Frontiers in Bioscience-Landmark (FBL) is published by IMR Press from Volume 26 Issue 5 (2021). Previous articles were published by another publisher on a subscription basis, and they are hosted by IMR Press on imrpress.com as a courtesy and upon agreement with Frontiers in Bioscience.
1 The Sol Goldman Pancreatic Cancer Research Center, Department of Pathology, The Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA
2 Department of Biophysics, Panjab University, Chandigarh, 160014, India
Abstract
Cyclooxygenase-2 (COX-2) is an inducible enzyme which triggers the biosynthesis of prostaglandins. COX-2 is activated in response to inflammatory stimuli and is one of the major molecules that is involved in the development and progression of colorectal cancer (CRC). Consistent with such a conceptual framework, it has been shown that COX-2 inhibitors prevent the carcinogenesis of CRC and aid in the treatment of sporadic or familial cases of CRC as shown by an overall increase in the survival rate. However, prolonged use of these inhibitors is associated with increase risk of development of cardiovascular complications, implying that further research is required to identify COX-2 inhibitors that are associated with lower risks of such complications.
Keywords
- Colorectal cancer
- Cyclooxygenase
- Inflammation
- Biomarker
- Review
Cyclooxygenase (COX) enzymes are a member of myeloperoxidase family, which regulates the prostaglandin (PG) biosynthesis. COX isozymes metabolized the arachidonic acid to prostaglandins and thromboxane (1). Prostaglandins regulates various physiological pathways such as tumor development, migration, differentiation, inflammation and cell to cell adhesion, while thromboxane triggers platelet aggregation, proliferation and vasoconstriction (2). COX has 3 different isoforms with distinct biological properties and functions: COX-1, COX-2 and COX-3. COX-1, a housekeeping enzyme is constitutively expressed in human cells and tissues, COX-2, a proinflammatory enzyme is predominantly associated with inflammatory diseases, while COX-3 is a splice variant of COX-1 and is non-functional in humans (3).
COX-2 is also known as prostaglandin-endoperoxide synthase 2 and is encoded by PTGS2 gene in humans. It is a homodimer of 581 amino acids with a molecular weight of 70kDa. The functional role of COX-2 is conversion of arachidonic acid to prostaglandins which further converted into prostaglandin H2 (PGH2), that finally metabolized by tissue-specific synthases into 5 major prostaglandins (PGD2, PGE2, PGF2α), prostacyclin (PGI2), and thromboxane A2 (TXA2) (3). In normal conditions, COX-2 is unexpressed in cells, while in response to stimuli such as inflammation; COX-2 is highly expressed (4). The role of COX-2 in inflammation and cancer is well known. Various studies reported that COX-2 promotes tumor proliferation, angiogenesis induction, tumor invasion, metastasis and apoptosis inhibition (3, 5, 6). It is revealed that the overexpression of COX-2 is associated with gastrointestinal malignancies such as colorectal, pancreatic, liver, gastric and esophageal cancers (7-9) (Figure 1). CRC is the third most common and deadly cancer with 1.8 million new cases diagnosed each year worldwide. The five-year survival rate of colorectal cancer is only 13% (10). The majority of CRC cases are sporadic (75%), around 20% cases are due to familial risk, and only 2-4% cases are due to lynch syndrome, familial adenomatous polyposis or other inherited mutations (11). Since, inflammation is a major risk factor for the colorectal cancer and COX-2 over expression is linked to the CRC development, so it is important to explore the role of COX-2 and its inhibition as a prognostic biomarker for early detection and prevention of CRC.
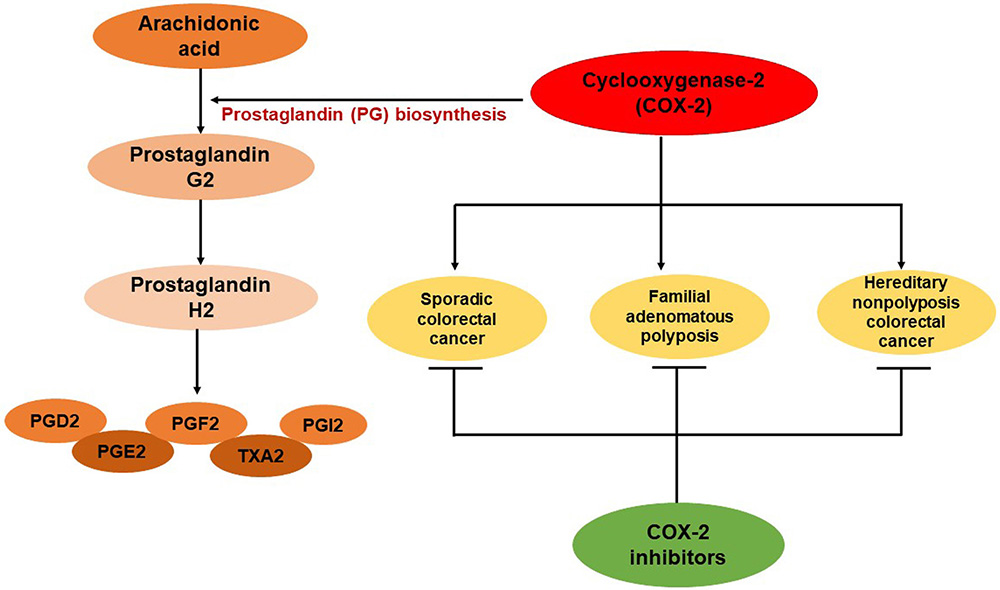 Figure 1
Figure 1Role of COX-2 in prostaglandin biosynthesis and colorectal cancer development. PGD2= prostaglandin D2, PGE2= prostaglandin E2, PGF2α= prostaglandin F2α, PGI2= prostacyclin and TXA2= thromboxane A2.
This review is focused on the role of COX-2 in colorectal cancer progression in various clinical studies which are associated with sporadic cancer, familial adenomatouspolyposis and Hereditary nonpolyposis colorectal cancer. Furthermore, we would also discuss the therapeutic implications of various COX-2-inhibitors in CRC treatment.
Sporadic colorectal cancer developed in adenoma-carcinoma sequence manner by accumulation of genetic alterations in oncogenes and tumor suppressor genes. Majority of the CRC cases are developed sporadically (11). CRC is a multifactorial disease and its one of the prominent factors is chronic inflammation which causes this deadly disease. It is already evident that COX-2 is inactive in humans under normal conditions; however, it is triggered in response to stimuli by various pro-inflammatory molecules such as cytokines. A major product of COX-2 is PGE2 and its increased expression is involved in the promotion of CRC progression (12). A study conducted by Roelofs and colleagues in the year 2014, reported the elevated mRNA levels of COX-2 in 80% of colorectal tumors as compared to their normal adjacent colorectal mucosa in 60 CRC patients (13). A meta-analysis study observed the overexpression of COX-2 in 4567 CRC patients by immunohistochemistry and also suggested the poor prognostic factor for CRC with worse overall survival (OS=1.19; 95% CI: 1.02 – 1.37) (14). Recently, our group explored the role of COX-2 in CRC patients and its association with clinic-pathological parameters. In this study, we collected colonic tumor tissues and its adjacent normal colonic tissues of 30 CRC patients after surgical intervention at PGIMER, Chandigarh. The mRNA expression of COX-2 was assessed by quantitative Real-time PCR and protein expression was analysed by immunohistochemistry. We observed a significant increased expression (p=0.001) of COX-2 mRNA in colonic tumor tissues in CRC patients as compared to their adjacent normal counterparts. While analyzing the association between clinicopathological parameters of CRC patients and COX-2 mRNA expression, we did not observe any significant change in COX-2 expression between low grade and high grade CRC (p=0.585). Moreover, the correlation between COX-2 expression and other parameters such as, age, tumor site, TNM stage (Tumor, Node, Metastasis) and histology grade was also non-significant. However, a significant high expression of COX-2 was observed in female CRC patients than male CRC patients. This is a first kind of study which reported a significant association between overexpression of COX-2 mRNA and gender of CRC. Further, we validated these results with immunohistochemistry and found that 70% of CRC cases were positive to COX-2 immunostaining and located in cytoplasm of the tumor tissues as compared to normal colonic tissues which showed negative immunostaining for COX-2 (8).
Our study is supported by work of others. Recently, Zhou and colleagues conducted the bioinformatics analysis of RNA-seq data on 647 CRC patients and reported a significant high mRNA expression of COX-2 (p<0.001) on the basis of TCGA-COAD (The Cancer Genome Atlas Colon Adenocarcinoma) dataset. Additionally, they confirmed the COX-2 expression in 229 CRC cases by immunohistochemistry and observed the cytoplasmic as well as nuclear expression of COX-2 in CRC tissues, which correlated with poor prognosis of CRC (15). In another study, COX-2 expression was detected in 145 CRC patients with I-IV stages by immunohistochemistry. The positive immunostaining of COX-2 was observed in 74% cases (107/145) which is significantly correlated with advanced tumors (p<0.045). Moreover, COX-2 overexpression is significantly correlated to poor differentiated tumors (grade III) with 89.5% cases (16). The immunohistochemical analysis was carried out in colorectal adenocarcinoma patients, which revealed the COX-2 expression in 56 out of 65 cases (86.2%), while 44 cases were strongly expressing COX-2. Also, a significant correlation between COX-2 overexpression and tumor invasion was observed (p=0.021). COX-2 higher expression was also significantly associated with tumor stages (p=0.05) (17). Recently, a study conducted on Greek population reported a significant difference in COX-2 overexpression between advanced stages of CRC tumors (p<0.05), and a positive immunostaining of COX-2 was also observed in dedifferentiated tumors (p<0.01) (18). Similarly, the case-control study of 213 CRC patients reported the increased mRNA and protein expression of COX-2 in colonic tumor tissues as compared to normal tissues. Further, high COX-2 expression was associated with CRC stages, infiltration, lymphatic metastasis and low survival rate (19).
Familial adenomatous polyposis (FAP) is a rare inherited condition in which multiple adenomatous polyps form in the colon and around 1% of CRC cases arise from this syndrome. This condition caused by a mutation in the adenomatous polyposis coli (APC) gene inherited from a parent (20). A study carried out by Brosens and colleagues in the year 2005, reported a significant high expression of COX-2 in colonic carcinomas from FAP patients as compared to normal colonic mucosa (p=0.0019); while comparing to adenomatous mucosa, a significant increased expression of COX-2 was also observed in colonic carcinoma with FAP (p=0.00045) (21). In another study, the gene expression of COX-2 was analyzed in 15 FAP patients by Real-time PCR. The mRNA expression of COX-2 was significantly increased (16.3-fold; p<0.01) in visible lesions as compared to normal mucosa in biopsies collected from FAP patients (22). Like familial adenomatous polyposis, there is a rare condition known as Juvenile polyposis coli (JPC) which is characterized by the growth of multiple polyps in large intestine before the age of 20. Since, JPC is associated with the risk of development of large intestine cancer; so it is worthwhile to explore the role of COX-2 in JPC patients. Thus, Gupta and colleagues performed immunohistochemical analysis of COX-2 on 8 JPC cases with age range from 7-16 years. They reported a significant high expression of COX-2 in JPC cases as compared to cases of solitary rectal polyps (SRP) (p<0.001). The strong immunostaining of COX-2 was observed in those JPC cases that have mild adenomatous changes (23).
Hereditary nonpolyposis colorectal cancer (HNPCC), also known as Lynch syndrome is another genetic condition that is associated with a high risk of colorectal cancer. Alterations in DNA mismatch repair genes are linked with Lynch syndrome which occurs in an autosomal dominant inheritance pattern. HNPCC accounts for 2-4% of all colorectal cancer cases (24). COX-2 immunostaining was observed in HNPCC patients and their frequency and intensity was compared with FAP patients and sporadic colorectal cancer cases. Cytoplasmic COX-2 positive staining was observed in 16 out of 24 (66.7%) HNPCC patients as compared to 92.3% sporadic patients (24 out of 26) and 2 out of 2 (100%) FAP cases. Though, the HNPCC cases show positive expression of COX-2 but their frequency was decreased as compared to sporadic cancer (p=0.035) (25). Similarly, the intensity of COX-2 immunostaining was also reduced in HNPCC cases with less number of tumors showing strong COX-2 expression (p=0.035). Similarly, Peng and colleagues reported the same frequency pattern of COX-2 expression in HNPCC patients relative to sporadic cancer. They observed COX-2 overexpression in 6 out of 14 (42.9%) HNPCC patients with carcinoma as compared to 22 out of 24 (91.7%) sporadic colorectal carcinoma patients. Interestingly, COX-2 high expression was found in mismatch repair (MMR) positive HNPCC carcinoma cases (p=0.05) (26).
COX-2 is one of the clinical parameters used in the diagnosis of colorectal cancer along with other markers such as carcino-embryonic antigen (CEA) and carbohydrate antigen (CA) 19-9. The study performed by Yang and colleagues detected serum levels of COX-2, CEA and CA antigen on 50 CRC patients and 50 healthy controls. Due to the non-specificity of the single tumor marker and improve the accuracy of the diagnosis, they observed the combined detection of all the 3 tumor markers in CRC patients and controls. The significant higher expression level (p=0.032) of COX-2 and other tumor markers was observed in CRC patients as compared to controls. The diagnostic coincidence rate was 88% (p=0.036) in combined detection of all three markers in CRC patients. The accuracy rate was 92.3% (p=0.043), specificity was 89.9% (p=0.072) and sensitivity was 90.1% (p=0.015) in combined detection of COX-2, CEA and CA which indicates that these markers can be used in early diagnosis of CRC (27). Similarly, a retrospective study conducted to detect the tissue and serum level of tumor markers (COX-2, IQGAP3 and B7-H4) in 118 CRC patients and 85 healthy controls. The high COX-2 immunostaining was observed in all the CRC cases, while high expression was observed in 63 cases and lower expression was observed in 55 cases out of 118 patients. The significant higher serum level of COX-2 was found in CRC patients (48.55±13.43, p<0.001) as compared to controls, along with other tumor markers. Serum levels of these markers were also correlated with TNM stage (p<0.05). While analyzing the ROC curve of COX-2 in CRC patients, they observed COX-2 AUC was 0.796 (95% CI 0.737–0.856, P < 0.001). The poor survival rate was also found in the patients with higher tissue or serum level of COX-2 which make it a prognostic factor for CRC (28). However, these studies clearly indicating that COX-2 give more efficient result if combined it with other tumor markers for diagnosis of CRC and further studies with large population is needed to evaluate its accuracy and specificity in early detection of CRC.
Several studies reported the efficacy of selected COX-2 inhibitors in the prevention of colorectal cancer. As mentioned in Figure1 and Table 1, there are two types of COX-2 inhibitors: COX-2 selective and nonselective NSAIDs. There are various studies which shows the effect of these inhibitors alone or in combination as a treatment of CRC. A population based retrospective cohort study revealed the less mortality rate in CRC patients with COX-2 inhibitors (celecoxib, rofecoxib, etoricoxib, nabumetone, meloxicam, etodolac, and nimesulide) before and after diagnosis of the cancer (29). A prospective study was conducted to analyze the efficacy of aspirin and COX-2 inhibitor on stage III colon cancer patients. The improved overall survival (OS) was observed with the use of aspirin (HR = 0.63, 95% CI = 0.35-1.12) in 799 patients, while with the use of COX-2 inhibitor (HR = 0.50, 95% CI = 0.23-1.07) in 843 patients. Besides overall survival, recurrence-free survival (RFS) and disease-free survival (DFS) was also improved with aspirin and COX-2 inhibitors. However, the P trend was not significant and the precise dose and duration of these drugs to show the potential effect on CRC patients was uncertain, but the study suggested that frequent use of aspirin and any dose of COX-2 inhibitors shows the low mortality and reduced recurrence of colon cancer (30). Although further studies were warranted to explore the efficacy of these inhibitors but some other studies are consistent with these results where the prolonged use of aspirin and other NSAID reduced the long-term incidence and mortality of colorectal cancer (31-34).
| Disease | Cox-2 inhibitors | References |
|---|---|---|
| Sporadic CRC | Celecoxib | 29, 35, 36, 37 |
| Rofecoxib | 29 | |
| Etoricoxib | 29 | |
| Nabumetone | 29 | |
| Meloxicam | 29 | |
| Etodolac | 29, 44 | |
| Nimesulide | 29 | |
| Aspirin | 30, 38-40 | |
| Sulindac | 44 | |
| Lynch Syndrome | Naproxen | 42 |
| Aspirin | 41 | |
| FAP | Sulindac | 43, 45 |
| Erlotinib | 45 |
A randomized adenoma prevention with celecoxib (APC) trial was conducted on 2457 patients from whom colorectal adenomas were removed who were susceptible to recurrent adenomas. Out of them, 679 patients were assigned to placebo, 685 assigned to 200mg of celecoxib dose and 671 assigned to 400mg celecoxib. The incidence of adenomas was 60.7% in placebo group, 43.2% in group received 200mg celecoxib dose (RR = 0.67, 95% CI = 0.59-0.77) and 37.5% in group received 400mg celecoxib dose (RR = 0.55, 95% CI = 0.48-0.64). Moreover, high dose of celecoxib (400mg) caused the reduction of recurrent adenomas by 45%. However, adverse effect of celecoxib was reported in 20.4% of patients with low dose (200mg) and 23% with high dose (400mg) as compared to the placebo group (18.8%). The serious cardiovascular events were also increased with celecoxib treatment in low dose (RR = 2.6, 95% CI = 1.1-6.1) and high dose (RR = 3.4, 95% CI = 1.5-7.9) as compared to placebo. Thus, the study suggested that celecoxib can be effective for the prevention of colorectal adenomas, however due to the side effects of this drug on cardiovascular risk; further studies were recommended (35). Recently, a prospective planned analysis of the APC trial was conducted on 1295 patients, which further divided into 2 groups viz. placebo (n=440) and celecoxib group (n=855). COX-2 expression was assessed in pre-treatment adenomas by immunohistochemistry and it was reported that patients with high COX-2 expression showed the significant reduction of adenomas with celecoxib treatment (RR = 0.37, 95% CI = 0.22-0.61; p=0.0001). Thus, COX-2 expression is an early indicator in colorectal adenomas that can be used to prevent colorectal adenomas with celecoxib treatment (36). A recent meta-analysis based on the systemic review of 3 RCTs (randomized controlled trials) which include 4420 patients and 3 post-trial of 2159 patients with history of colorectal adenomas where they compared the effect of different doses of celecoxib (400-800mg/day) with placebo. They observed that any dose of celecoxib for a duration of 1-3 years significantly decreased the incidence of recurrence of advanced colorectal adenomas (RR = 0.42, 95% CI = 0.34-0.53) and other adenomas (RR = 0.67, 95% CI = 0.62-0.72) as compared to placebo. However, celecoxib at a dose of 800mg/day caused the increased risk of adverse events (RR = 1.2, 95% CI = 1.0-1.5) and serious cardiovascular events (RR = 3.42, 95% CI = 1.56-7.46), while it was observed that celecoxib once daily at a dose of 400 mg/day did not cause the increase risk of cardiovascular events (RR = 1.01, 95% CI = 0.70-1.46) as compared to placebo. The post-trial studies indicated that after discontinuing the celecoxib treatment for >2 years, the treatment effect was also disappeared on recurrent advanced colorectal adenomas (RR = 1.15, 95% CI = 0.67-1.99) and other adenomas (RR = 1.15, 95% CI = 0.88-1.49). This study was the analysis of 3 RCTs including APC trial and this comprehensive evaluation suggested that celecoxib at a dose of 400mg/day once daily could be a potent chemopreventive agent for patients with high risk of colorectal adenomas but with low risk of cardiovascular events. However, long-term trials of celecoxib with ≤400 mg dose are warranted to explore the safety and efficacy of this drug (37).
There are various other NSAIDs such as aspirin, naproxen and sulindac which exhibit anti-tumor, anti-inflammatory and chemopreventive properties by targeting various cancer-promoting molecules including COX-2. It was demonstrated that aspirin inhibiting COX-1 and COX-2 activity resulted in the inhibition of PGE2 synthesis. Further, a randomized double-blind trial was conducted by Benamouzig and colleagues on 272 patients with colorectal adenomas. They reported that daily intake of aspirin (low dose) significantly reduced the recurrence of adenomas at 1 year (38). Some other clinical studies also suggested that low-dose aspirin is very effective in the prevention of colorectal adenomas (39, 40). The ongoing clinical trial finding the best dose of aspirin for the treatment of lynch syndrome cancer by comparing the effect of different doses of aspirin (100mg, 300mg, 600mg) in cancer patients (41). Like aspirin, naproxen and sulindac also targeted COX-2 and effectively reduced the number and size of colorectal adenomas in patients with Lynch syndrome and FAP (42, 43). In a randomized trial, sulindac treatment significantly lowered the ACF (aberrant crypts foci) number (p=0.0075), hyperplastic polyps and adenomas by its daily intake (44). Similarly, a double-blind, placebo-controlled trial on FAP patients reported that combined treatment of sulindac and erlotinib (epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor) significantly lowered (69%) the colorectal polyp count as compared to placebo (p=0.009) (45). These clinical studies strongly suggest that COX-2 inhibitors may provide a promising approach for the prevention of colorectal cancer. However, further studies are needed to determine the long-term use of these drugs to lower the adverse effects.
It is well known that COX-2 is a proinflammatory molecule and highly expressed in inflammatory diseases. The main function of this enzyme is to mediate prostaglandin biosynthesis and convert arachidonic acid to prostaglandins, which are overexpressed during inflammation and may cause gastrointestinal diseases. COX-2 is one of the key molecules which involved in the progression of sporadic and familial colorectal cancer and the use of COX-2 inhibitors open up a new vista for the colorectal cancer treatment.
COX-2
Cyclooxygenase-2
colorectal cancer
Cyclooxygenase
prostaglandin-endoperoxide synthase 2
prostaglandin D2
prostaglandin E2
prostaglandin F2α
prostacyclin
thromboxane A2
Tumor, Node, Metastasis
The Cancer Genome Atlas Colon Adenocarcinoma
Familial adenomatous polyposis
Juvenile polyposis coli
solitary rectal polyps
Hereditary nonpolyposis colorectal cancer
mismatch repair
disease-free survival
Hazard ratio
overall survival
adenoma prevention with celecoxib
relative risk
confidence interval
number

