgical delivery no longer has the opportunity the newborn to have a colonization of the microorganisms present in the mother that constitute the first microbiota development. Subsequently, the growth of intestinal germs begins immediately after birth and depends on the bacteria of the mother and the environment in which the child grows. In fact, these exposures to the microbiota belong to the first initial development of the newborn microbiota. This will have its importance in the health its subsequent, because from a bleached colonization of species and microbial number have been observed the development of diseases such as various allergic forms, bronchial asthma, increase of the corporeal fat up to infantile obesity, type 1 diabetes mellitus, neurological disorders and more [10, 11]. The composition of the microflora is therefore influenced mainly by age, environmental factors and the homeostasis of the immune system. After two years and in adulthood the microbiota remains almost constant and is characteristic of every individual. Several studies show that the composition of the microbiota is not similar to that of young age. There are no limits of time or age in which the composition of the microbiota changes, changes occur gradually over time. Perhaps the most important but also changing factor are the eating habits that shape the microbiota, which in turn affects the health of the elderly. Bacteroidetes predominate in newborns, while over time the composition changes gradually and in the elderly the species of the genus Firmicutes predominate (Fig. 4) [6, 12, 13, 14].
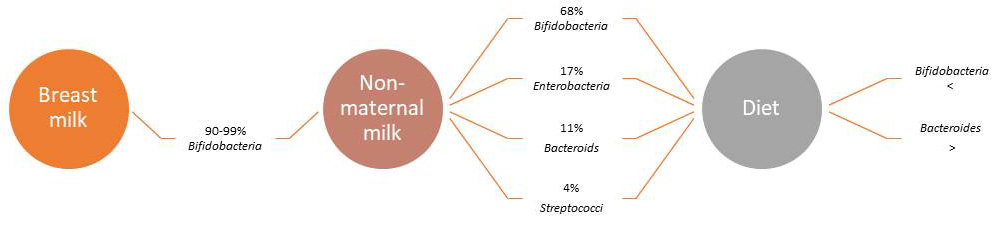 Fig. 4.
Fig. 4.The development of the gut microbiota (main genus and populations%) after birth.
Several studies show that the composition of the microbiota is not like that of
young age. The enteric microbiota, under conditions of normal interaction and
operation (eubiosis), provokes a continuous stimulus to the immune system and
this has because of a condition of “light normal intestinal inflammation”. This
creates a directed organized activity barrier against “bad” germs that is
opportunistic pathogens. In addition, the “good” bacteria of enteric biomass
release both molecules that block proliferation and consuming the nutrients
necessary for the survival of pathogenic germs, thus playing a second protective
role [15]. In fact, it has been shown that in the presence of the some bacterial
species (such as rectal Eubacterium rectale), these are able to harden
the secretion to the level of the intestinal mucosa of the
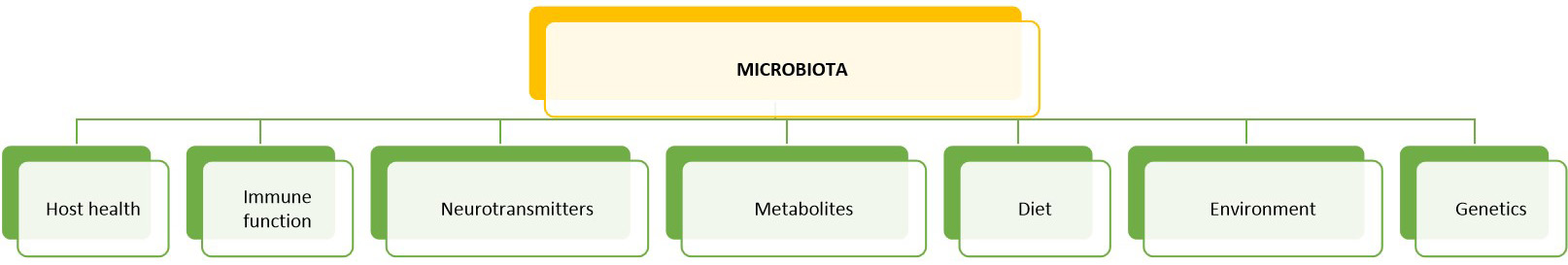 Fig. 5.
Fig. 5.Factors affecting the composition of the gut microbiota: the intestinal microbiota is of particular importance for the maintenance of human health and vice versa for the mutually beneficial dynamic interaction between host microbes in the intestine. This happens with a cross-talk between the immune system and the microbial biomass that is recognized not “dangerous” (not pathogenic). In addition, diet, and the environment, especially after birth, played an important and precise role in the growth and selection of “good” microorganisms. The interaction takes place as well as through intestinal metabolites and the nervous system (through the secretion of neurotransmitters) which can modulate the normal microbial enteric biomass.
Immune system works by learning, that is, at the beginning of life it has the necessary components (cells, intercellular mediators, etc.). But it does not have available data from the environment that acquires the newborn during the first period of life by contact with the mother’s and other persons microorganisms and the surrounding habitat. Indeed, on that first period years an inadequate data, the mechanisms of regulation of the host immunity defense could be inadequate. Hence, the immune system will be against not only pathogenic microorganisms but also other factors such as pollen, various types of dust or food and more, causing acute and chronic allergic reactions. Microorganisms along with digestive enzymes, mucus layer, intestinal peristalsis and epithelial barrier help the body’s immune response. The activity of the microorganisms of the microbiota in eubiosis is to defend the organism are on the one hand to influence in a determining way the development of the intestinal immune mechanism (for which we have made reference to the trophic role) and on the other hand to prevent the possible invasion of pathogens by effect on them and/or “activating” the immune mechanism of the host [16, 17, 18].
In terms of natural immunity, it can distinguish “bad” from the “good” germs
by recognizing the molecular models associated with PAMPs (Pathogen Associated
Molecular Patterns) on microorganisms. More specifically, natural immunity cells
using PRP (Pattern Recognition Receptors) detect PAMPs. PRPs (Pattern Recognition
Receptors) are also involved in the activation of acquired immunity and release
of cytokines. It is worth noting that there are many species PRPs, with Toll-like
(TLR) receptors on the front line, are found in macrophages, neutrophils,
dendritic cells, and epithelial cells of the intestinal mucosa. PAMPs recognized
by PRP receptors are nothing more than, microorganism’s polysaccharides or
monosaccharides, structural peptides, nucleic acids, lipoic acid (present in gram
+ bacteria) or fungal lipoproteins and glucans. However, since these molecules
are also found in symbiotic microbes, we characterize them as MAMP
(Microbe-Associated Molecular Patterns). So, through these MAMPs, it seems that
symbiotic germs change the expression of Toll-like receptors (TLRs) in
non-specific, immune response cells. Therefore, recognition of MAMPs triggers the
activation of the Nuclear factor kappa-light-chain-enhancer of activated
B cells (NF-
The human body as host and the gut microbiota have evolved so that there is benefit to both sides. On the one hand, the host provides space, adequate conditions, and food to the microbiota to grow and this in turn generally contributes to the supply of useful substances and induces resistance to various infections. The enteric active microorganisms flora have a great value for the human’s health preservation. It carries out processes that the human body has not evolved and therefore does not have the ability to act autonomously. The “supreme organism” theory has generally been formulated to note the mutually beneficial interaction between host microbes in the gut. Microorganisms have protective and trophic roles and are also involved in host metabolic pathways and immune functions. Several studies have shown that, in order for the intestinal mucosa to assume its complete structure, it needs to be colonized by microorganisms. For example, mice raised in sterile environments have developed fewer vessels in their intestinal villi. Growth in a sterile environment has also shown that defective growth occurs in lymphoid tissue associated with the intestine and in the production of antibodies. In addition, fewer Peyer patches develop in the sterile environment and less plasma cells in the lymph nodes germinal centers (GC) of mesentery organ than growth data in a non-sterile environment [1, 16, 21].
The gut microbiota acts as a bio-metabolic interconnecting “network” which
interconnects with the human body to perform many of the primary functions
necessary to maintain its health. The breakdown of many food ingredients that are
not digested—fibers, some lipids and proteins, bile acids, cholesterol,
endogenous mucus—are some of the most important actions of the intestinal
microbiota (7–10% of the daily energy requirement of the host). In this way
bacteria provide energy but also produce main metabolites such as the short-chain
fatty acids (SCFAs) which are another additional source of energy for the host.
Some species synthesize and secrete vitamins such as K, B
According to clinical studies conducted in recent years, changes in the composition of gut microbiota is associated with a number changes in the normal microbiological synthesis of the enteric biomass can present a number of serious pathological conditions such as low immune system health, allergy, irritable bowel syndrome (IBS), autoimmune diseases such as idiopathic inflammatory disease, diabetes (type 2), weight gain (which can lead to obese) and obesity, asthma, and chronic sinusitis, gastroesophageal reflux disease, constipation or diarrhea, dermatological problems, mental health disorders, Alzheimer’s disease and other [1, 16, 24].
In irritable bowel syndrome (IBS), several studies have shown a change and
imbalance in the composition of the gut microbiota and suggest that dysbiosis can
lead to impaired immune activity, which could lead to a continuous slight
intestinal inflammation. The alleged cause of this could be caused by exogenous
or endogenous trigger factors; however, the pattern of immune activity in IBS is
complex and most likely involves both inborn and acquired immunity mechanisms. On
the one hand, there is a reduced diversity of the community with a characteristic
decrease of the strains of the genus Firmicutes (such as
Bifidobacteria, Lactobacilli and Faecalibacterium
prausnitzii) and on the other an increase of the microbes that are attached to
the mucus such as the Bacteroidetes, while the remaining patients had a
normal composition of the intestinal microbiota. Firmicutes strains are
the main SCFAs producers, such as the butanoic acid, which has immunomodulatory
properties. Individuals with IBS with diarrhea (IBS-D) appear to have less
Lactobacillus spp. instead, those suffering from irritable bowel
syndrome with constipation (IBS-C) have more Veillonella spp. In the
environment of the intestinal flora the quality (the presence of different
species) and the quantity allow a complete functioning of the am bio-network in
patients with IBS seems to have a reduced settlement of “good” microorganisms.
About innate immunity, that of macrophages plays an important role. Currently,
that relationship between an increase or a decrease in the number of macrophages
observed in IBS is still the subject of various observations. In fact, the
presence of reduced chemokines produced such as CXC Motif Chemokine Ligand 9
(CXCL-9) and monocyte chemoattractant protein-1 (MCP-1/CCL2) has been discovered
that engage some immune cells (such as dendritic cells). However, an increase in
MCP-1 was noted, thus also questioning the data on the expression of intestinal
chemotactic factors. Furthermore, an increase in serum of the cytokines such as
the tumor necrosis factor alfa (TNF
“Idiopathic” inflammatory bowel disease (IBD) is a chronic immunologically
direct disorder disease and is represented by Crohn’s disease and Ulcerative
colitis. The IBD is currently thought pathophysiologic derive from a hostile
immune reaction to endogenous intestinal symbiotic microbes with or without
involvement of the autoimmune process. In non-pathological conditions intestine
enclose a vast number of immune cells that are in a state of activation such that
they do not have a complete immune response to normal microbiota microbes and
food antigens. This is achieved through very powerful regulatory pathways, such
as that of suppressor T cells (Tregs) that express the transcription of the
forkhead box P3 factor (Foxp3) and suppress inflammation. However, when there is
a real infection or other environmental stimuli, in a normal organism, there is a
complete activation of the intestinal immune system, but it is quickly suppressed
[16, 24, 25]. However, in the case of patients with IBD, this process of
extinction of the immune response may not be adequately regulated. The creation
and maintenance of the composition and function of intestinal microbes is under
the control of the host (e.g., immune, and epithelial responses), the environment
(e.g., through diet and the use of antibiotics) and possibly genetic. In turn
microbes, through their structural components and their metabolic activity, have
notable repercussions on both epithelial and immune function of the host; through
epigenetic efforts, these functions can be permanent. From an early age, when the
gut microbial community is established, these microbial effects on the host can
be important in determining the risk of developing IBD in the distant future. In
most studies the rate of idiopathic inflammatory bowel disease increases
particularly in the second to fourth decade of life, while some studies report
even a second high increase in the third age. In particular, therefore, the
components of microbes can promote or protect against diseases [22, 25, 26]. In
patients suffering from ulcerative colitis or Crohn’s disease, the germs
community have been shown to be different from those without infection, a
condition of dysbiosis: the presence of pathogenic microorganisms (e.g., directs
the immune response and/or the loss of microorganisms that inhibit inflammation
(e.g., Firmicutes such as Faecalibacterium prausnitzii). But
many changes and inflammations cause changes in the microbial community. In
addition, antibiotics such as metronidazole, ciprofloxacin and some diets alter
the gut microbiota and can improve symptoms of Crohn’s disease. Indeed, treatment
with antibiotics finds its usefulness in Crohn’s disease. The intestinal mucosa
immune system activity, does not normally respond to food [24, 27]. The
mechanisms involved in tolerance are multiple and include unemployment or
elimination of T cells that respond to antigens or giving the initiative to act
the T helper cells (T
In IBD patients, several studies have shown a significant increase in microbial species belonging mostly to the Enterobacteriaceae family (especially E. coli and Shigella). E. coli is the most abundant microbe in optional anaerobic intestinal bacteria. The development of E. coli when the intestinal mucosa is inflamed is due to its short replication time, its flexibility in metabolism and the variety of catabolic pathways. In addition, the presence of nitric oxide (NO), in addition to community microbes, seems to favor the development of E. coli in murine models. There is also a large variation in the genetic material of E. coli with those that have Adherent-invasive E. coli (AIEC) properties that appear in abundance in colonies in the final ileum tract mucosa more frequently in patients with Crohn’s disease [27, 28, 29]. However, the Carcinoembryonic antigen cell adhesion molecule 6 (CAECAM6) and the cellular heat shock protein Gp96 there are two important receptors on the enteric epithelial (particularly in the final ileum tract) that are responsible for selective colonization, penetration and retention of E. coli (AIEC). Also noteworthy is the ability of E. coli (AIEC) to survive and multiply in macrophages. It has been found that the E.coli (AIEC) series has the ability to cause chronic inflammation in genetically sensitive hosts [29, 30, 31, 32]. Dysbiosis of the intestinal microbiota in situations of infections such as viral ones can lose its eubiosis. Even in the case of the current SARS-CoV-2 pandemic, the infection has led to an alteration of the normal intestinal flora causing dysbiosis conditions. This means that the infection leads to extensive inflammatory reactions which can worsen the symptoms and therefore the prognosis of the patients. Changes in the fecal microbiota were observed compared to healthy controls. Indeed, in these patients they found that some genera were overrepresented such as Streptococcus, Clostridium, Morganella morganii and others. Instead, Coprococcus, Parabacteroides merdae, Firmicutes, Bacteroidetes were less present and others. It has also been noted that Bacteroidetes and Firmicutes with their action during viral infection is protective for the host. Therefore, further alterations in the enteric microbiota leads to worsening of the patient’s immune homeostasis [33, 34, 35].
Human tumors can be caused not only by genetic factors, food (excess of red meat, fat, etc.), lifestyle (smoking, alcohol, drug abuse etc.) and the environment (radiation, etc.), but also by chronic inflammation and persistent infections. In fact, the infections caused by Helicobacter pylori can cause gastric carcinoma instead a chronic inflammatory bowel disease such as ulcerative colitis which for about 5% can evolve into cancer. In fact, a quantitative and qualitative alteration in the microbes of the normal intestinal bacterial flora can lead to carcinogenesis (both through the diet and through its anti-inflammatory action on the intestinal mucosa). Indeed, the intestinal microbiota in patients with colorectal cancer (CRC) is characterized by an increased variety of Clostridium spp. As well as enrichment of the intestine with Bacteroides and Bifidobacterium spp. acid, such as Lactobacillus spp. and Eubacterium aerofaciens [33, 36]. Animal studies led them to propose a carcinogenicity model. These scientists found that mutations in the intestinal epithelial cells cause the intracellular ligaments to relax and the mucus to shrink, affecting the integrity of the intestinal mucosa. This results in the transfer of bacteria from the lumen to the skin where microbial products bind to tumor-associated macrophage receptors and release Interleukne-1, Interleukine-6 and especially Interleukin-23 which in turn stimulates T-helper lymphocytes that secrete interleukin IL-17 [37, 38, 39]. The latter activates the transcription factor STAT3 in epithelial cells, which increases the survival and proliferation of epithelial cells with the consequent addition of additional mutations that lead to dysplasia and possible carcinoma [39, 40, 41, 42, 43, 44]. These changes in the epithelium aggravate the already disturbed integrity of the epithelium, worsening the bacterial alteration and contribute to the vicious pathogenic cycle between bacterial translocation-inflammation-dysbiosis that can lead to oncogenesis. It is therefore understood that “good” bacteria can be the cause of carcinogenesis only if there is an alteration of the host’s immune response, indicating the special role of the mucosa in preventing the development of cancerogenesis and its complications [38, 45, 46, 47, 48].
There are several studies in which the microbiota has been associated with the development of diabetes (particularly, type 2), which is characterized by a decrease in concentrations of Clostridial bacteria (genera Roseburia and Faecalis). Variations in the number of Bifidobacterium, Lactobacillus, Clostridium, Firmicutes, Bacteroidetes have also been observed in the gut microbiota of young patients with type 1 diabetes [49, 50].
The increase in energy accumulation in obese individuals is related to the
transport of hydrogen between taxonomic groups of micro-organisms, since they
observed a simultaneous increase of the Prevotellaceae producing
hydrogen (H
The interaction between the gut microbiota and the brain constitutes the so-called crosstalking Gut-brain axis. This interaction is therefore bidirectional and occurs through endocrine, neural, immune, and humoral signaling connections pathways from the gut microbiota to the brain and from the brain to the gut microbiota. This communication network includes the and central nervous system (CNS) and the peripheral nervous system (PNS), and the hypothalamic pituitary adrenal (HPA or HTPA) axis. Through the pneumogastric nerve (component of the parasympathetic nervous system) the interaction takes place in a bidirectional world. This neuro-intestinal system includes the nerve part with its motor neurons, primary intrinsic afferent neurons and glial cells contained in the Auerbach’s plexus and tela submucosa that extend along the intestine. The CNS through the autonomic nervous system (ANS) and the HPA axis affects the gastrointestinal tract to its functions such as through motility its secretory capacity and its permeability (such as motility and secretion and more). In clinical practice, the evidence for GBA- microbial interactions stems from a dysbiosis condition between nervous system disorders (such as depression, autism and others) and functional gastrointestinal disorders [53, 54, 55]. The central nervous system through the autonomic nervous system but also through the HPA axis affects its function gastrointestinal tract through the motility of the digestive tract, its secretory capacity and its permeability. These effects in turn affect its microbiota. On the other hand, germ products can reach the brain and affect its function. In addition, it has been observed in experimental animals that the intestinal microbiota affects the formation of synapses in the brain and the production of neurotransmitters. So, we notice that there is a substantial interaction between the microbiota and the nervous system. It is believed that changes in the bidirectional interaction between the brain and the gut may be the cause but also for its evolution over time of irritable bowel syndrome. The pathogenesis of functional gastrointestinal disorders. Therefore, as we have previously mentioned, beyond the pathogenesis of functional gastrointestinal disorders, neurological disorders are involved (such as Parkinson’s disease, disorders on the affective sphere, mood and emotions, chronic pain and others). Intestinal microbes with their metabolites influence the permeability of the intestinal barrier, the immune system, motility and activity of the intestinal nervous system. Preclinical data also show that they can regulate brain behaviors and functions, including responses to stress, emotions, pain control, eating behavior and generally brain biomolecular functions [56, 57]. The gut microbiota therefore it can interfere with the neural pathways and behavior in the face of a stressful condition. Social stresses increase the risk of inflammatory diseases, promoting the expression of pro-inflammatory genes and the differentiation of monocytes. Therefore, the alterations that cause inflammatory processes induce the alteration of the intestinal microbiota and thus can further favor the ability of pathogens to colonize the intestine. It has also been shown that a condition of continuous stress can influence the secretion of IgA, the inflammatory response subsequently leading to dysbiosis and thus disturbing intestinal homeostasis. Several on animals studies are conducted for research on the interaction on stress-inducing stimuli between the intestinal microbiota and the HPA axis. These provide us with information on the importance of the gut microbiota in the development of the HPA axis. It has been noticed, in the germ-free (GF) mice, in front of a mild contentious stress stimulus there is an increase in the secretion of ACTH and corticosterone compared to mice lacking for particular pathogens. It was noted that this condition of increased hormone release had been partially normalized in these animals by the introduction of the fecal microbiota from lacking for particular pathogens animals. It was also noted that everything normalized with the introduction of the Bifidobacterium infantis strain, during their early childhood. On the other hand, in another study the administration of B. infantis improved the response to the stressful stimulus in gf mice. Therefore, for a response to the same stimuli in adult life, the development of an effective intestinal microbiota from birth is important. This is what will ensure the correct development of the HPA axis in the future [52]. In fact, in addition to the increase in hormones during stress in these animals, it was also noted the reduction in the levels of the Brain-derived neurotrophic factor (BDNF), which is a neurotrophin involved in both growth and neuronal survival. Finally, in many studies on gf animals, alterations in the expression in the hippocampus of the serotonin 1A receptor (5-HT1A) and N-methyl-D-aspartate (NMDA) receptor have been found. By influencing the release of corticotropin-releasing hormone (CRH) from the hypothalamus and therefore a modified response of action of HPA. Stress also leads to high levels of the secretion of Interleukin-6 and of the chemotactic monocyte protein-1 (MCP-1), which were associated with changes caused by the stress-induced stimulus in certain bacteria such as Coprococcus and other [53, 57]. In another study, observed the changes with B. infantis treatment, but not a reduction in corticosterone, while later with the use of a similar model found that adding Lactobacillus to the diet reduced corticosterone levels [58, 59, 60]. It is also unclear whether changes in the gut microbiota in patients with such disorders as irritable bowel syndrome are due to primary changes involving only germs and/or changes associated with bowel-brain communication. In addition, although there are rare cases of patients who develop psychotic symptoms after using a wide range of antibiotics, there are not enough clinical data showing that a sudden change in the gut microbes has a clinically obvious effect on the individual’s symptoms. The first study in experimental animals has already shown that the absence of a normal gut microbiota can significantly affect the response to stress, and this can be reversed by re-colonization of the gut. The (GF) mice are thinner than the free specific pathogen mice (SPF), although they consume more calories. The metabolic changes that occur in them can affect the brain development and alter the activity of neural circuits associated with eating behavior and metabolism. Changes in barrier permeability in germ-free mice can lead to significantly different access of microbial metabolites to the brain. There are therefore two-way interactions of the gut microbes with the central nervous system. The CNS regulates the intestinal tract and the intestinal nervous system through sympathetic and parasympathetic pathways of the ANS as well as with the HPA axis (Fig. 6). The CNS effects now can affect the intestinal microbiota indirectly by changing their environment and directly through a variety of signaling molecules. The ANS regulates functions such as motility, acid secretion, production of bicarbonate and mucus, retention of epithelial fluids, intestinal permeability, and mucosal immune response. Most of these functions are under the influence of sympathetic and parasympathetic in the intestinal nerve circuits systemic [53, 61, 62].
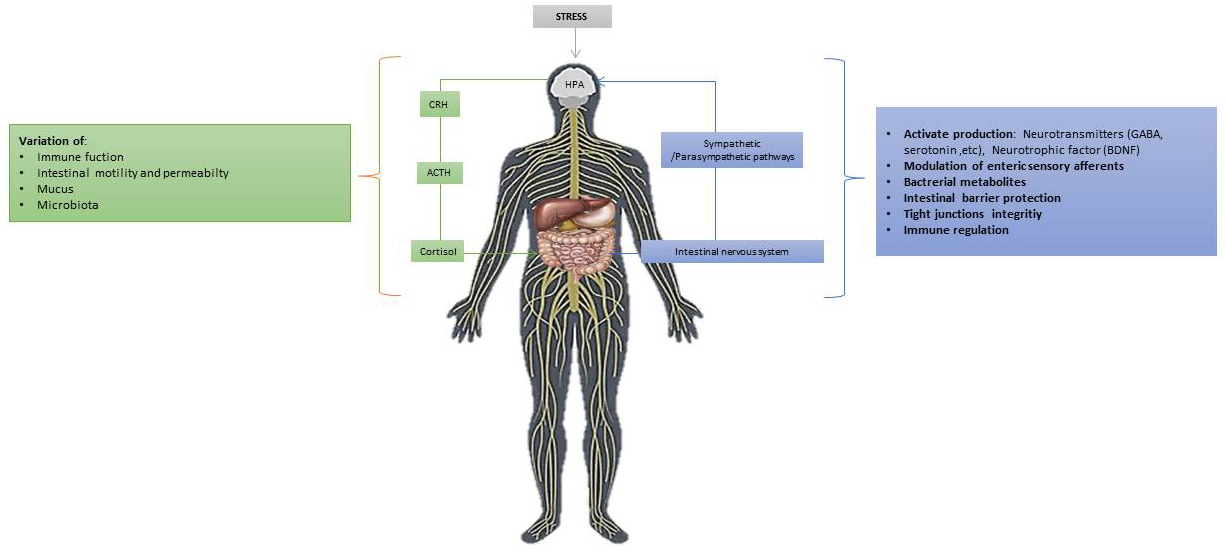 Fig. 6.
Fig. 6.Main Gut/brain axis bidirectional mechanisms and environmental stressed conditions: stress stimulates the HPA axis which, through the release of the hypothalamic CRH leads to stimulates the secretion of the hormone of ACTH. Subsequently, it in turn leads to the secretion of cortisol (adrenal glands). This in turn affects the brain and, therefore, both communication pathways, hormonal and neural, interact so the brain is able to modulate the actions of intestinal effector cells.
Another important systemic axis is the communication between the lung microbiota
and the intestinal microbiota (Gut-lung axis). This is another type of crosstalk
with the exchange of immunological information between the two systems. This will
result in the possibility of influencing the functional behavior of the lung
microbiota under certain conditions. Thus, the gut microbiota can regulate and
modify the immunological activity of the lung via bacterial lipopolysaccharides
(LPS) and various bacterial metabolites (such as SCFAs and other). Subsequently,
after stimulation with the production of dendritic cells that they cause the
activation of various T lymphocytes (particularly T-reg, T-h17, Th1) migrating to
the lower respiratory tract through the circulatory flow. Instead, bacterial
metabolites cause tumor necrosis factor (TNF-a) to decrease via activation of the
activated B cell light chain kappa-enhancer nuclear factor (NF
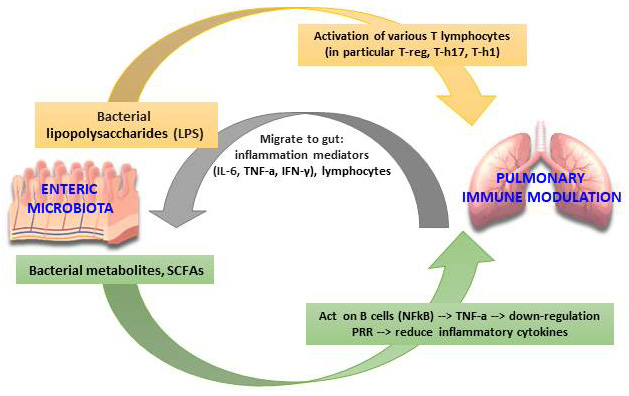 Fig. 7.
Fig. 7.The Gut-lung axis microbiota immunomodulation hypothesis. (Adapted from Santacroce et al. The Human Respiratory System and its Microbiome at a Glimpse. Biology (Basel). 2020 Oct 1; 9 (10): 318).
Finally, we reported that the interconnection between enteric microbes and host immune functions promotes the proper functioning of the intestinal immune system, but a condition of severe intestinal dysbiosis that leads to not only intestinal inflammation with the integumentary system involvement. This is the crosstalk between the Gut-skin axis. Consequently, we can have various pathological manifestations of the skin such as eczema, atopic dermatitis, acne, and others. It is reported that in some cases of dysbiosis conditions of the microbiota, there is an increase in the final products of the metabolism of aromatic amino acids (i.e., free phenol and p-cresol from the Clostridium difficile. This can lead to changes in the immune response (modification of the production between Teff and Treg lymphocytes) in the intestine which may involve the skin. Finally, most likely during SARS-CoV-2 skin manifestations may be due to dysbiosis and the disregulation of the crosstalk Gut-skin axis [69].
Probiotics (Greek =
 Fig. 8.
Fig. 8.The main probiotics in relation to their main use (
The choice of strains depends on their safety, efficacy for health and their ability to benefit humans. Indeed, e.g., probiotics that act on the large intestine must be resistant to sialic enzymes, acid secretion of stomach (changes of pH), bile secretions, small intestine enzymes such as those of the pancreatic secretions (lipase and amylase) and the environment of other foods and drinks encountered during their passage along the gastrointestinal tract. In addition, they must be able to compete with microbial flora. The manipulation of probiotics as oral therapeutics has also been shown to be useful in reducing the small intestinal bacterial overgrowth linked with anxious-depressive disorders. In one study, probiotics from 2 strains L. helveticus and B. longum in healthy volunteers were involved in the therapy for anxiety and depression. After two weeks the improvement of the symptoms was noticed. The same happens with the use of Lactobacillus rhamnosus which it was noticed that it acts on GABA (gamma-aminobutyric acid) which in cases of anxiety and depression its production is modified (Fig. 9).
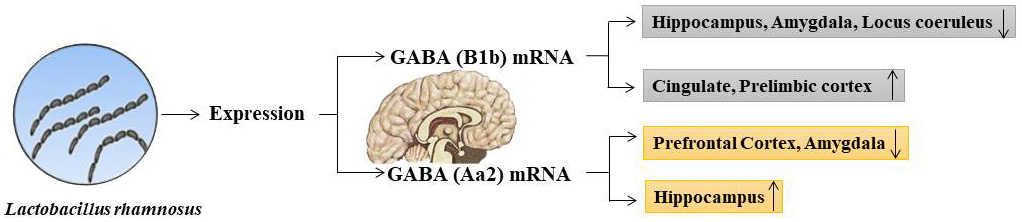 Fig. 9.
Fig. 9.The effect after oral administration of Lactobacillus rhamnosus in the brain in relation to the variation of the expression of GABA mRNA.
Therefore, some studies report that as adjuvant therapy and to prevent psychiatric disorders (such as addiction to substances of abuse, bipolar disorder and others) it would be appropriate to use some selected bacterial strains such as Bifidobacterium infantis (increase in the levels of the 5-HT precursor tryptophan) has been studied in its efficacy in relieving IBS-associated depression and anxiety. Indeed, researchers now refer to these living organisms as “psychobiotics” which, if administered in well-established quantities they can have beneficial effects on the health of patients suffering from psychiatric diseases; and it has already been proposed as an additional treatment in patients suffering from depression or in mild depressive states in general. In addition to these that we have mentioned, other psychobiotics can be E. Coli, Bacillus and Saccharomyces (facilitate the increase of norepinephrine), Candida, Streptococcus and Enterococcus (increase the production of 5-HT) Bacillus and Serratia (stimulate the secretion of dopamine) [69, 72, 73, 74, 75]. The Anandamide and 2-Arachidonoylglycerol (2-AG) of the Endocannabinoid System (ECS) which have neurotransmitter and neuromodulatory capabilities, a strain of Lactobacillus acidophilus can modify the function of their receptors in the spinal cord [76, 77, 78]. Thus, probiotics in general can now be effective in therapy and prevention as an adjunct in various infectious and non-infectious diseases. Probiotics in general can now be effective in various infectious and non-infectious diseases [79, 80, 81, 82, 83]. Prebiotics are foods that the body is unable to digest but which lead to a selective and specific function leading to the development and/or activity of one or a limited number of bacterial species already present in the large intestine. Therefore, they are mainly made up of indigestible carbohydrates and the most used may be inulin, fructo-galacto- and xyloligosaccharides. These prebiotics once found in the large intestine undergo fermentation by the SCAFs of the local bacterial flora. Furthermore, it has been noted through several studies that they also have benefits in comparison with similar probiotic strains (such as Bifidobacterium spp. and Lactobacillus spp.). In chronic inflammatory bowel disease. The benefit of this treatment has been shown to be highly dose-dependent. In fact, high levels of prebiotics can often intensify problems like bloating and flatulence. Finally, the prebiotics could be useful in combination with probiotics [69, 84].
Actually, much “light” has been made about the microbial biomass that colonize our body and particularly for the intestinal microbiota. Its pathophysiological involvement in various diseases such as non-gastrointestinal has been extensively studied and continues to be studied. The composition of the gut microbiota differs in health and disease conditions. It contributes to the pathophysiological processes associated with the three axes: Gut/Brain, Gut/Pulmonary and Gut/Skin. Therefore, in conditions of eubiosis of the intestinal microbiota it acts in favor of human health thus preventing the development of “bad” germs (pathogens). Moreover, by modulating the local immune defenses, it puts the immune system in an equilibrium for an effective and adequate response against the pathogenesis of various diseases. Finally, due to the intake of antibiotics, diet, infections such as the viral pandemic SARS-CoV-2 potential pathogens favoring the growth and/or transmission in the various other systems of pathogenic germs that will lead to various local or systemic diseases. Furthermore, this microbial imbalance can compromise local intestinal health such as allergies, inflammatory diseases, pre-cancer conditions and more. Thus, probiotics and prebiotics restore the balance and the functional homeostasis of the intestinal microbiota which with its cross-talking axes not only creating effective immune defenses against various diseases but can also be an additional help for mental ones (Psychobiotics).
All the authors equally contributed to conceiving the study, literature investigation and project management. Dr. Charitos wrote the manuscript and all the authors reviewed it and agreed to its publication.
Not applicable.
None.
No public funds or private grants available for this paper.
The authors declare that there is no conflict of interest.









