Frontiers in Bioscience-Landmark (FBL) is published by IMR Press from Volume 26 Issue 5 (2021). Previous articles were published by another publisher on a subscription basis, and they are hosted by IMR Press on imrpress.com as a courtesy and upon agreement with Frontiers in Bioscience.
1 Laboratorio de Neuroproteccion, Facultad de Farmacia, Universidad Autonoma del Estado de Morelos, Morelos, Mexico
2 Division de Ciencias Basicas e Ingenierias, Universidad Popular de la Chontalpa, H. Cardenas, Tabasco, Mexico
3 Instituto de Bioingenieria, Universidad Miguel Hernandez, Elche, Alicante, Spain
Abstract
Some organophosphorus compounds (OPs), which are used in the manufacturing of insecticides and nerve agents, are racemic mixtures with at least one chiral center with a phosphorus atom. Acute exposure of humans to these mixtures induces the covalent modification of acetylcholinesterase (AChE) and neuropathy target esterase (NTE) and causes a cholinergic syndrome or organophosphate-induced delayed polyneuropathy syndrome (OPIDP). These irreversible neurological effects are due to the stereoselective interaction of the racemic OPs with these B-esterases (AChE and NTE) and such interactions have been studied in vivo, ex vivo and in vitro, using stereoselective hydrolysis by A-esterases or phosphotriesterases (PTEs) and the PTE from Pseudomonas diminuta, and paraoxonase-1 (PON1) from mammalian serum. PON1 has a limited hydrolytic potential of the racemic OPs, while the bacterial PTE exhibits a significant catalytic activity on the less toxic isomers P(+) of the nerve agents. Avian serum albumin also shows a hydrolyzing capacity of chiral OPs with oxo and thio forms. There are ongoing environmental and bioremediation efforts to design and produce recombinants as bio-scavengers of OPs.
Keywords
- Chiral organophosphorus
- Paraoxonase-1
- Albumin
- Stereoselectivity
- Phosphotriesterases
- A-esterases
- Hydrolysis
- Calcium. copper
- Nervous agent
- Review
OPs are amide esters or thiol derivatives of phosphoric acid or phosphorothioic acid; they have been synthesized since 1940. OPs have become the most used insecticides around the world because of their great ability to inhibit B-esterases (1, 2). The acute toxic effect of OPs on biological systems occurs through acetylcholinesterase (AChE) inhibition. Therefore, this cholinergic syndrome is caused by the acetylcholine accumulation in the peripheral synapsis (neuromuscular) and central synapsis (neuron-neuron) that primarily induce salivation, lacrimation, gastrointestinal stimulation, trembling, and convulsions. Abdominal respiratory paralysis is the main cause of human deaths caused by intoxication with these compounds. The cholinergic syndrome caused by OPs appears clinically during the first 72 h of the intoxication (3–5). In some cases of acute intoxication by OP racemic insecticides, such as phosphates, phosphonates, and phosphoramidates, irreversible and delayed symptoms with signs of ataxia appear between the second and third week after the exposure. This neurodegenerative syndrome is known as organophosphate-induced delayed polyneuropathy (OPIDP) (6, 7) and correlates with the inhibition and aging of the so-called neuropathy target esterase (NTE) of the central nervous system (8).
The morbidity and mortality that occur in humans due to OP intoxication due to agricultural activities and the continuous threat of the use of nerve agents in wars have encouraged the search for enzymatic systems that can hydrolyze these compounds. This action would neutralize adverse effects to the human population. The current pharmacological treatment for cholinergic syndrome consists of the administration of atropine (an ACh muscarinic antagonist) and oximes (reactivation of AChE), which have been ineffective in most of the severe acute intoxication cases (9–11). There is no drug of choice to OPIDP. Consequently, treatment involves the application of biomolecules that interact with OPs to protect human life. Therefore, the administration of human serum butyrylcholinesterase (HuBuChE) has been suggested. This B-esterase may represent a promising scavenger biomolecule for OP intoxication (12). However, its main limitation is its stoichiometry: A large quantity of the enzyme is required to achieve protection from OP intoxication in vivo. Some proposed second-generation bio-scavengers include A-esterases or phosphotriesterases (PTEs), such as bacterial PTEs, and, mainly, mammalian serum paraoxonase-1 (PON1). Notably, variant recombinant PON1s have increased the enzyme’s specificity for more toxic OPs insecticides (13-15). These recombinant proteins could be a therapeutic alternative in the intoxication by racemic mixtures of these compounds.
Some OP pesticides have been commercialized as active ingredients in insecticides; the chemical structures have at least one chiral center in the pentavalent phosphorus atom (Figure 1). The biological activity of these racemic compounds is the result of the detoxification of one enantiomer. The other enantiomers can have adverse effects due to their interaction with target biomolecules (16–21). Johnson and coworkers (22) examined the stereoselectivity of cholinergic and delayed neurotoxicity with racemic OP mixtures. They evidenced stereoselective neurotoxicity of O-ethyl O-4-nitrophenyl phenylphosphonothioate (EPN) and methamidophos insecticides. Other commercial insecticides, such as ruelene, trichoronate, and fenamiphos, require biochemical studies with regard to their stereoselective interaction with B-esterases. Given that these insecticides represent an environmental risk for intoxication (19, 20, 23, 24), their differential toxicity among biological species and individuals may be due to the stereoselective hydrolysis of these racemic mixtures by A-esterases (16). The limited scientific information about stereoselective OP hydrolysis may be attributed to the reduced number of chiral separation methods and the lack of OP enantiomer standards. The separation and collection of enantiomers of some commercial chiral OPs with chiral liquid chromatography have allowed stereoselective toxicological evaluation (25–37). The enantiomer (–)-trichloronate is 8–11 times more toxic than (+)-trichloronate, and it contributed 68% and 72% of the harmful activity against the microorganisms Ceriodaphnia dubia and Daphnia magna, respectively. Profenophos and fonofos showed stereoselective toxicity for (–) enantiomers in C. dubia (92–94%) and D. magna (87–94%). However, the stereoselective toxicity of the chiral OPs is not exclusive to the (–) isomers, because the (+) enantiomers of fenamiphos and leptophos were 2.0–3.8 times more toxic to D. magna than the (–) isomers (38, 39). This selective poisonous effect for the (+) enantiomers has been observed for phosphoramidates such as methamidophos and acephate, which show 97% effectiveness against domestic flies versus their racemic mixtures (40).
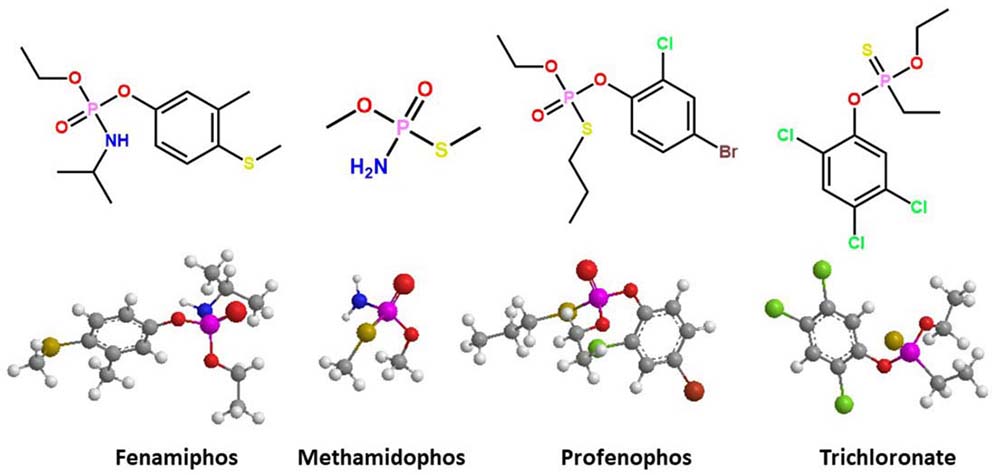 Figure 1
Figure 1Three-dimensional representation of the docked complex of chiral structure of organophosphorus pesticides; *denotes chiral center.
Stereoselective hydrolysis of racemic OPs is defined as the preferential hydrolysis of one stereoisomer over the other (41). The enantiomers of chiral OPs induce different toxic effects: Stereoselective hydrolysis is one of the biological factors that determines their toxicity. The adverse impact corresponds to the isomer that withstands in vivo hydrolysis by A-esterases or PTEs. The toxic relevance of degradation has been demonstrated for EPN (42), methamidophos (43, 44), and methamidophos analogs, specifically the S-methyl series and dichlorophenyl phosphoramidates (45–48). PTEs that hydrolyze chiral OPs have been identified in invertebrates, mammals, and—mainly—bacteria (49–57). PTEs are divalent metallic cation–dependent hydrolases (53, 58–60). The structure of these metalloproteins shows binding sites for hydrophobic OPs due to a three-pocket conformation that binds the leaving group and the other two substituent groups of the molecule to position the phosphorous center and perform the catalysis (58, 61–66). The crystalline structures of several bacterial PTE have been obtaining, and their catalytic capacities have been associated with structural folding, like TIM barrel, "pita-bread," β-lactamase, and β-propeller (61, 62, 67, 68). Paraoxon is considered to be a good substrate for PTEs, due to the catalytic efficiency: Kcat 104 s-1 and Kcat/Km of approximately 108 M-1 s-1 in the usual laboratory in vitro assay (59). However, PTEs can be considered nonspecific for OPs (69–76). In particular, bacterial PTEs are efficient enzymes for substrates with phenolic (77), thiol, and halogenide electron acceptors (72, 77). This broad substrate affinity for these esterases is explained by the nonspecific nature of the substrate-binding site: There are three ester groups of the substrate interact with the three pockets hydrophobic site in the enzyme surface (78). The stereoselectivity of the wild-type PTE from Pseudomonas diminuta for chiral organic phosphates depends on the substituent bound to the central phosphorous atom (63, 71). Researchers confirmed the substrate specificity of this PTE with a library of 16 paraoxon analog compounds; the authors concluded that combinations of methyl, ethyl, isopropyl, and phenyl groups as structural substituents X and Y were substrates for this PTE, with values from 18000 s-1 for dimethyl p-nitrophenyl phosphate to 220 s-1 for diisopropyl p-nitrophenyl phosphate (79). The enzyme hydrolyzed the SP-enantiomer, preferably in the racemic mixtures of chiral OPs (80, 81). The stereoselectivity activity was evident for methyl isopropyl p-nitrophenyl phosphate; the hydrolysis of the SP enantiomer was 100 times higher than its corresponding RP enantiomer (79). The efficient hydrolysis of the EPN (81), acephate, and methamidophos insecticides (73), as well as other racemic mixtures of phosphate, phosphonate, and phosphinate esters, constitute examples of the stereoselective hydrolysis of PTEs. In some cases, this stereoselectivity is around five orders of magnitude higher compared with non-stereoselectivity (Table 1) (71).
| Chiral OP compound | Bacterial phosphotriesterase | Stereoselectivity (Fold) | References |
|---|---|---|---|
| Acephate | PTE from E. coli (wild-type) | SP > RP (100) | 73 |
| Methamidophos | PTE from E. coli (wild-type) | SP > RP (100) | 73 |
| EPN | PTE from P. diminuta (wild-type) | SP > RP | 81 |
| Paraoxon analogue | PTE from P. diminuta (wild-type) | SP > RP (100) | 79 |
| Sarin analogue | PTE from P. diminuta (wild-type) | Rp > Sp (9) | 88 |
| Sarin analogue | PTE from P. diminuta (I106A/F132A/H254Y mutant) | SP > RP (10) | 88 |
| Sarin analogue | PTE from P. diminuta (G60A mutant) | Rp > Sp (50) | 88 |
| Soman analogue | PTE from P. diminuta (wild-type) | RPRC > RPSC, SPRC, SPSC (10-1200) | 88 |
| Soman analogue | PTE from P. diminuta (I106A/F132A/H254Y mutant) | SPRC > SPSC, RPRC, RPSC, (5-37) | |
| Soman analogue | PTE from P. diminuta (G60A mutant) | RPRC > RPSC, SPRC, SPSC (4-6000) | 88 |
| Acetylphenyl methyl phenyl phosphate | PTE from P. diminuta (wild-type) | SP > RP (1.2 x 102) | 71 |
| Acetylphenyl methyl phenyl phosphate | PTE from P. diminuta (G60A mutant) | SP > RP (3.7 x 105) | 71 |
| Acetylphenyl methyl phenyl phosphate | PTE from P. diminuta (I106G/F132G/H257Y mutant) | RP > SP (9.7 x 102) | 71 |
| Sarin analogue | OPAA from Alteromonas sp. (wild-type) | RP > SP (2) | 86 |
| Soman analogue | OPAA from Alteromonas sp. (wild-type) | RPSC > RPRC, SPRC, SPSC (29-7260) | 86 |
| Cyclosarin | PTE from P. diminuta (wild-type) | (+)GF > (–)GF (21) | 89 |
| Cyclosarin | OPAA from Alteromonas sp. (wild-type | (+)GF > (–)GF (12) | 89 |
| Cyclosarin | OPAA from A. haloplanktis (wild-type | (+)GF > (–)GF (24) | 89 |
| Cyclosarin | PTE from P. diminuta (H254G/H259W/L303T mutant) | (–)GF > (+)GF | 89 |
| VX nerve agent | PTE from P. diminuta (wild-type) | SP = RP (1) | 91 |
| VX nerve agent | PTE from P. diminuta (H254Q/H257F mutant) | SP > RP (12) | 91 |
| VX nerve agent | PTE from P. diminuta (L7ep-2b mutant) | RP > SP (12) | 91 |
| VR nerve agent analogue | PTE from P. diminuta (wild-type) | RP < SP (25) | 85 |
| VR nerve agent analogue | PTE from P.diminuta (G60A mutant) | RP > SP (7600) | 85 |
| VR nerve agent analogue | PTE from P.diminuta (I106A/F132A/H257Y mutant) | SP > RP (270) | 85 |
Site-directed mutagenesis has allowed modification of the rate of catalytic hydrolysis, increasing or decreasing it, as well as the transformation of the PTE stereoselectivity of P. diminuta for OPs. Glycine (Gly) residues of the substrate hydrophobic union site to PTE play a crucial role in providing the stereoselectivity to these chiral compounds (82). The mutant protein G60A of PTE showed a 100-fold reduction in its Kcat/Km value for the RP enantiomer of methyl phenyl p-nitrophenyl phosphate. Consequently, the ratio of stereoselective hydrolysis increased 13000 times in favor of this enantiomer versus wild-type PTE (63, 79, 83). This same recombinant PTE protein rose from 1–3 orders of magnitude the stereoselectivity for other chiral phosphoric esters (71). Furthermore, mutant PTE I106G/F132G/H257Y reversed the stereoselectivity, because the RP enantiomer of 4-acetyl phenyl methyl phenyl phosphate was hydrolyzed preferably (with a factor of 9.7 x 102). By contrast, the wild-type protein and mutant PTE G60A preferentially hydrolyzed the SP enantiomer (1.2 x 102 and 3.7 x 105, respectively) (71).
The most neurotoxic racemic OPs have been synthesized for use as agents of war; among the best known are sarin, soman, and VR (Figure 2). The adverse effects of these nerve agents depends on the stereochemistry in the phosphorous center (84). All contain a chiral center at the phosphorus atom, and some present a second chiral center in one carbon atom, such as soman. Bacterial PTEs hydrolyze most of the nerve agents in a stereoselective manner in favor of the RP isomers (85), which are less toxic for mammals. The detoxifying property of bacterial PTE on these warfare agents has been of great interest in medical toxicology and biotechnology for environmental bioremediation. Hoskin and coworkers (86) identified three enzymes in Escherichia coli that can hydrolyze soman, but only one of them showed stereoselectivity. The racemic mixture of soman was hydrolyzed with two-phase kinetic behavior: a fast initial phase and a subsequent slow phase, which approximates the observed non-enzymatic detoxification rate for soman. Hill and coworkers (87) demonstrated that organophosphorus acid anhydrolase (OPAA) from Alteromonas sp. hydrolyzed the isomer RPSC 7000 times more than the isomer SPSC from the soman analogs. The P(+) isomers of p-nitrophenyl sarin analogs were hydrolyzing by OPAA (2–4 fold) faster than bacterial wild-type PTE from P. diminuta, for chiral analogs of sarin and soman. the RP enantiomer of one sarin analog (Kcat = 2,600 s-1) had a higher affinity compared with its corresponding SP enantiomer (Kcat = 290 s-1). This stereoselectivity hydrolysis was reversed with the PTE mutant I106A/F132A/H254Y, with markedly reduced Kcat values: 410 s-1 for the RP enantiomer (6 fold smaller) and 4200 s-1 for the SP enantiomer (14 times greater) (88). Other similar enzymatic reactions were obtained for the hydrolysis of other chiral OPs with a thiolate leaving group. The comparison of the rates between soman and sarin analogs demonstrated that the pinacol substituent has less affinity than the isopropyl group in the active site of the PTE enzyme wild-type of P. diminuta, because the sarin RP isomer is hydrolyzed faster (by two orders of magnitude) than the soman RPRC isomer (Table 1).
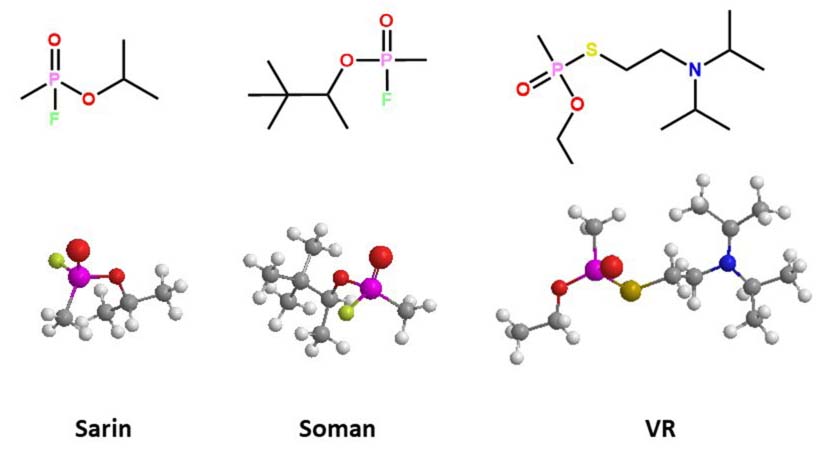 Figure 2
Figure 2Three-dimensional representation of the docked complex of chemical structure of toxic OP nerve agents; *denotes chiral center.
Harvey and coworkers (89) confirmed the stereoselective hydrolysis property of PTEs from P. diminuta and Alteromonas sp., as well as OPAA from Alteromonas haloplanktis. Specifically, those wild-type proteins showed a hydrolysis ratio of 21.5, 12 and 24, respectively, in favor of the (+) isomer of cyclosarin. The H254G/H259W/L303T mutant reversed the stereospecificity of the native PTEs because it preferentially catalyzed the hydrolysis of the (−) isomer of cyclosarin ((+)/(–) ratio = 0.16), which is two times more toxic to AChE than the racemic compound. Tsai et al. (85, 90) have demonstrated that the inherent stereoselectivity of wild-type PTE from P. diminuta on the enantiomers RP of sarin, soman, and cyclosarin increased with the size of substituent group bound directly to phosphorous atom. The mutants of this wild-type protein have an increased stereoselective capacity for the R/S ratio (Kcat/Km) from 22 to 760 times for a cyclosarin analog. In other cases, the PTE mutants reversed the stereoselectivity. In particular, the substitution of alanine with a glycine residue (mutant G60A) in the small pocket of this bacterial PTE improved the stereochemical preference with an R/S ratio (Kcat/Km) from 25 to 7600 times in VR compound analog. The PTE I106A/F132A/H257Y mutant showed a stereoselective reversion for sarin, soman, cyclosarin, VX, and VR. Those mutant proteins showed levels of enzymatic activity up to 15,000 times greater for the SP isomers compared with wild-type PTE. The results are relevant from a toxicological perspective because they are the enantiomers that show the highest toxic potency for mammals.
The catalytic activity of the wild-type PTE bacterial (from P. diminuta and Flavobacterium sp.) toward V-type nerve agents is approximately three orders of magnitude lower than G-type nerve agents (Kcat/Km < 103 M-1 s-1) (75, 76). Tsai and coworkers (85) observed that wild-type PTE showed a slight hydrolysis preference for the SP enantiomer of VX (S/R ratio, Kcat/Km = 2). Nevertheless, Bigley and coworkers (91) did not observe stereoselective hydrolysis when they used the bacterial wild-type enzyme. However, the double mutant of bacterial PTE QF (H254Q/H257F) hydrolyzed the more toxic SP enantiomer 12 times more than the RP enantiomer of VX. This result is similar to the one obtained by Tsai and coworkers (85) with a VX analog. These authors also observed stereoselective catalysis: Hydrolysis of the RP isomer (reverse hydrolysis) was 12 times higher than its corresponding SP enantiomer with the mutant L7ep-2b (CVQFL + H254R/N265D/A270D/L272M/S276T) (91). Studies of PTE from P. diminuta have suggested their potential role as a therapeutic intervention for nerve agent intoxication to attenuate the neurological deficit in humans who are chronically exposed due to wars. With this objective, mutant PTEs have been designed to provide stereoselectivity on the more toxic isomer (91).
Serum aryldialkylphosphatase (EC 3.1.8..1.), also known as PON1, is the best-known protein of the family of enzymes called paraoxonases from mammalian tissues (mainly liver and human serum). Its name is derived from its capacity to hydrolyze paraoxon, which is a metabolite of the neurotoxic insecticide parathion (92); in addition, it was the first of the three PON members discovered. Molecular studies have revealed that PON1 messenger RNA (mRNA) is widely distributed in the brain, kidney, liver, small intestine, and lungs of animals. Nevertheless, PON1 only reaches significant levels of toxicological interest in the liver and serum (93–97). PON1 is a calcium-dependent A-esterase that comprises 354 amino acids; its molecular weight is 45 kDa (95, 96, 98–100). It is synthesized in the liver (98) and secreted into the bloodstream to bind high-density lipoprotein (HDL) (101). PON1 is an A-esterase with paraoxonase, arylesterase, and lactonase activity. Phenylacetate is one substrate for which PON1 has high affinity (102, 103). Since its identification in animal tissues, researchers have proposed that PON1 is involved in protection against OP toxicity (104–107). In vitro studies have demonstrated that PON1 hydrolyzes toxic aryl esters and OP insecticides (in the oxo form), such as parathion, chlorpyrifos, diazinon, and chiral nerve agents, including sarin and soman (108–110). Despite the broad affinity for ester substrates, PON1 shows different catalytic hydrolysis rates that depend on the chemical’s structural conformation (108, 110, 111). PON1 is considered to be a relevant protein for in vivo OP detoxification because species like birds and fish, which have low serum PON1 activity, are very susceptible to OP insecticide intoxication (112). By contrast, species with greater levels of this protein, such as mammals, are more resistant to the toxicity of these compounds (108, 113, 114). The most direct evidence of the protective role of PON1 against OP toxicity has been demonstrated in rodent models. The exogenous administration of purified PON1 protected against the toxicity of OPs in the oxo form. The extension of this protective effect was dependent on the efficiency catalytic of PON1 versus OP (115–118). The catalysis rate of human serum PON1 (HuPON1) on chiral nerve agents has been considered low in toxicological terms. Therefore, PON1 might be a PTE with limited protection against OP toxicity because it only hydrolyzed a few OPs and it is unable to act on racemic mixtures. For this reason, protein engineering has been applied to this A-esterase to increase specificity and rate of catalytic hydrolysis for OPs, including racemic warfare agents and chiral insecticides.
The relatively low expression of A-esterases, like PON1, during neurodevelopment increases the susceptibility to OP pesticide toxicity in human populations (119, 120). The high toxicity of OP insecticides correlates with PON1 levels and carboxylesterases related to age (120, 121). Experiments with rodents have demonstrated that the ontogeny of this esterase correlates with the toxicity of these compounds (119–121). Specifically, clinical studies have shown that newborn children have one third to one quarter of the PON1 serum levels that are found in adults (122–124), as well as variability in the active protein levels among individuals (125). The combination of genetic variability and PON1 expression in human development can mean up to 160-fold greater susceptibility to OPs (123). A longitudinal epidemiological study concluded that PON1 activity in children increased 3.5 times from birth to 7 years. Nevertheless, these PON1 levels at the age of 7 years were significantly lower (1.8%) than the maternal levels, and the difference was more evident for mothers and children with genotypes (QQ) associated with one PON1 copy with low activity (see section 3.4.2.) (124). In a longitudinal study of the cohort, González and coworkers (126) found that the PON1 levels in the serum of 9-year-old children were lower (1.7–7.9%) than the levels in their respective mothers. However, these differences were not statistically significant; hence, PON1 levels in children at this age may reach the levels found in adults.
Chiral OPs induce adverse effects in the biological systems related to their stereochemical structure and the affinity to B-esterases from the nervous system (127). Johnson and coworkers (43) established that the administration of 5–7 times the median lethal dose (LD50) of D (+)-methamidophos induced OPIDP in hens. By contrast, the administration of L(–)-methamidophos at similar doses did not induce OPIDP (128). The ratios of rate constants for the inhibition of AChE/NTE for D (+)-methamidophos were 2 for hens and 3 for humans; the same ratios for L(–)-methamidophos were to 900 for hens and humans (129). This same group previously demonstrated that the enantiomers of one OP showed a different capacity to inhibit AChE and NTE in hen’s brain, with consequent stereoselective OPIDP (22). These results suggested stereoselective hydrolysis when racemic mixtures of OPs that include phenyl phosphonothioates like EPN (130) and phosphoramidothioates like methamidophos and its analogs when administered in vivo (45, 131). Other pre-clinical studies on aquatic and cellular toxicity have corroborated the stereoselective toxicity of the chiral OPs pesticides (132).
Fenamiphos is widely using as a nematicide in the production of fruits, vegetables, grains, and tobacco, among other crops (133, 134). This racemic OP is highly toxic to aquatic and terrestrial organisms (135). Aquatic toxicity studies in D. magna have demonstrated that (+)-fenamiphos is 20 times more toxic than (–)-fenamiphos. This stereospecific aquatic toxicity was corroborated with isomers of other chiral insecticides in D. magna and C. dubia (136), as well as in ex vivo inhibition studies of BuChE from horse serum (38) and AChE from PC12 rat cells (137). Notably, the racemic insecticide profenophos showed a stereoselective effect in vitro on AChE from animal serum, and (+)-profenophos inhibited this B-esterase up to 23 times more than (–)-profenophos. Nevertheless, in vivo studies have clearly shown that this isomer is approximately 23 times less potent as an inhibitor of AChE (138). The authors explained this contrary, adverse effect by suggesting a stereospecific bioactivation in vivo of enantiomer (–)-profenophos (139), as was observed for (–)-isofenphos in rat liver microsomes (140). Since the first reports of inhibition and aging in vivo and in vitro by chiral OPs of AChE and NTE esterases, it has been suggested that stereoselective metabolism (hydrolysis) should be considered in the neurotoxicity of these compounds. For this reason, the characterization of the hydrolyzing role of A-esterases or PTEs, including PON1 from human serum (HuPON1), on the chiral OPs, insecticides, and warfare agents, is relevant in terms of public health and environment protection (Table 2).
| Chiral OP compound | Protein source | Stereoselectivity (Fold) | References |
|---|---|---|---|
| IMP-pNP | Mammalian serum PON1 (wild type) | P(+) > P(–) | 155 |
| IMP-pNP | Mammalian serum PON1 (V346A mutant) | P(+) > P(–) | 155 |
| Ciclosarin | Mammalian serum PON1 (wild type) | P(+) > P(–) | 155 |
| Ciclosarin | Mammalian serum PON1 (V346A mutant) | P(+) > P(–) | 155 |
| Soman | Mammalian serum PON1 (wild type) | P(+)C(+), P(+)C(–) > P(–)C(+), P(–)C(–) | 155 |
| Soman | Mammalian serum PON1 (V346A mutant) | P(+)C(+), P(+)C(–) > P(–)C(+), P(–)C(–) | 155 |
| Soman | Guinea-pig skin | P(+)C(+), P(+)C(–) > P(–)C(+), P(–)C(–) (~11) | 156 |
| Soman | Mouse skin | P(+)C(+), P(+)C(–) > P(–)C(+), P(–)C(–) (~15) | 156 |
| Soman | Human skin | P(+)C(+), P(+)C(–) > P(–)C(+), P(–)C(–) (~34) | 156 |
| Soman | Rat liver (soluble fraction) | P(+)C(–), P(+)C(+) > P(–)C(+), P(–)C(–) | 161 |
| Soman | Human serum | P(+)C(+), P(+)C(–) > P(–)C(+), P(–)C(–) (~40) | 162 |
| Soman | HuPON1 (wild-type) | P(+)C(+), P(+)C(–) > P(–)C(+), P(–)C(–) (~3) | 163 |
| EMP–MeCyC | HuPON1 (wild-type and mutants) | P(+) > P(–) | 164 |
| VX and VR | rHuPON1 (recombinant) | P(+) > P(–) | 165 |
| HDCP | Rabbit serum | S(–) > R(+) | 142 |
| HDCP | Rabbit serum | S(–) > R(+) (8-10) | 145 |
| HDCP | Rat serum | S(–) > R(+) (5) | 145 |
| HDCP | Hen, rata or rabbit liver (particulate or soluble fraction) | S(–) > R(+) (2-12) | 145 |
| HDCP | Hen, rata or rabbit kidney (particulate or soluble fraction) | S(–) > R(+) (1-12) | 145 |
| HDCP | Human serum | S(–) > R(+) (2-3) | 33 |
| HXM | Rabbit serum | R(+) > S(–) | 143 |
| Trichloronate | Human serum | (+) = (–) | 171 |
| Fenamiphos | Human serum | (+) = (–) | 36 |
| Profenofos | Human serum | (+) = (–) | 172 |
PON1 activity in rabbit serum was first identified with paraoxon as a substrate in the presence of calcium (141), so ethylenediaminetetraacetic acid (EDTA) can inhibit it. Currently, this activity in animal tissues, such as liver and serum, is defined as calcium-dependent and EDTA-sensitive PTE activity. Albumin is a vertebrate serum protein with paraoxonase activity; it hydrolyzes paraoxon in the absence of calcium in mammalian serum (142). Studies carried out with neuropathic phosphoramidates, like methamidophos and its analogs, were among the first performed on stereoselective hydrolysis of OPs. In particular, the incubation of racemic O-hexyl-S-methyl phosphoramidite (HXM) with rabbit serum confirmed a slow but specific elimination of the R-(+)-HXM enantiomer (143). The stereoselectivity of this catalytic reaction was evident with its compound analog, O-2,5-dichlorophenyl phosphoramidate (HDCP) in several animal tissues (144, 145). These ex vivo assays showed stereospecific calcium-dependent hydrolysis of S-(–)-HDCP in the microsomal fraction of rabbit liver and mainly in rat and rabbit serum. The EDTA-resistant hydrolysis of HDCP was not stereoselective in these tissues and animal species (145, 146). These results, suggest that the blood concentration of R-(+)-HDCP will remain higher than S-(–)-HDCP and induce the neurotoxic effects.
Mammalian serum PON1 hydrolyzes calcium-dependent OPs in the glutamine/arginine polymorphism at position 192 (Q192R). The Q192R polymorphism affects the hydrolysis levels of some racemic OPs substrates (98, 110, 115, 147). In our laboratory, the stereoselective hydrolysis of fenamiphos and HDCP by different PON1 Q192R alloforms from human serum has been characterized by chiral chromatographic methods (32, 37). The stereoselective hydrolysis has been quantified by measuring the residual concentration (µM) of each insecticide enantiomer, after a specified incubation time with 2.5 mM calcium or 5 mM EDTA at physiological pH and temperature. There were significant differences for the stereoselective hydrolysis of fenamiphos by the different PON1 Q192R alloforms from human serum (36). The average residual concentration (μM) of (+)-fenamiphos and (–)-fenamiphos for each one of the three PON1 Q192R alloforms from adults and children were in a range of 166–200 μM. The highest level of hydrolysis (12%) was not significant (p > 0.05, Student’s t-test). The lack of significant hydrolysis for the fenamiphos enantiomers in human serum suggests that age may not influence the catalytic efficiency of PON1 Q192R alloforms for the hydrolysis of chiral insecticides in vivo. These results contradict those reported for sarin, which established a greater susceptibility to chiral OPs intoxication associated with the age and to PON1 Q192R alloforms from human serum (104, 121, 148, 149).
For HDCP hydrolysis by human serum samples, after a 60-min incubation at physiological conditions three PON1 Q192R alloforms showed an exclusive and significant stereospecific calcium-dependent hydrolysis of S-(–)-HDCP. Indeed, the remaining concentrations of this isomer were lower than R-(+)-HDCP in 47 human adult serum samples that were diagnosed by the PON1 192 polymorphism (33). This stereoselective calcium-dependent S-(–)-HDCP hydrolysis is inhibited with EDTA and is independent of the PON1 Q192R alloform, which shows a tendency for greater hydrolysis compared with the RR alloform. This research reinforces that R-(+)-HDCP (the isomer that inhibits NTE and causes its aging) might be the enantiomer that induces delayed neuropathy caused by this chiral phosphoramidate. In summary, the insecticide fenamiphos is not hydrolyzed by PON1 Q192R alloforms from human serum. These data reinforce the hypothesis that PON1 has an irrelevant detoxifying role for OPs in vivo because of the limited number of chiral OPs that it hydrolyzes. When it does hydrolyze compounds, it prefers the less-toxic enantiomers, as is in the case of HDCP.
The stereospecific calcium-dependent hydrolysis of warfare agents by mammalian tissues has been studied for more than 50 years to determine the toxic properties of its isomers on human health. The first study reported by Augustinsson (150) identified stereoselective degradation of tabun by pig kidney tissue. Cristen (151, 152) observed the stereoselective hydrolysis of P(+) sarin in the plasma of several species, which included rats and humans. Subsequently, other researchers used chromatographic techniques and corroborated the stereoselective hydrolysis of soman in animal tissues (153, 154) that included the skin homogenates of the mouse, human, and guinea pig. Those results showed the hydrolysis of the less toxic isomer (C(+/–) P(+)) of this compound, with a speed constant of 0.127 min-1 g-1. The stereospecificity of PON1 on nerve agents has been studied to determine the toxic properties of their isomers. Wild-type PON1 and their recombinants have shown metal-dependent stereospecific hydrolysis for the less toxic soman isomers (89, 155). Different studies have demonstrated the stereospecific hydrolysis of soman in rats, guinea pigs, and marmots (153, 154). Van Dongen (156) examined the stereoselective hydrolysis of soman using skin homogenized from guinea pigs, mice, and humans; it hydrolyzed the less toxic isomer (C(+/–) P(+)) of soman, with a rate constant of 0.127 min-1 g-1 l-1. In 1957, Augustinsson reported that phosphorylphosphatase from pig liver had a stereospecific effect toward D-tabun. While, Cristen and coworkers (152) demonstrated the stereoselective hydrolysis of sarin in serum from several species; they also reported higher hydrolysis of P(+)-sarin than its corresponding P(–)-isomer (151). Later, researchers demonstrated that the P(+)-sarin isomer was rapidly hydrolyzed (157, 158). On the other hand, Herbert and coworkers (159) showed stereoselectivity in fraction IV of human serum towards soman C+P+ isomer. Likewise, Benschop et al. (158) and De Jong and coworkers (160) reported that the tissues with a high PON1 content, such as mammalian serum and liver, preferably hydrolyzed the C(±) P(+) soman stereoisomers. These results for stereoselectivity were corroborated with a variant of the gen-shuffled (LR1) of HuPON1, expressed in bacteria, which exhibited stereoselective hydrolysis in vitro for the less toxic isomers soman and cyclosarin. Although the catalytic efficiency of HuPON1 against warfare agents, like sarin, VX, and soman, was low, its capacity to hydrolyze these toxic nerve agents in vivo makes it an attractive protein as a biological scavenger of chiral OPs (155). Meanwhile, Little and coworkers (161) reported significant non-stereoselective hydrolysis of the four soman isomers in the rat liver, data that suggest the presence of a 40-kDa enzyme that is not PON1. The stereoselective hydrolysis capacity of PON1 on chiral OP esters has been demonstrated against racemic substrates, like the sarin analog known as O-isopropyl-O-(p-nitrophenyl) methyl phosphonate (IMP-pNP). Amitai (155), showed the stereoselective hydrolysis of cyclosarin, soman, and O-(isopropyl)-O-(p-nitrophenyl)methyl phosphonate (IMP-PNP); these data corroborate that PON1 is less active toward the less toxic isomers.
De Bisschop (162) identified the stereoselective hydrolysis of soman in human serum. Yeung and coworkers (163) characterized the catalytic activity of HuPON1 toward each of the four soman isomers simultaneously by chiral-gas chromatography coupled to mass spectrometry. The Kcat/Km values ranged from 625 to 4130 mM-1 min -1, with the following order for the isomers: C(+) P(+) > C(–) P(+) > C(+) P(–) > C(–) P(–). These data indicate that the soman hydrolysis by HuPON1 is stereoselective. Wild-type HuPON1 showed low activity, but its catalytic hydrolysis of the four soman stereoisomers was stereospecific. A critical assumption in the analytical model developed to determine the kinetic constant for each stereoisomer is that each one behaves as an independent substrate, but competitive in the hydrolysis reaction.
The reversal of stereoselective hydrolysis through recombinant mammalian PON1 has been developing by Amitai and coworkers from the Israel Institute for Biological Research. They have proposed that research should aim to increase enzymatic detoxification toward the more toxic P(–) isomer of nerve agents because wild-type mammalian PON1 have shown calcium-dependent stereoselective hydrolysis toward the less toxic P(+) enantiomers of those neurotoxic compounds. Previous studies on OP hydrolysis with recombinant PON1 have allowed the identification of Leu69, Val346, and His115 of PON1 as key positions that enhance the hydrolysis of cyclosarin, soman, and other nerve agents. However, residual AChE inhibition studies have suggested their stereoselective hydrolysis toward the less toxic P(+) isomer of these chiral OPs similar to the stereoselectivity of wild-type PON1. For these studies, the authors designed and synthesized other PON1 variants as well as new asymmetric fluorogenic OPs analogs of VX, cyclosarin, and soman. Finally, they demonstrated through AChE inhibition assay that the recombinant HuPON1 L69V/S138L/S193P/N287D/V346A showed reverse stereoselectivity toward the more toxic P(–) isomer of an analog compound of these warfare agents (164).
On the other hand, Otto and coworkers in 2010 (165) demonstrated the stereoselective hydrolysis of P(+) enantiomer of VX and VR by HuPON1 purified from Trichoplusia ni larvae. This HuPON1 variant was resistant to the hydrolysis of non-chiral insecticides, such as chlorpyrifos. The P(+) isomer of VX was fully hydrolyzed after 240 min, but its corresponding P(–) isomer was not hydrolyzed after 360 min. The stereoselectivity of the VR racemic mixture was similar to the observed for VX; the P(+) isomer was fully hydrolyzed after 420 min. The preferential hydrolysis of rHuPON1 on one particular isomer is indicative of the conformational restrictions of the active site of PON1 variant, where the fixation and catalysis are dependent on the three-dimensional arrangement of substituents O-alkyl around chiral phosphor atom in OPs. The lack of hydrolysis toward P(–) isomers of VX or VR by rHuPON1 might result from the enzyme's inability to bind the substrate, a phenomenon that inhibits the catalysis. Kirbli and coworkers (166) synthesized recombinant single (H115W) and double (H115W/F347W) PON1s to generate variants that hydrolyzed class G and V warfare agents. They reported that H115W PON1 recombinant increased the catalytic activity levels of chiral OPs to wild-type PON1 protein. The double variant H115W/F347W PON1 showed a light stereoselective hydrolysis toward the tabun P(–) isomer ((–)/(+) ratio = 1.34) versus simple mutant H115W PON1 ((–)/(+) ratio = 1.06). These results suggest the participation of the amino acid tryptophan at position 347 near the active site residues promotes higher affinity binding of the P(–) isomer compared with the P(+) isomer. Goldsmith and coworkers (167) have used direct evolution to increase PON1 activity toward the more toxic SP isomer of nerve agents G-type (tabun, sarin, soman, and cyclosarin). The PON1 variants showed a ≤ 340 increase in the ratios and catalytic efficiencies of 0.2–5 × 107 M-1 min-1. Additionally, there was PON1 stereospecificity reversal, from an enantiomeric ratio of (E) < 6.3 × 10−4, in favor of the RP isomer of the cyclosarin analog and wild-type PON1, to E > 2,500 for the SP isomer in an evolved variant. PON1 variants can hydrolyze the toxic SP isomer of cyclosarin (≤ 1.75 × 107 M-1 min-1) efficiently. This result had been previously obtained using directed evolution (168).
Research has identified a phosphotriesterase activity that is different from PON1 in birds. This activity is not calcium-dependent and EDTA-resistant in hen serum; this activity was named HDCPase due to the ability to hydrolyze HDCP. Sogorb and coworkers (169) identified albumin as the protein responsible for this PTE activity. Subsequently, ex vivo experiments with particulate fraction of the hen, rabbit, and rat liver, as well as serum from rabbit and rat, demonstrated the stereoselective activity of S-(–)-HDCP (146), which was in a range of 1–3 fold greater when the microsomal fraction of the liver was used in the assay (10–80 mg) (147). Toxicological research has demonstrated that employing high concentrations of subcellular fractions for in vitro or ex vivo tests achieved a more realistic approximation of the stereoselective hydrolytic processes of OPs in biologic systems in vivo. Our research group recently identified novel PTE activity in chicken (34) and turkey serum (37). It is 20 fold higher than calcium-dependent HDCP activity, which is copper-dependent and stereoselective to the enantiomers of higher toxicity of HDCP and trichloronate; R-(+)-HDCP) and (–)-trichloronate (a chiral compound in thio form), respectively. This new A-esterase activity has been identifying in chicken and turkey serum albumins (35, 170).
The results of studies on stereoselective hydrolysis by bacterial wild-type PTEs and their recombinants on chiral warfare agents have allowed researchers to suggest potential uses in the clinical toxicology treatment against racemic OPs toxics. In the field of biotechnology, these proteins can be used in the elaboration of bioreactors in environmental bioremediation. Human serum PON1 is a PTE with a limited role in OP hydrolysis because it only hydrolyzes a few non-chiral OP insecticides. Indeed, the main limitation comes from the limited hydrolysis of chiral OP insecticides, or its preference for hydrolyzing the isomers with low toxicity. Biotechnological production of recombinant PTEs with stereoselective hydrolysis of OPs is required in the field of the clinic and veterinary toxicology for the treatment of intoxications caused by chiral OP insecticides. Finally, the toxicological field needs to continue in the discovery and design of new A-esterases or PTE proteins that hydrolyze chiral OPs compounds or their neurotoxic enantiomers.
This manuscript has been supported by project SEP/CONACYT 257092.
OP(s)
Organophosphorus compound(s)
Acetylcholinesterase
neuropathy target esterase
Organophosphate-induced delayed polyneuropathy syndrome
Phosphotriesterase(s)
Paraoxonase-1
Acetylcholine
Acetylcholinesterase
Butyrylcholinesterase
Human plasma butyrylcholinesterase
O-ethyl O-4-nitrophenyl phenylphosphonothioate
VR nerve agent
Organophosphorus acid anhydrolase
VX nerve agent
High-density lipoprotein
Paraoxonase-1
Human serum PON1
Recombinant human serum PON1
Lethal dose 50%
Ethylenediaminetetraacetic acid
O-hexyl-S-methyl phosphoramidite
O-2,5-dichlorophenyl phosphoramidate
O-isopropyl-O-(p-nitrophenyl) methyl phosphonate
O-(isopropyl)-O-(p-nitrophenyl)methyl phosphonate
Enantiomeric ratio


