1 Department of Emergency Medicine and Intensive Care, Shanghai Songjiang District Central Hospital, 201600 Shanghai, China
2 Department of Emergency Medicine and Intensive Care, Shanghai Songjiang Clinical Medical College of Nanjing Medical University, 201600 Shanghai, China
3 Department of Pharmacy, Shanghai Songjiang District Central Hospital, 201600 Shanghai, China
Academic Editor: Chuanjin Wu
Abstract
Caspase recruitment domain-containing protein 9 (CARD9) is highly expressed in myeloid cells and has been identified as a central regulator of innate immunity. Increasingly, studies demonstrate that CARD9 also plays a critical role in the development of lung cancer. This review focuses on the clinical significance and potential molecular mechanisms that CARD9 plays in lung cancer.
Keywords
- CARD9
- Lung cancer
- Clinical significance
- Molecular mechanism
- Therapy target
Lung cancer remains a challenging disease largely due to its high mortality and morbidity. Data gathered in the USA indicate that in 2018 approximately 230,000 new cases of lung cancer were diagnosed and that this accounted for approximately 13% of all cancer cases [1, 2, 3]. Despite great advances in effective prevention, early diagnostic detection, and more effective therapeutics, many patients still experience disease recurrence without significant improvements in survival. The current therapeutic options for lung cancer include radiation, chemotherapy, and surgery. Each of these approaches has limitations, and patient prognosis remains dismal. Specifically, the 5-year survival rate for lung cancer is estimated at 18%, and lung cancer is responsible for approximately 25% of all cancer-related deaths, more than breast, colorectal, and prostate cancer [4, 5]. Therefore, it is critical to further explore molecular mechanisms that could improve diagnosis and therapy for lung cancer patients.
Emerging studies have emphasized the critical role of the tumor microenvironment (TME) in tumor progression. Myeloid-derived suppressor cells (MDSCs), a heterogenous group of immune cells derived from myeloid lineage, attenuate host immune responses through production of suppressive components within the TME [6, 7, 8]. In the context of the tumor, MDSCs can facilitate tumor escape, abnormally activate tumor cells, and suppress T cell function by increasing levels of reactive oxygen species, arginase, and nitric oxide synthase production [6, 8]. Additionally, MDSCs contribute to tumor angiogenesis, tumor cell invasion, and premetastatic niche formation through activation of non-immunologic mechanisms [9, 10].
It is clear that understanding MDSC biology is critical for improving clinical prognosis and improved treatment strategies in lung cancer patients [11]. Interestingly, CARD9 is highly expressed in MDSCs and exhibits a central role in regulating innate immunity [1]. Recently, it was reported that CARD9 function in MDSCs might drive a novel mechanism of lung cancer development [1, 12, 13]. This review focuses on our current understanding of CARD9 function in lung cancer and discusses CARD9 clinical significance, its molecular mechanism of action, as well as its potential as a therapeutic target.
CARD9 is highly expressed in myeloid cells especially macrophages, dendritic
cells, and MDSCs [14, 15, 16]. CARD9 is known as the downstream effector molecule of
the pattern recognition receptors including Toll-like receptors and C-type
Lectins. Upon activation of these receptors, CARD9 triggers activation
NF-
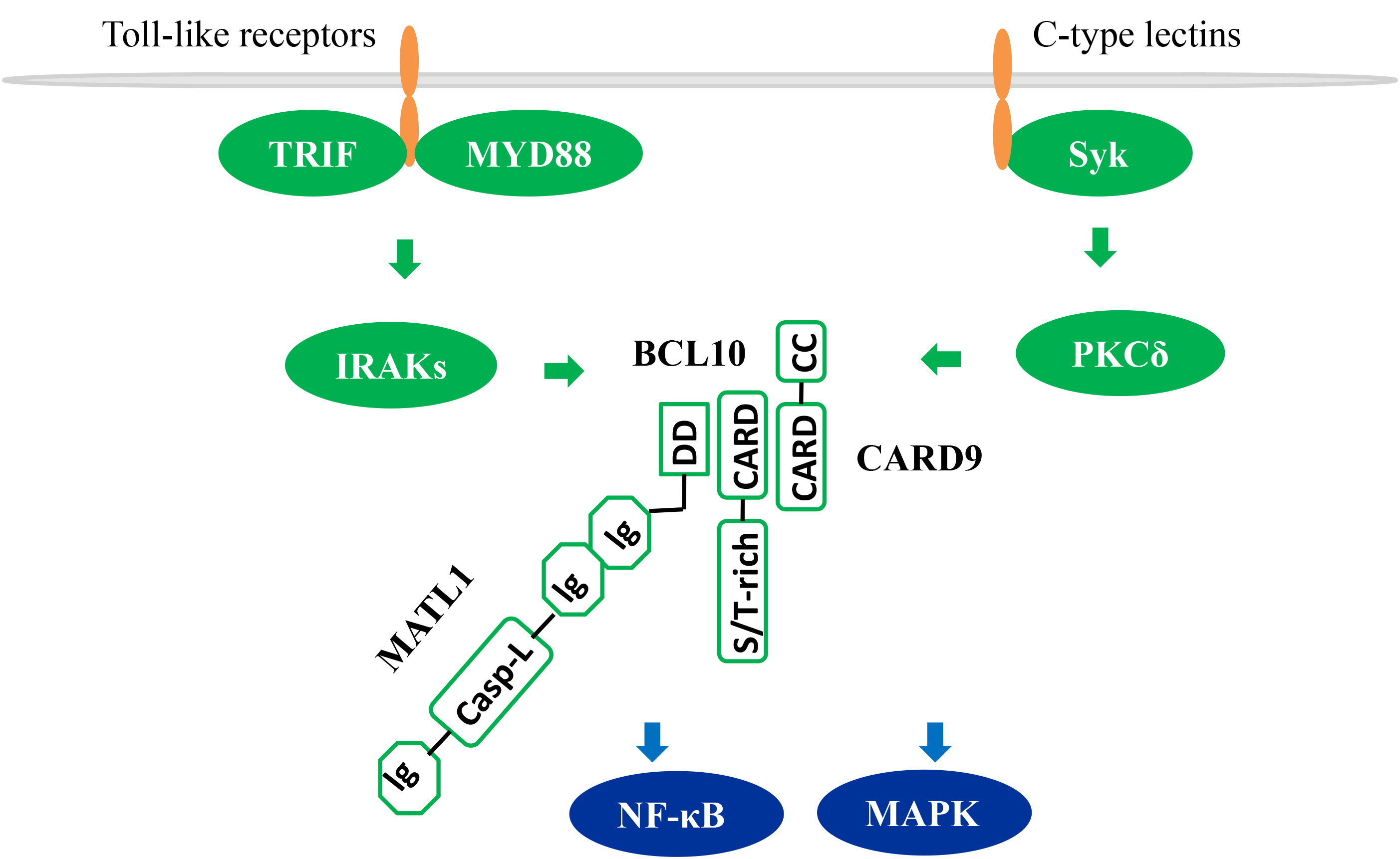 Fig. 1.
Fig. 1.
CARD9-dependent NF-
Through its myeloid cell functions, CARD9 has emerged as an essential player in
tumor initiation and progression. We previously summarized the clinical
significance of CARD9 in a variety of tumor types (Table 1) including
hepatocellular carcinoma, intestinal carcinoma, gastric carcinoma, kidney
carcinoma, and malignant pleural effusion [16, 17]. These studies suggest that
high CARD9 expression is associated with tumor progression and poor survival rate
[18, 19, 20, 21, 22]. However, preclinical studies in tumor-prone mouse models indicate that
CARD9 plays a dual role in the carcinogenic process as it exhibits both a
pro-tumor and/or anti-tumor activity [23, 24, 25, 26]. Specifically, CARD9 promoted tumor
progression in kidney and intestinal cancer by activating NF-
| Gene | Tumor | Function | Mechanisms |
| CARD9 | Intestine | Pro-tumor | IL-1 |
| CARD9 | Intestine | Pro-tumor | IL6, G-CSF, RANTES, T-cell, macrophage |
| CARD9 | Intestine | Pro-tumor | IL-12, IL-10, IL-1 |
| CARD9 | Kidney | Pro-tumor | Card9 linked pVHL to NF-κB biology |
| CARD9 | Kidney | Pro-tumor | Card9-induced JNK hyper-activation |
| CARD9 | Melanoma | Anti-tumor | Dectin-1-mediated cross-priming of CD8+ CTLs |
| CARD9 | Leucocythemia | Anti-tumor | leukemic cell apoptosis |
Macrophages are a prominent constituent of the tumor microenvironment where they can either promote, or suppress, tumor growth and metastasis depending on their biological state [27, 28, 29]. Further, CARD9-induced tumorigenesis was reported in a small cohort of patients, but exact evaluation in a large patient cohort is likely needed to confirm these findings.
A recent study conducted by Qu et al. [1] studied 31 patients diagnosed with lung cancer. To address the relationship between CARD9 and MDSCs in these patients, CARD9 expression level and types of cells expressing CARD9 in lung tissues were examined. Compared with normal tissues, expression of CARD9 was significantly unregulated in both tumor and paracancerous tissues. The study documented that CARD9 was principally expressed in myeloid cells resident within the tumor tissue, but not tumor cells themselves. Furthermore, an increased population of MDSCs was observed in larger tumors and this was closely associated with higher expression of CARD9. Moreover, clinical pathology data indicated that CARD9 expression was positively correlated with tumor size [1]. In sum, this study provides a framework for understanding the contribution of CARD9 to lung cancer development in humans.
Miwa and colleagues reported that CARD9 served as a prognostic marker of poor outcome in lung cancer patients [13]. In this investigation, a total of 74 lung cancer patients diagnosed with adenocarcinoma were enrolled. Of these, 24 patients harbored CARD9-low expression, and the remining 50 cases were characterized as CARD9-high expression. Clinicopathologic analysis demonstrated that CARD9 expression was positively associated with poor overall survival, but not vascular and lymphatic invasion. This implicates CARD9 expression as an independent prognostic factor in lung cancer patients. Of note, in contrast to other studies that documented CARD9 expression uniquely in MDSCs [1], CARD9 was found to be expressed in adenocarcinoma cells in this study.
Pan and co-workers also identified CARD9 as a potential prognostic indicator in lung cancer [30]. Contrary to previous findings [1, 13], CARD9 expression was reduced in lung cancer and exhibited anti-tumor activity in this study. A total of 94 cases diagnosed with non-small cell lung cancer were studied in this report and CARD9 expression, at both protein and mRNA levels, indicated a clear decrease in cancer tissue in comparison with adjacent normal tissues. Clinicopathologic analysis indicated that CARD9 was negatively correlated with lymph node metastasis, T category, histological differentiation, tumor node metastasis (TNM) stage, and remote metastasis. Low CARD9 expression was associated with poor overall survival and progression-free survival of patients. Additionally, CARD9 in clinical specimens was found to be expressed in both tumor cells and macrophages.
Two studies reported that CARD9 regulated lung cancer through the control of
MDSCs activation and indoleamine 2, 3-dioxygenase (IDO) production [1, 12]. In
the Lewis lung cancer mouse model, CARD9 deletion induced tumor cell
proliferation, increased tumor size and weight, and aggravated
splenomegaly. The expression level of MDSCs-related functional genes in tumor
tissues of CARD9-deficient mice, such as iNOS-2, Arg-1, S100A8/A9, GP91phox and
IDO, was significantly increased compared with that of wildtype mice. This result
suggested that CARD9-deficiency in the TME may impair anti-tumor immune response
through the activation and accumulation of immunosuppressive MDSCs. MDSCs are
identified as the main obstacle in anticancer immunity and immunotherapy [31, 32, 33].
To test the pro-tumor function of MDSCs in CARD9-deficient mice, anti-Gr-1
monoclonal antibody, which effectively removes MDSCs, was injected into mice. As
expected, this antibody decreased the number of MDSCs and coordinately increased
the number of tumor-associated CD8
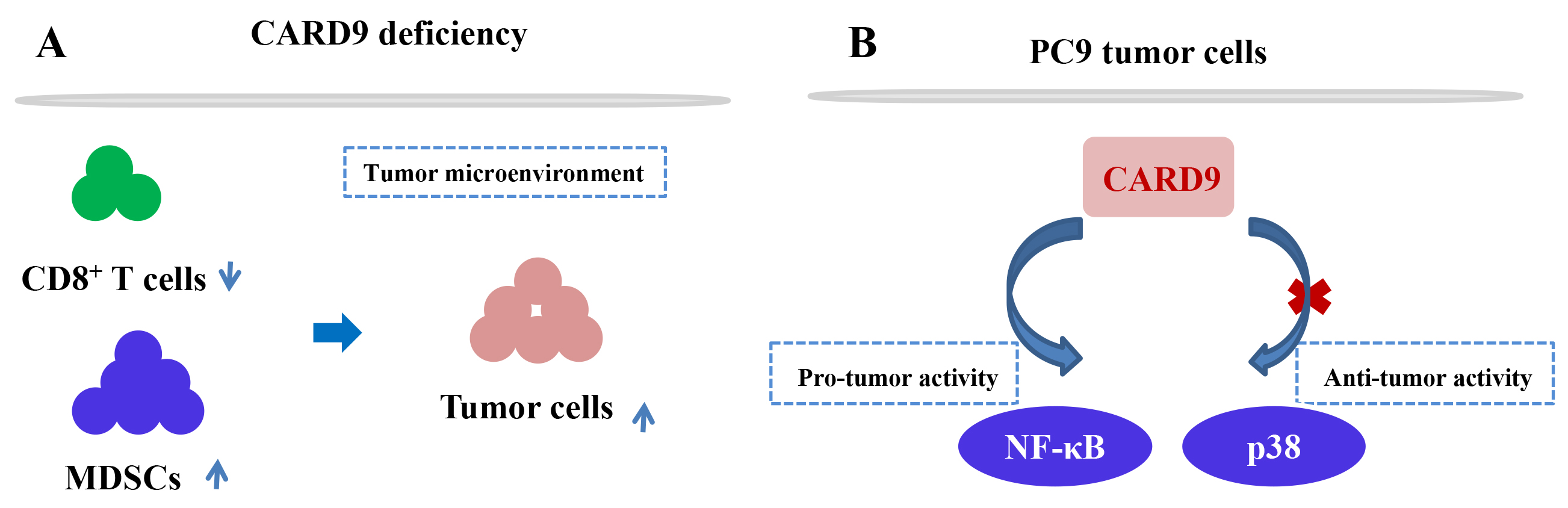 Fig. 2.
Fig. 2.The molecular mechanism of CARD9 in lung cancer. (A) CARD9-mediated immune response in the tumor microenvironment; (B) CARD9-induced signalling pathway in the PC9 cell line.
CARD9 protein was also found to be expressed in lung cancer cell lines PC9, H1299, and HBE [30], suggesting its potential participation in tumorigenesis. In vitro experiments showed that CARD9 knockdown in PC9 cells significantly promoted cell proliferation, invasion, migration, and inhibited cell apoptosis. Furthermore, reduced CARD9 expression activated p38 and contributed to tumor development in a p38-dependent manner. Contrary to previous findings regarding CARD9-induced p38 activation [37, 38, 39], in this study CARD9 deficiency upregulated p38 expression and the underlying molecular mechanism for this finding will need to elucidated in the future. Nevertheless, CARD9 has a significant anti-tumor response in lung tumors via downregulation of p38 signaling.
In this study, the investigators also showed that CARD9, in lung cancer cell
lines, had a critical role in facilitating the progression of lung cancer [13].
CARD9 was upregulated in various lung cancer cell lines including PC9, A549 and
II18. Furthermore, CARD9 was found to activate the canonical NF-
CARD9 expression in myeloid cells has been linked to tumor-related inflammation, tumor immune microenvironment, and intestinal microecology, suggesting that CARD9 may represent a potential target for anti-tumor immunotherapy [17].
Ganoderma lucidum polysaccharide (GLP), isolated from the fungus
Ganoderma lucidum, serves as an effective anti-tumor agent that
significantly inhibits tumor growth in the Lewis lung cancer mouse model.
Moreover, GLP administration to these mice [40] can effectively suppress the
accumulation of MDSCs in the spleen and tumor tissues, increase the percentage of
CD4
CARD9 is a central integrator of innate immunity in myeloid cells and plays a key role in lung cancer pathogenesis. However, some important questions regarding CARD9 remain unknown. First, it is unclear whether CARD9 is protective against lung cancer development. A total of three papers have been published that report on the clinical significance of CARD9 in lung cancer. Of these, two indicate CARD9 possesses pro-tumor activity, and one paper suggests anti-tumor activity. Of note, in vivo studies in mice support an anti-tumor function for CARD9 and this contradicts clinical data obtained from humans. This discrepancy may be due to high expression of CARD9 in patient samples because of the increased proportion of myeloid cells within the tumor [1]. Future studies will be needed to fully understand CARD9 pro-tumor versus anti-tumor functions using a large cohort of patients.
Second, the cellular distribution of CARD9 in lung cancer remains controversial; specifically at question is whether CARD9 is expressed in MDSCs and/or lung tumor cells. As previously reported [42, 43, 44], investigators documented that CARD9 expression was limited to tumor-infiltrating macrophages in colon cancer. In contrast, studies of oral squamous cell carcinoma [45] and renal cell carcinoma [44] revealed CARD9 expression within tumor cells. Others documented CARD9 expression, at both the protein and mRNA levels, is upregulated in various cultured lung cancer cell lines, suggesting that CARD9 is not only expressed in MDSCs but also in lung tumor cells. In contrast, some clinical data gathered from lung cancer patients clearly show that CARD9 was only expressed in MDSCs rather than in tumor cells [1].
Despite the uncertainties outlined in this review, the bulk of studies support an anti-tumor function for CARD9 in lung cancer. More studies, focused on the biological function of CARD9, will be required to better determine how MDSCs interact with tumor cells in a CARD9-dependent mechanism.
RS—writing-original draft preparation; ZY—writing-review and editing. All authors have read and agreed to the published version of the manuscript.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.


