Frontiers in Bioscience-Landmark (FBL) is published by IMR Press from Volume 26 Issue 5 (2021). Previous articles were published by another publisher on a subscription basis, and they are hosted by IMR Press on imrpress.com as a courtesy and upon agreement with Frontiers in Bioscience.
1 Departamento de Inmunologia, Instituto de Investigaciones Biomedicas, Universidad Nacional Autonoma de Mexico, AP 70228, Ciudad de Mexico, 04510, Mexico
2 Departamento de Farmacologia, Facultad de Medicina, Universidad Nacional Autónoma de Mexico, Ciudad de Mexico, 04510, Mexico
3 Departamento de Parasitologia, Facultad de Medicina Veterinaria y Zootecnia, Universidad Nacional Autonoma de Mexico, Ciudad de Mexico, 04510, Mexico
Abstract
The communication between neuroendocrine and immune system maintains a bidirectional complex network. Both systems jointly act during a parasite infection to maintain homeostasis and to eliminate such pathogens. Parasites interfere with the synthesis, secretion, metabolism, action, and elimination of endogenous hormones, as well as with the immune system in the host. Here, we aim to address as how parasite colonization disrupts the normal homeostasis of endocrine organs of the host, likely due to the exacerbated immune response, or by the impact of the parasite directly affecting endocrine tissues.
Keywords
- Parasites
- Neuroimmunoendocrine host-parasite network
- Neuroendocrine effect
- Helminths
- Protozoan
- Biological pollutants
- Endocrine disruptors compounds
- Behaviors
- Review
The immune and neuroendocrine systems are integrated through a complex network of hormones, neurohormones, cytokines, chemokines, neurotransmitters and neuropeptides, which serve to maintain homeostasis (1, 2). Two of the main components of this network, are the hypothalamic-pituitary-adrenocortical (HPA) axis (3) and the hypothalamic-pituitary-gonadal axis (HPG) (4). The relationship between the HPA and HPG axes and the immune system during acute and chronic inflammatory responses due to parasite infection have been well established (5–8). An important aspect of cellular communication that has emerged from studies of the neuroendocrine and immune system is the redundancy in the chemical messengers. The identification of a relatively high number of cellular messengers responsible for coordinating interactions between these homeostatic systems may herald a fundamental shift in our understanding of important physiological processes such as neurotransmission, reproduction, the stress response, or neuromodulation or the host immune response. Thus, there is an extremely complex neuroimmunoendocrine (NIE) network involving many molecules that foresees potent interactions in events generally attributed to the exclusive operation of single systems in response to simple precepts (neurotransmission, reproduction, defense). So much plasticity and multi-functionality in a network are not without a risk. In recent years it has been discovered that a large amount of parasitic infections, have strong effects at the central nervous and endocrine system, a fact that undoubtedly has a significant impact on the behavior of the host that can provide and, in some cases, prevent the advent of the infection (5). Also, it is well known that parasites induce many neuroendocrine changes in the infected host, which may point out to the fact that parasites are interfering with the endocrine system by inducing this changes, either, directly themselves producing factors capable of altering the host neuroendocrine response, or indirectly through the activation of the immune response, that in turns, affect the neuroendocrine response.
All pollutants have direct and indirect effects in almost every ecosystem. No matter what their impact is, pollution affects every species on this planet. Wildlife is prone to suffer same symptoms and diseases than humans (9). Forest, wild-life, parasites and plants can be harmed by acid rain; all these issues imply growth and photosynthesis difficulties and more vulnerability to diseases or bad weather (10). Global warming is changing some ecosystems faster than the capability of animals and plants to adapt, leading to possible extinction of a huge amount of species, including parasites (11). For example, due to the rising amount of carbon dioxide emissions and acid rain generation, the surface of oceans and water bodies has increased its acidity. This phenomenon is called ocean acidification and can lead to harmful consequences, such as depressing metabolic rates in jumbo squid, depressing the immune responses of blue mussels, and coral bleaching. Furthermore, ocean and lakes acidification makes water toxic to aquatic animals (12–14). Toxicity of the water, reduced amount of oxygen in deeper layers and difficult adaptability to the new substances may cause several damages into indigenous fauna and flora.
Environmental parasitology (EP) addresses the question which ecologically relevant information can be obtained by identifying or analysing parasites in the environment. In the common understanding, two different branches of EP exist. Whilst the more ecologically based approach is interested in parasites as indicators for environmental health, the more medically oriented understanding of EP specifically concerns the occurrence and diagnosis of human and animal parasites in the environment (15). Parasites have long been considered biological pollutants, since they affect human and animal health. Most of the effects related to this statement are immunologic reactions, gastrointestinal, hematological or tissue affections (16). However, due to their potential role in the interruption of many neuroendocrine functions of the host, they also act like endocrine disrupting biologicals, interfering with host homeostasis (17). We will delve into the neuroendocrine regulation that parasites can trigger in their hosts, in as much as hormonal modulations due to these microorganisms.
Here, we show some relevant examples of parasitic (helminths and protozoa) disruption of host's endocrine pathways, induced by parasites.
Many parasites are able to alter the growth and the reproduction of their hosts. For instance, some cestodes (like Ligula intestinalis) alter the gonadal development inducing reproductive dysfunction. A decrease in serum hormones levels like estrogens or testosterone, as well as, its receptors and enzymes related to their biosynthesis, were also affected on an in vivo roach model (18). Taenia crassiceps, is able to cause a process known as feminization when male mice are chronically infected (19, 20). The parasite induced an outstanding estradiol serum levels increase while those of testosterone and dihydrotestosterone were declined; these changes leads on sexual behaviour alterations such as the loss of ejaculation, the decrease in the number of mounts and intromissions in the murine model (20). Interestingly, the mRNA expression of interleukin (IL)-6 was also enhanced, leading into a positive feedback on the estradiol biosynthesis that results in the promotion of the immune response in favor of infection (21, 22). In the female host, T. crassiceps chronic infection, induce an interruption of the estrous cycle, decreasing in estradiol levels, and a result, the lordosis behavior, that it is part of the reproductive cycle of the infected host, it is interrupted (23).
Furthermore, trematodes as Trichobilharzia ocellate likewise alter the function of the reproductive tract. The schistosome parasite inhibits the reproduction of its host, the snail Lymnaea stagnalis, and induces it a giant growth (24). ). In this snail, the regulation of the reproduction and growth are regulated by gonadotropic hormones and the molluscan insulin-related peptides (MIPs), respectively (24). The parasitic infection inhibits the development of the reproductive tract of the snail, and suppresses the secretion of the gonadotropic hormones (25).
Furthermore, some of the substances that are secreted by parasites are chemically identical or similar to those secreted by the hosts, altering in a direct manner their signalling pathways (26). Nevertheless, parasites can affect also in an indirect manner some host’s neuromodulator such as dopamine, octopamine and serotonin (27, 28). For example, the trematode Schistosoma mansoni secretes opioid peptides such as endorphin (26). In infected hosts, opioid levels increase in the serum and central nervous system (CNS) affecting the host behaviour (26). Even more, schistosome nervous system is modulated by classical neurotransmitters such as acetylcholine (ACh), glutamate and biogenic amines (BAs) (29, 30). BAs are the largest subset of neurotransmitters in the animal kingdom (31) and in schistosome contribute to the motility of the parasite (30, 32). Octopamine (OA) and its precursor, tyramine (TA) are BAs that in locusts and molluscs are involved in motor control (33, 34), in some arthropods perform the functions that in vertebrates are perform by adrenaline and noradrenaline (35), and in mammals are present in trace amounts (36). In humans OA is present in nerve tissues (37) and in blood in detectable (ng/mL) amounts that variate in some pathological conditions (38). ). Different to invertebrates humans don’t express OA receptors, nevertheless OA can bind with presumably low affinity to alpha-1, alpha-2, beta-1, and beta-2 adrenergic receptors of mammals (39).
Protozoan parasites also interfere with hormonal homeostasis, even promoting sexual dysfunction. In this regard, T. gondii produces an enzyme that is involved in the synthesis of dopamine (DA) and norepinephrine (NE), provoking an increase of dopaminergic activity in rodents (40); these result in the reduction of fear perception of the rodents, by the diminution of the innate fear of cat odor, enhancing the chance of predation (41). In humans, the increase in dopamine by Toxoplasma infection has been related to schizophrenia (42, 43). Moreover, it has been demonstrated that Toxoplasma can also augment testosterone levels by increasing the expression of luteinizing hormone receptors on Leydig cells; these receptors regulate the synthesis of testosterone in testis (41). This fact supports the observations that Toxoplasma only increases testosterone levels in men but not in women, neither in castrated male rats (41, 44). Furthermore T. gondii induce apoptosis of placental cells and fetal resorption with a concomitant increase of progesterone and estradiol levels in pregnant female mice and pregnant women (45, 46). Interestingly, this phenomenon can be prompt by modifications in the immune system response that might adjust the immune response against the parasite to the fetus; the above might be inferred by the increase on mRNA indoleamine 2,3-dioxygenase (IDO) expression. Another overwhelming parasitic disease, Plasmodium infection, causes a decrement in sperm motility, sperm count and viability in male albino mice; jointly, serum testosterone levels have also been negatively affected (47). Of note, in humans, protozoa parasitic infections also cause major health conditions; infertility, miscarriage, sperm reduction and susceptibility to HIV transmission are examples of them (48).
Interestingly, it has been postulated that parasitic infections by protozoa, nematodes, trematodes, even arthropods have a higher prevalence in men than in women (49). The afore mentioned, leads us to reflect that regardless of the type of parasite, it affects the host's hormonal homeostasis. The information of some of the strong endocrine effects of parasites on their hosts it is summarized in Table 1.
| Type of parasite | Effect | Hormones involved | Model | |
|---|---|---|---|---|
| Helminths | ||||
| |
• Alteration of the gonadal development |
• Estrogen |
• Decreased |
|
| |
• Induction of feminization |
• Estradiol |
• Increased |
|
| Schistoma mansoni | • Inhibition of development of the reproductive tract | • Gonadotropic hormones |
• Decreased |
|
| Protozoa | ||||
| Toxoplasma gondii |
• Increase in the testicular steroidogenesis and sexual behavior in rats |
• Testosterone |
• Increased | |
| • Hemorrhage |
• Any hormone was evaluated, Probably Estradiol | • Increased? | ||
| • Not measured | Testosterone |
Testosterone |
Comparison of patients infected with Toxoplasma and men and women free of infection (34) | |
| • Miscarriage |
• Progesterone |
Increased |
Pregnant women (46) | |
| |
• Decreased in motility, count and viability of sperms | • Testosterone | • Decreased | Male albino mice (47) |
| • Primary testicular failure |
• Testosterone |
• Decreased |
HIV infected men (49) | |
In nature, the population size of each species is regulated by natural environmental factors. While molecular studies indicate that various chemicals can alter endocrine system-mediated processes in parasites, studies on individual species, populations, and communities show that these effects may be ecologically relevant. From an ecological point of view, parasites represent a large part of the world's biodiversity and fulfil many crucial ecological roles in each ecosystem; this implies that its current decline is a clearly worrying issue that needs to be dealt with shortly (50). Based on the fact that certain groups of endoparasites are excellent accumulators of toxic metals and organic pollutants (51, 52), parasites should be added to the list of already existing accumulation indicators, since in order to detect contaminants in e.g. acanthocephalans and cestodes, these contaminants had to pass through the integument and membranes of these parasites and therefore deduce that they were biologically available. This is in contrast to detecting contaminants found in filter-feeding organisms, such as mussels, it is unclear whether the substances are flexibly attached to the gills or absorbed at the cellular level. Parasites can often absorb chemicals at very high levels. Therefore, they can bioconcentrate contaminants that are present in very low concentrations in the environment and make them detectable and quantifiable using conventional analytical techniques and assess to what extent they are available for uptake by biota. To date, more than 50 species of metazoan parasites, belonging mainly to the four major endohelminth taxa (Acanthocephala, Cestoda, Digenea, and Nematoda) have been considered and suggested as sentinels for metal contamination (15).
By definition, parasites are not neutral with respect to their interaction with their hosts. These interactions affect the host's physiological homeostasis, which often leads to negative effects on their health. In addition to this, if parasites and hosts face contaminants, deviations from physiological homeostasis also occur. The effects of contaminants and parasites can be synergistic, antagonistic, or additive, depending on the specific contaminants and parasites. The parasitic host ratio in a contaminated environment can alter homeostasis of contaminant levels in infected hosts compared to uninfected hosts. Since reduced concentrations of contaminants are generally correlated with fewer adverse effects, it might be advantageous to become infected if hosts are faced with environmental contamination. On the other hand, parasites can increase the toxic effects of contaminants by interfering with host protection mechanisms. In these cases, a parasitosis would have exclusively negative effects on the physiological homeostasis of its hosts (52). Consequently, the effects of parasites have variable implications for the health of their hosts, and it is not possible to reach a general conclusion about their effect on the health of the host. This may alter the definition of parasites as exclusively harmful to their hosts (Figure 1).
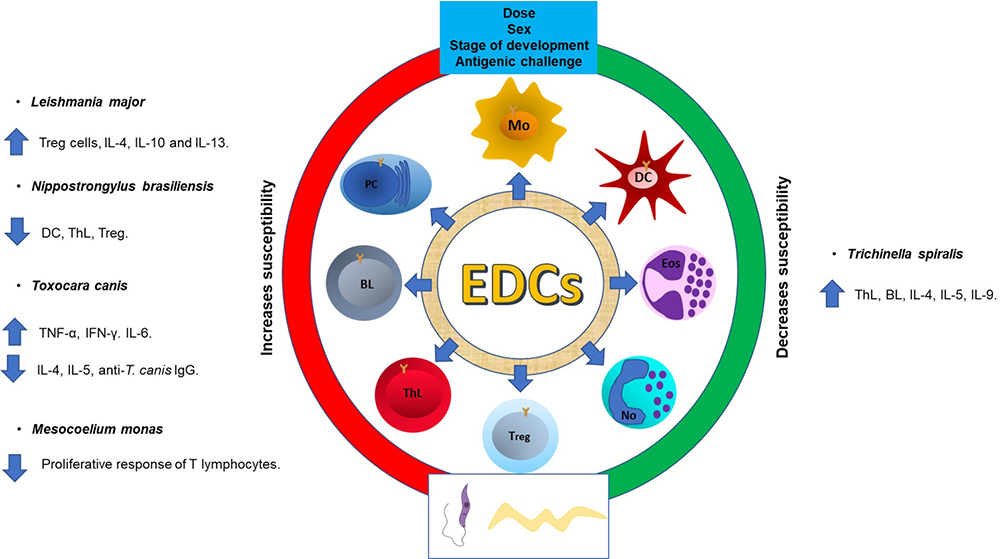
 Figure 1
Figure 1Endocrine Disrupting Compounds (EDCs) modify susceptibility to parasitic infections. The dose, sex, stage of development at which they are exposed, as well as antigenic challenge, among other factors, they can modify the susceptibility to infection. Macrophages (Mo), Dendritic cells (DC), Eosinophils (Eos), Neutrophils (No), T regulatory cell (Treg), T helper lymphocytes (ThL), B lymphocytes (BL), Plasmatic cells (PC).
Currently, few studies have evaluated the effect of endocrine disruptors on the immune response associated with an infection caused by parasites. The results differ because there are several factors that must be considered, such as: the dose of the pollutant, the period of development in which they are being administered, age, sex, and the antigenic challenge used. All of the above is reflected in different changes in the cellular subpopulations of the immune system or in its effector mechanisms (53). To date, the most studied endocrine disruptor in this regard is bisphenol-A (BPA). BPA is widely used as a monomer in the production of polycarbonate plastics, epoxy resins and dental sealants. The main BPA exposure source in animals and humans is through food and beverages that have been in contact with materials manufactured with BPA, which is detached from its matrix. BPA is classified as an EDC with estrogenic character since it can bind to nuclear estrogen receptors (ERα and ERβ) and trigger signalling pathways (54). Different BPA effects have been reported on the immune system cells; however, many reports do not consider that the immune response must be studied by challenging the immune components, so there is little information about the BPA effects on the immune response during a parasite infection (53).
Prenatal BPA treatment in the drinking water, or adult subcutaneous (SC) administration, one week before to infection with Leishmania major, induced an increase in the inflammation in the food pad of mice in a dose-dependent manner. The Treg cells number were also reduced at splenic level in both, mice exposed to BPA at prenatally or in the adult stage (53). Of note, animals exposed to BPA in the adult stage had an IL-4, IL-10 and IL-13 increased level expression after L. major infection. BPA effect in Trichinella spiralis infection has also been reported. Neonatal male and female syngeneic BALB/c mice that were expose to a single dose of BPA, harboured fewer parasitic loads on the duodenum, when they were infected orally with Trichinella spiralis in adulthood (55). Furthermore, neonatal administration of BPA in female mice induced host protection against Taenia crassiceps infection, denoted by the reduction of 40% parasite loads as compared to untreated and vehicle-infected mice groups. Interestingly, in infected animals, the BPA treatment modulated some lineages of the innate immune response and caused slight changes in cells belonging to the adaptive immune response at the peripheral and mesenteric lymphoid nodes, respectively. Additionally, the estrogenic compound enhanced the Th2 cytokine profile (56). On the contrary, Ménard et al. (2014) reported an increase in susceptibility to Nippostrongylus brasiliensis infection, when BPA was administered to rats in the drinking water previous to infection (57). In Toxocara cannis infection, the effect of BPA it is detrimental. Del Rio-Araiza et al (2020), exposed male Wistar rats to BPA during the perinatal period, and found a significant increase of the percentage of Toxocara canis larvae in the lungs and liver in the adulthood (58). Additionally, the exposure to BPA caused a dramatically decrease in the production of specific antibodies against Toxocara; a downregulation of Th2 cytokines (IL-4, IL-5 and IL-13), and upregulation of Th1 cytokines (IFN-γ and TNF-α) (58). The evidence shown is indicative that endocrine disruptors can permanently alter the immune response when evaluated using an infection caused by parasites as an antigen challenge (Figure 2).
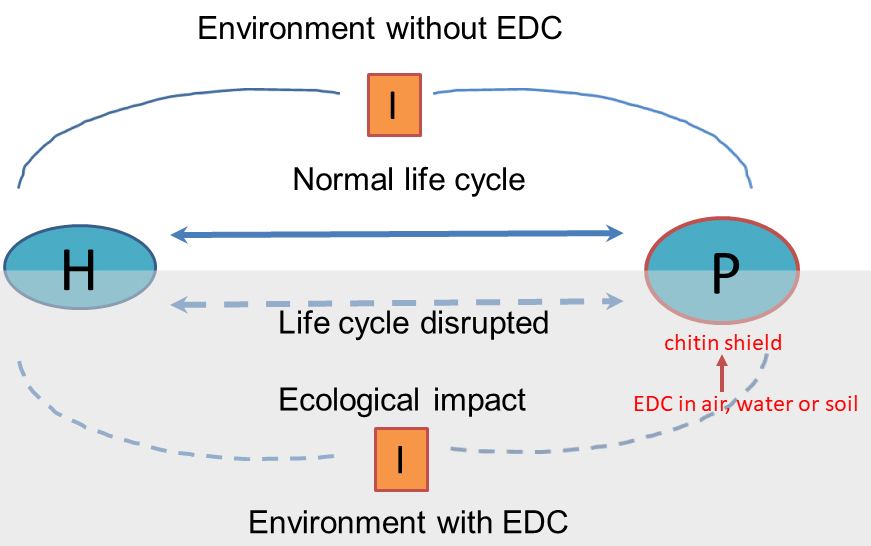
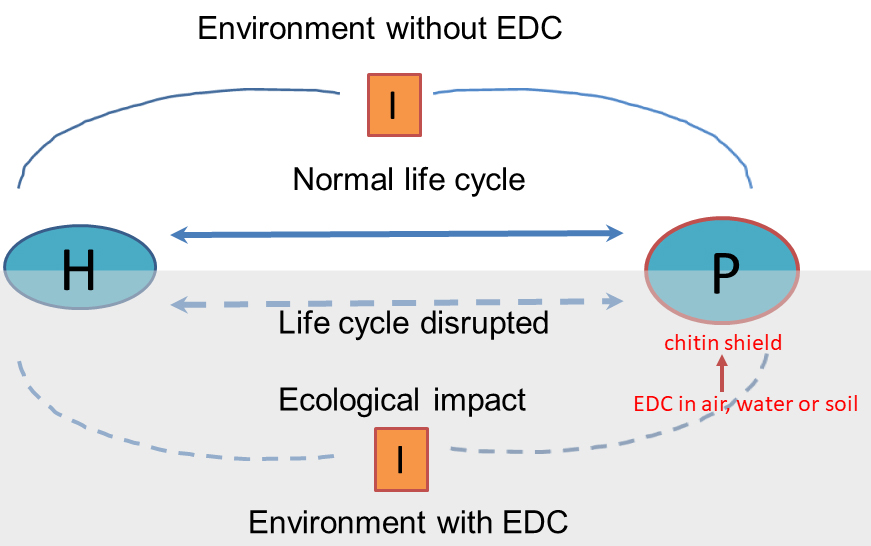 Figure 2
Figure 2Schematic representation showing the possible ecological disruption that can cause the effect of EDCs on the life cycle of the host-parasite relationship. On the other hand, the affinity between chitin and certain EDCs in the environment is highlighted, making parasites as possible indicators of environmental contamination. P: parasite H: host I: intermediate hosts or Intermediate stages of free life.
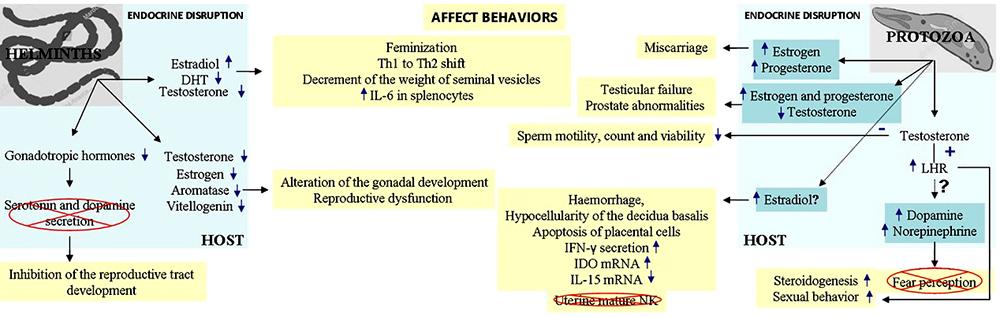
Parasites that co-exist inside of a host are bound to interact with the host environment, where parasites secrete substances that, depends on where they are established, act directly or indirectly on the host cells or tissues. In most of the cases these substances alter the neuroendocrine microenvironment and the behaviour of the host (59–61). Some relevant examples of parasitic (helminths and protozoa) disruption of host's endocrine pathways were shown in the above paragraphs, and are resumed in Figure 3. The ability of a parasite to affect a female or male host of the same species differentially can be mediated by hormonal regulation of the immune response of the host or by direct hormonal effects on the parasite. Understanding the contribution of each of these effects and the characterization of the parasite molecules involved might facilitate the development of drugs that counteract the effects of hormones on the host immune system or the parasite.
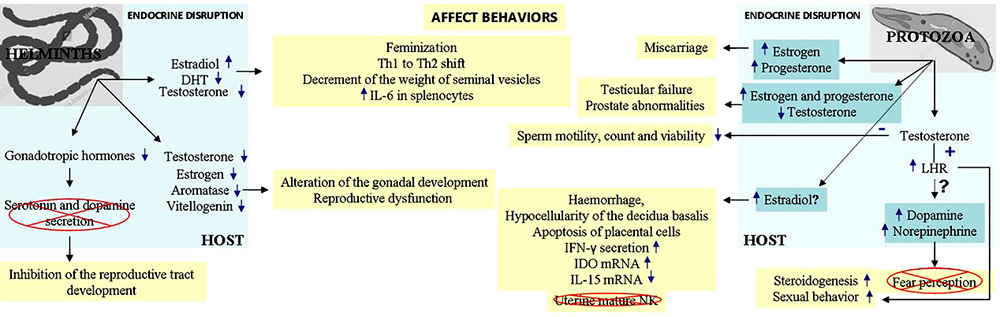 Figure 3
Figure 3In light of this evidence, it is clear that the host endocrine system can not only influence the course of parasitic infection by modulating the immune system but the parasite interferes and modulates neuroendocrine functions in the host. The ability of a parasite to affect a female or male host of the same species differentially can be mediated by hormonal regulation of the immune response of the host or by direct hormonal effects on the parasite (see Outstanding Questions). Furthermore, all endocrine effects that parasites induce in a host make them endocrine disruptors, and as such, they deserve to be included in this classification as biological endocrine disruptors (BEDs). Understanding the contribution of each of these effects and the characterization of the parasite molecules involved might facilitate the development of drugs that counteract the effects of hormones on the host immune system or the parasite.
This work was supported by Programa de Apoyo a Proyectos de Innovación Tecnológica (PAPIIT), Dirección General de Asuntos del Personal Académico (DGAPA), Universidad Nacional Autónoma de México (UNAM) (Grant IN-209719); Fronteras en la Ciencia, Consejo Nacional de Ciencia y Tecnología (CONACYT) (Grant FC-2016-2125), both to JMM. CTGDL has a Posdoctoral fellowship from project FC-2016-2125, CONACYT.
EDCs
Endocrine Disrupting Compounds
axis Hypothalamic-pituitary-adrenocortical
axis Hypothalamic-pituitary-gonadal
Neuroimmunoendocrine
Environmental parasitology
Interleukin
Central nervous system
Acetylcholine
Biogenic amines
Octopamine
Tyramine
Dopamine
Norepinephrine
Bisphenol-A
Nuclear estrogen receptors