1 Department of Pharmacology, School of Pharmacy, Southwest Medical University, 646000 Luzhou, Sichuan, China
2 Rizhao Hospital of Traditional Chinese Medicine, 276826 Rizhao, Shandong, China
3 Department of General Biophysics, Faculty of Biology and Environmental Protection, University of Lodz, 90-236 Lodz, Poland
Abstract
Backgroud: Protein kinases play an important role in cell proliferation, differentiation, mobility and cell cycle arrest etc. These enzymes act as important targets in developing anticancer agents. Over the years, a large number of protein kinase inhibitors have been discovered and developed as anticancer agents for the treatment of cancers clinically. However, the drug-resiatance and off-targeting limit their effeciancy for the treatment of human cancer. Materials and methods: Alkaloids are an important class of natural products with broad spectrum biological activities. In the past decades, numerus alkaloids with significant anticancer activity by inhibiting protein kinases were identified. In the present mini-review, we will present the key enzymes including mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) and janus-activated kinases/signal transducer and activator of transcription (JAK/STAT) targeted by alkaloids and highlight the special sites targeted by alkaloids on protein kinases and/or reversing drug resistance. Additionally, the challenge and prospect of developing alkaloids as new anticancer agents are also discussed. Conclusion: Alkaloids suppressed tumor growth through targeting different signaling pathways mediated by protein kinases of cancer cells. It is conceivable that novel alkaloids anticancer agents with promising clinical value will be developed in the future.
Keywords
- Alkaloids
- Protein kinase inhibitors
- Anticancer activity
- MAPK
- PI3K/AKT/mTOR
- JAK/STAT
Protein kinases catalyze the transfer of a phosphate group to a specific amino acid in a protein molecule. Since the phosphate groups are charged negatively, the 3D structures of the specific protein will be changed when adding the phosphate group, leading to activation and inactivation of the specific proteins. There are several kinds of protein kinases with different selection towards substrates, and tyrosine protein kinases and serine threonine protein kinases are the main subfamilies [1]. It is well established that protein kinases function as a kind of important regulators in cellular events involving DNA damage-repair, cell proliferation, motility and apoptosis, etc. [2, 3]. Most protein kinases such as PI3K, Akt, EGFR and MAPK are usually highly expressed and/or activated in tumor cells, and these enzymes have been acted as major targets in developing novel anticancer agents [4]. Nowadays, almost a quarter of all newly developed anticancer drugs belongs to protein kinase inhibitors. Gefitinib (Iressa), a selective EGFR inhibitor, was approved in 2015 by the US Food and Drug Administration (FDA) to treat non-small cell lung cancer [5, 6]. In 2005, a multi-target tyrosine kinase inhibitor, Sorafenib (Nexavar; Onvx/Bayer), was approved by FDA to treat advanced primary liver cancer and primary kidney cancer (advanced renal cell carcinoma) [7, 8, 9]. However, the off-targets and drug resistance of protein kinase inhibitors limit their efficiency and application in the treatment of cancer patients [10, 11]. Therefore, it is urgent to develop novel agents overcoming the drawback of protein kinase inhibitors.
Alkaloids, a class of nitrogen-containing organic compounds usually with complex ring structures, are widely distributed in natural organisms. Alkaloids display diverse biological activity, including antiviral, antimicrobial, antinflammatory, and anticancer etc. [12]. Over the past decades, a large number of alkaloids have been applied clinically for the treatment of different malignant tumors [13, 14]. Taxol and vincristine, a kind of microtubule inhibitors are used to treat human cancers [15, 16]. Irinotecan and topotecan developed from camptothecin are also commonly used in cancer treatment [17]. Over the years, numerous alkaloids have been found as protein kinase inhibitors, and some of them display potent anticancer activity [18]. We discussed and highlighted the therapeutic targets according to their pathways (Fig. 1) and the special sites targeted by alkaloids on protein kinases and/or reversing drug resistance. In addition, the challenge and prospect of developing alkaloids as new anticancer agents were also presented in this mini-review.
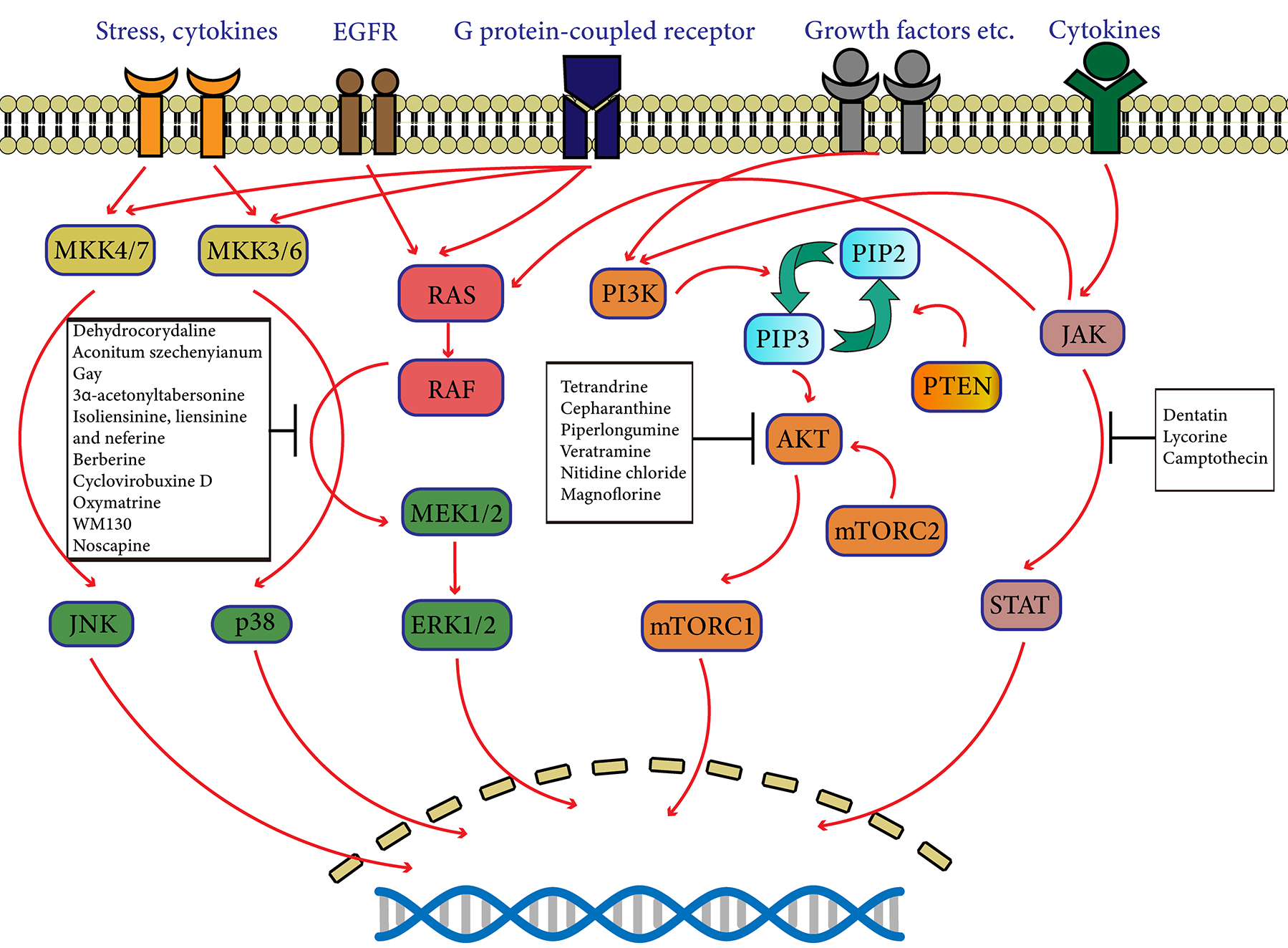
 Fig. 1.
Fig. 1.Schematic diagram of protein kinase pathways targeted by natural alkaloids. The protein kinase pathways including MAPK, PI3K/AKT/mTOR, JAK/STAT pathways play a significant role in the proliferation, survival and differentiation of cells. Natural alkaloids are able to interfere with some specific molecules related to protein kinase.
MAPK, a class of serine-threonine kinases, mediates intracellular signal
transduction related to cell proliferation, differentiation, death, survival and
transformation [19]. The classical MAPKs consist of extracellular
signal-regulated kinase (ERK), p38 MAPK and c-Jun NH2-terminal kinases
(JAK)/stress-activated protein kinases (SAPK). There are many subtypes of these
kinases, including ERK1-8, p38-
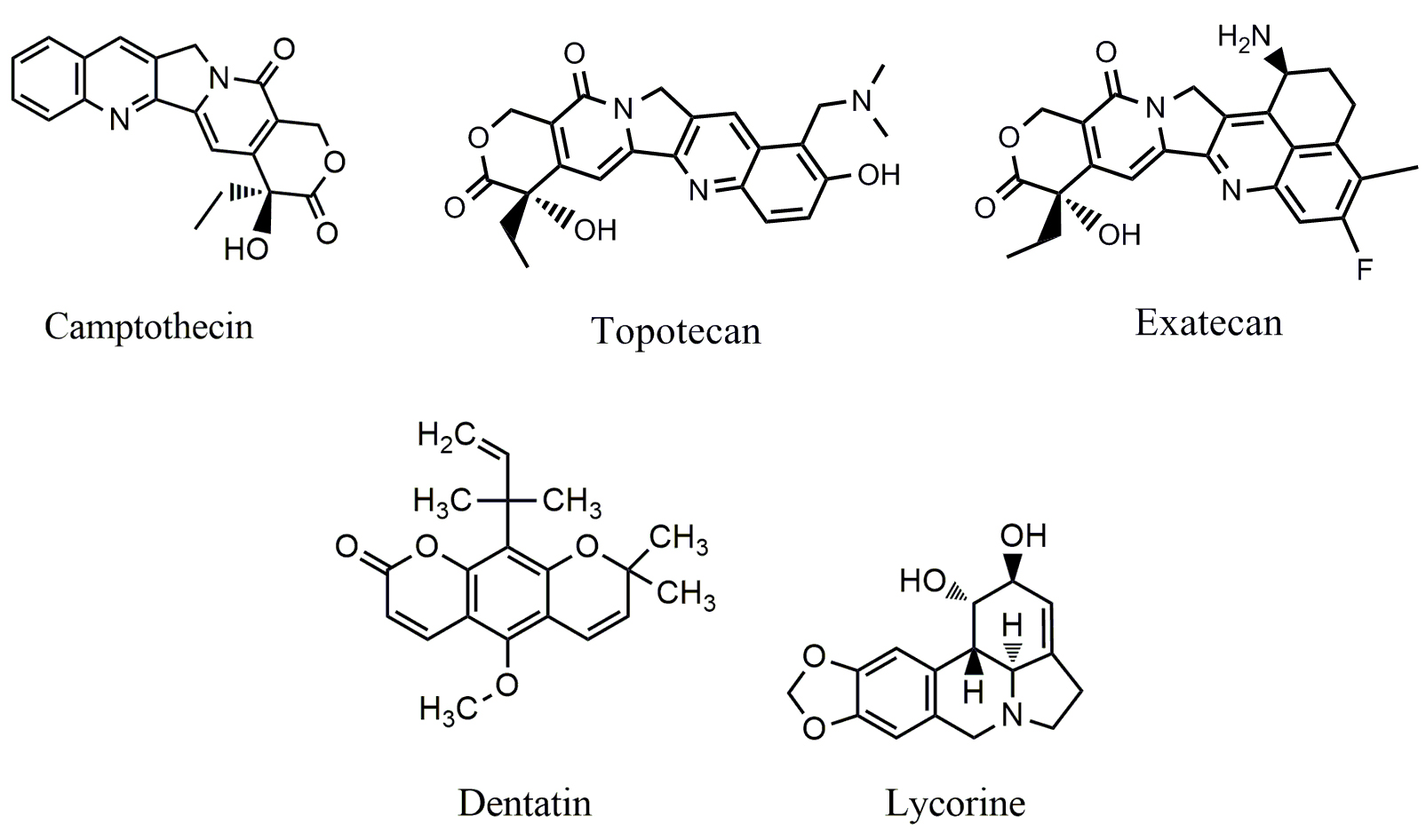
Over the years, numerous alkaloids affecting MAPK signaling have been
identified. Dehydrocorydaline (DHC) (Fig. 2), an isoquinoline alkaloid isolated
from Corydalis yanhusuo, Corydalis tuber or Corydalis
bulbosa [25], displays diverse biological activities, including inhibition of
antibody-mediated and cell-mediated allergy, inhibition of proinflammatory
cytokine expression, and promotion of myoblast differentiation [26]. In recent
years, the anticancer effects of DHC attract great attention; DHC was able to
induce apoptosis [27], and inhibit tumor metastasis [28]. Recent study showed
that DHC could inhibit the proliferation and metastasis of melanoma cells by
down-regulating the MEK1/2-ERK1/2 cascade and the phosphorylation levels of
MEK1/2 and ERK1/2 were down-regulated by DHC in a time-and dose-dependent manner
[29]. DHC significantly inhibited the proliferation of melanoma cells with
IC
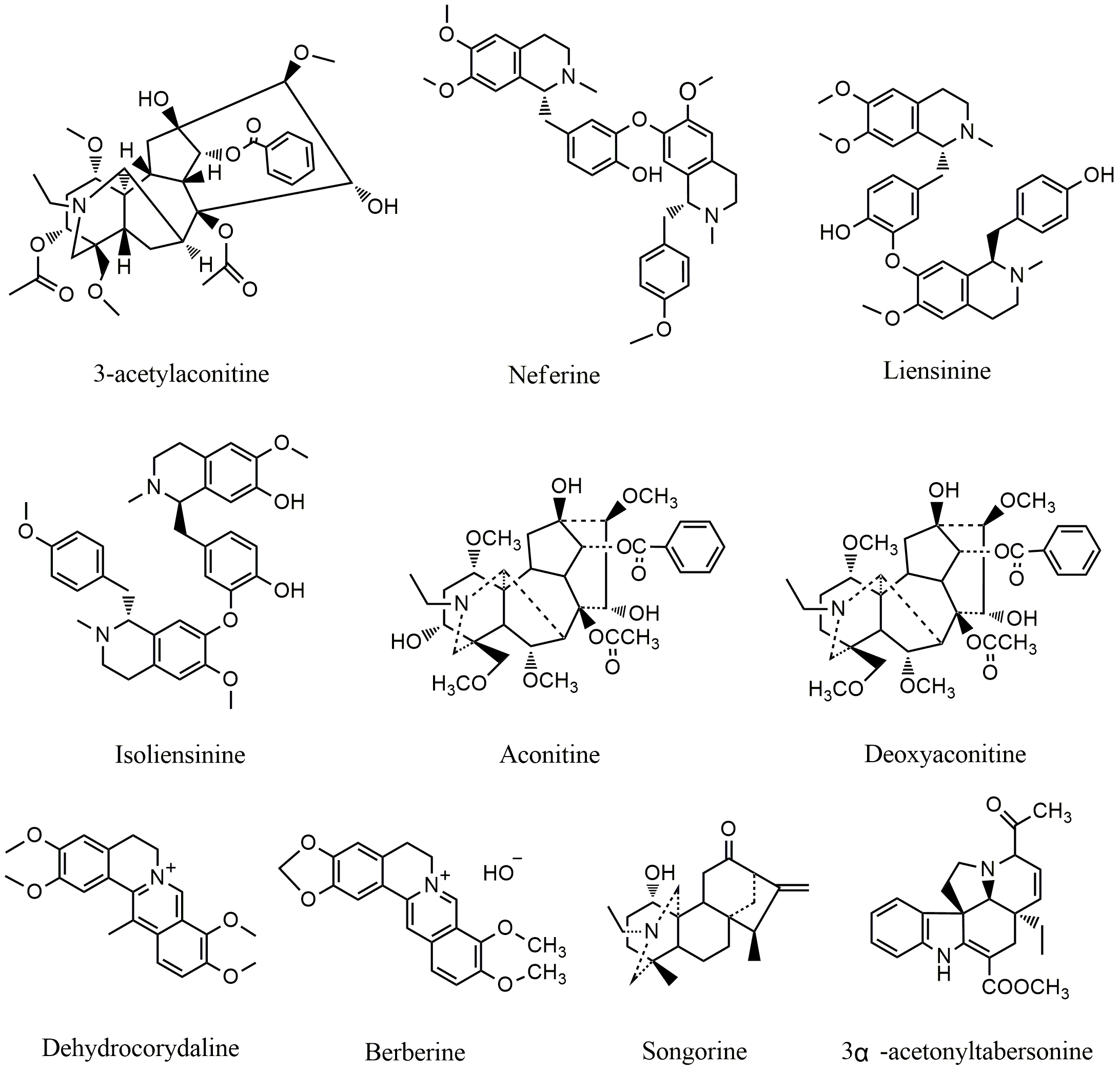
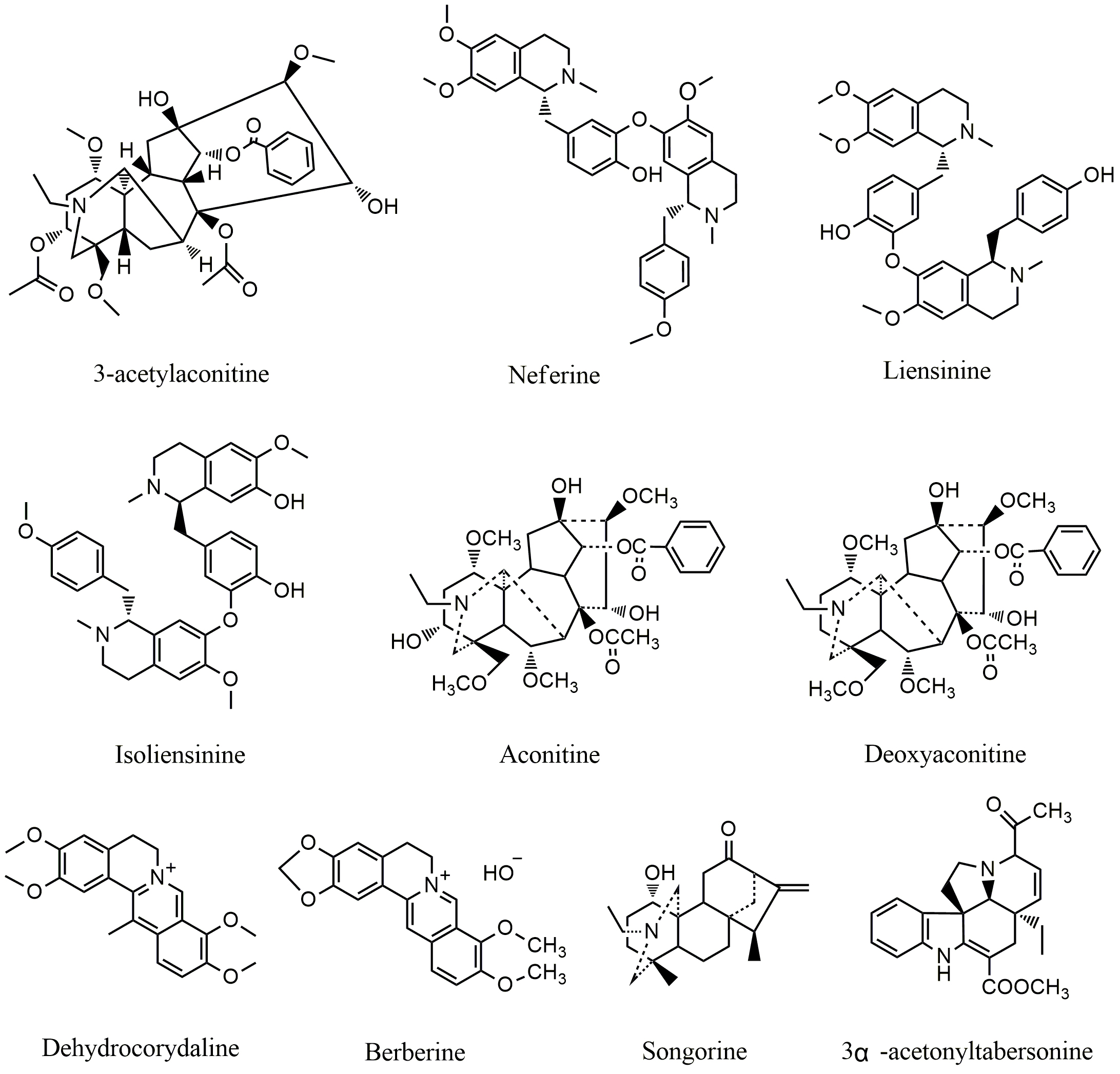 Fig. 2.
Fig. 2.Chemical structures of alkaloids targeting MAPK pathway.
Aconitum szechenyianum Gay, a traditional Chinese medical herb, is used
to treat inflammation related diseases in China for more the 500 years [30]. Its
extract displays potent anticancer activity in several cancer cells including
hepatoma SMMC-7721, gastric cancer SGC-7901 and esophageal cancer Eca-109 cells
both in vitro and in vivo [31]. Recent studies showed that the
extracts of Aconitum szechenyianum Gay were able to induce apoptosis
via targeting p38 MAPK pathway in hepatoma HepG2 cells, cervical cancer
Hela cells and lung cancer A549 cells; treatment with the extract down-regulated
the level of p38 MAPK phosphorylation, and induced mitochondria-dependent
apoptosis. Four alkaloids (Fig. 2) responsible for the anticancer effect were
isolated from its dried roots including 3-acetylaconitine
(C
3
Several alkaloids including isoliensinine (Fig. 2), liensinine (Fig. 2), and
neferine (Fig. 2), are isolated from the seed embryo of lotus (Nelumbo
nucifera Gaertn), and display potent anticancer activities in several cancer
cells [36, 37, 38]. Neferine inhibited the proliferation of human osteosarcoma cells
by inducing G1 phase arrest [36]. Isoliensinine induced apoptosis in triple
negative human breast cancer cells through targeting p38 MAPK and JNK pathways
[38]. Treatment with isoliensinine (20
PI3K/AKT/mTOR pathway, activated by tyrosine kinase receptor or other cytokines, plays an essential role in regulating cell proliferation, growth, survival, exercise, metabolism and immune response [42, 43]. It is well documented that PI3K/AKT/mTOR pathway is over activated in up to 60% of cancers [44]. The activation of PI3K/AKT/mTOR pathway promotes uncontrolled proliferation, genomic instability and metabolic reprogramming of cancer cells [45]. Over the years, numerous anticancer agents are used for the treatment of human malignancies in clinic via inhiting PI3K/mTOR signaling pathway.
Tetrandrine (TET) (Fig. 3), a bisbenzylisoquinoline alkaloid and calcium channel
blocker isolated from Stephania tetrandra S.Moore, is used for the
treatment of silicosis and rheumatoid arthritis as a traditional Chinese medicine
[46]. Recent studies have shown that PI3K/AKT/mTOR Pathway plays a critical role
in TET induced apoptosis in several cancer cells; treatment of human gastric
cancer cells with TET resulted in inactivation of AKT/mTOR phosphorylation [47].
TET was also capable of inducing autophagy via targeting PI3K/AKT
signaling in MCF-7 and MDA-MB-231 cell [48]. Clinical trial showed that the
combination of TET and traditional cancer drugs including daunorubicin, etoposide
and cytarabine (150 mg/m
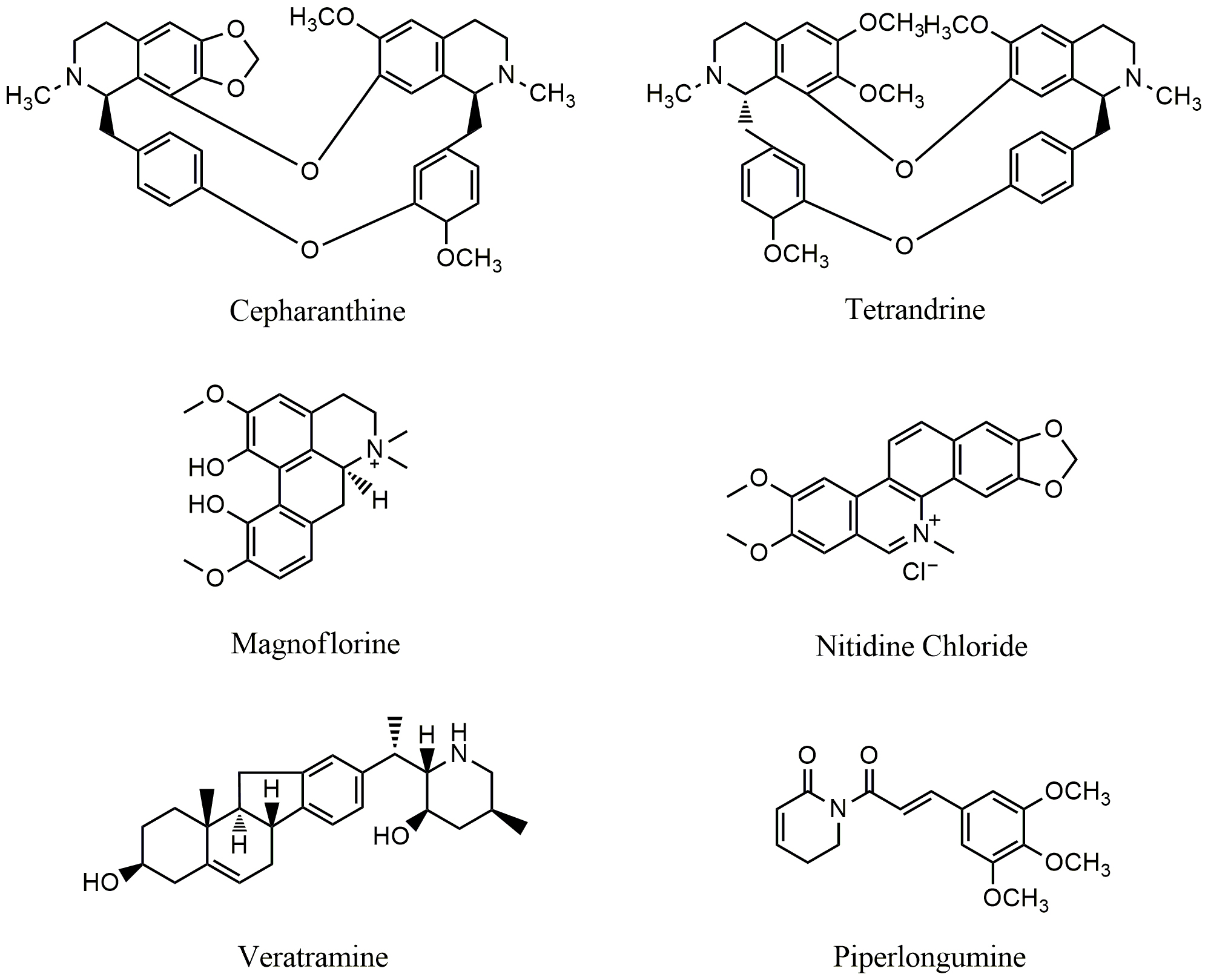
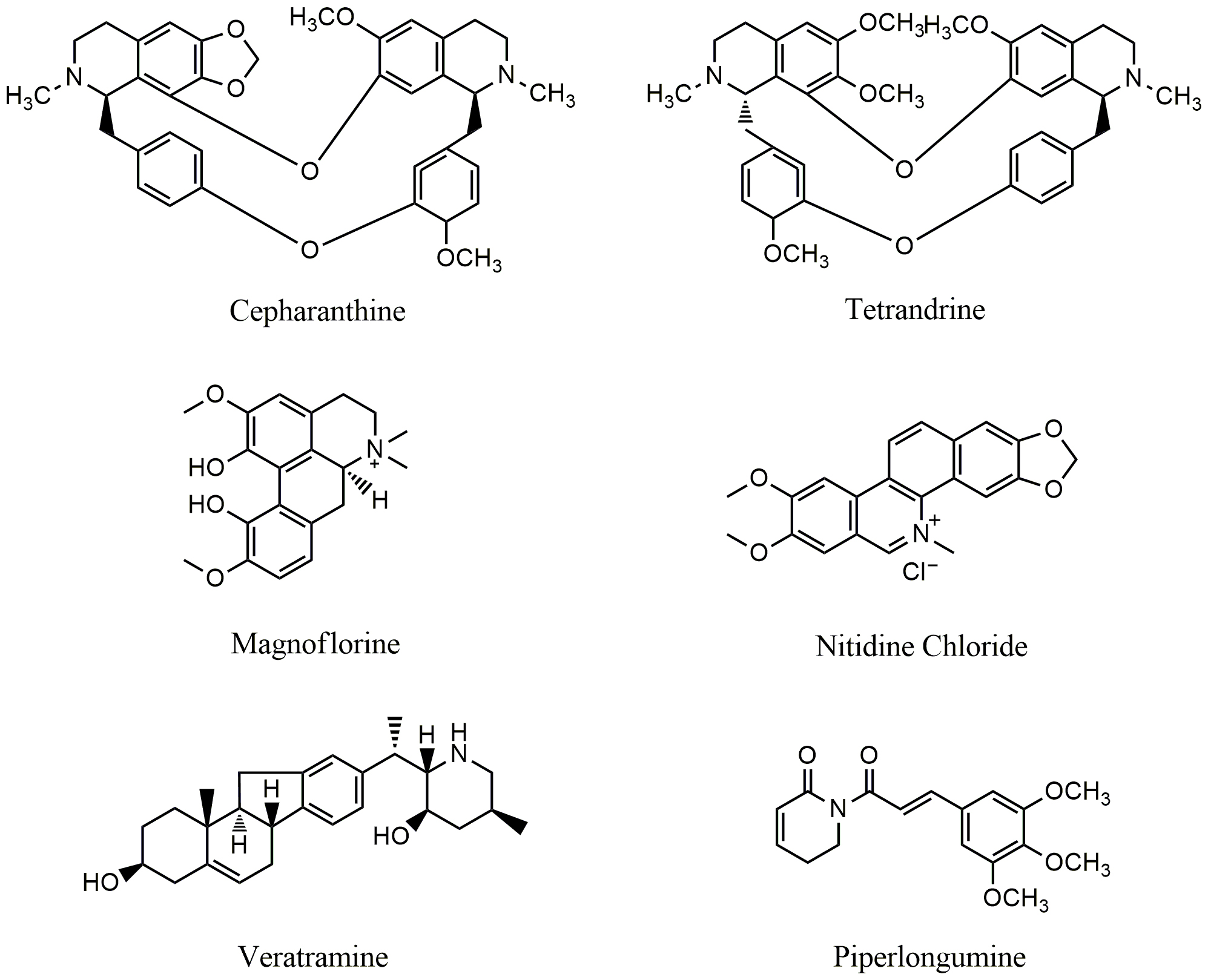 Fig. 3.
Fig. 3.Chemical structures of alkaloids targeting PI3K/AKT/mTOR pathway.
Piperlongumine (Fig. 3), a natural pyridine alkaloid, is extracted from
Piper longum [52]. Studies have shown that piperlongumine displays broad
biological activities including antianxiety, antiangiogenic, antidepressant,
anticancer, antibacterial, and antidiabetic activities [53]. Piperlongumine
exhibits strong cytotoxicity in various cancer cells, but it does little harm to
normal human cells [54]. PI3K/AKT/mTOR play an important role in piperlongumine
induced apoptosis; treatment with the compound led to down-regulation of p-Akt
and p-mTOR in both A549 and U937 cancer cells [55, 56]. Studies also found that
p38 was involved in piperlongumine-induced cancer cell growth inhibition;
treatment with the compound (20
Veratramine (Fig. 3), a natural steroidal alkaloid, is isolated from the plants
of lily family [60, 61]. Veratramine is able to inhibit cell growth both
in vitro and in vivovia targeting PI3K/Akt/mTOR;
treatment with veratramine (19.81
Nitidine chloride (NC) (Fig. 3), a benzoanthraquinone alkaloid isolated from Zanthoxylum nitidum (Roxb) DC, shows significant anticancer activity against a variety of cancer cells including ovarian cancer, colorectal cancer, liver cancer, and renal carcinoma [63, 64, 65, 66]. NC treatment led to down-regulation of AKT phosphorylation and suppressed the metastasis of renal cell carcinoma 786-O and A498 cells as well as glioblastoma U87 and U251 cells [63]. NC also inhibited the activation of STAT3 and ERK pathways and suppressed the expression of Bcl-2, Bax, cyclin D1, CDK4, VEGF-A and VEGFR2 in human hepatocellular carcinoma HepG2, HCCLM3, and Huh7 cells [64]. Magnoflorine (MAG) (Fig. 3), an apomorphine alkaloid, is isolated from Coptidis Rhizoma [67]. MAG exhibits wide spectrum activities including anti-inflammatory, anti-anxiety, anticancer etc. [68, 69]. MAG induced apoptosis and autophagy via targeting AKT/mTOR and p38 MAPK pathways [70]. Treatment with MAG led to decreased expression of p-PI3K, p-AKT and p-mTOR. In vivo study showed that MAG inhibited the growth of MCF-7 tumors bearing in nude mice and reduced the side effects of doxorubicin (DOX) in experimental animals. The tumor inhibitory rate has been increased from 30% (DOX alone) to 70% when the mice were treated with the combination of Mag/DOX (both at 3 mg/kg) [70].
Epidermal growth factor receptor (EGFR), a 170 kDa transmembrane glycoprotein, is composed of a single polypeptide chain. The occurrence and development of various cancers are associated with its mutations, overexpression or signal regulation disorders [71]. Therefore, EGFR and its related signal factors have become the critical targets for the discovery and development of novel chemotherapeutic drugs MCF-7.
Cyclovirobuxine D (CVB-D) (Fig. 4) is the main active ingredient extracted from
Buxus microphylla. CVB-D is widely used to treat cardiovascular diseases
such as coronary heart disease, angina pectoris and arrhythmia in China [72, 73].
In recent years, there are numerous reports to show that CVB-D inhibited EGFR.
CVB-D can inhibit the proliferation, colony formation, cell cycle process, and
induced apoptosis through mitochondrial-mediated pathway in human gastric cancer
MGC-803 and MKN28 and hepatocellular carcinoma HepG2 and HCCLM3 cells [74].
Previous study revealed that CVB-D induced autophagic death through targeting
AKT/mTOR signal pathway; treatment with CVB-D led to upregulation of p-AKT and
p-mTOR in MCF-7 cells [75]. Recent study showed that both EGFR and PI3K/AKT were
involved in CVB-D induced growth inhibition of cancer cells; treatment with CVB-D
(10–40
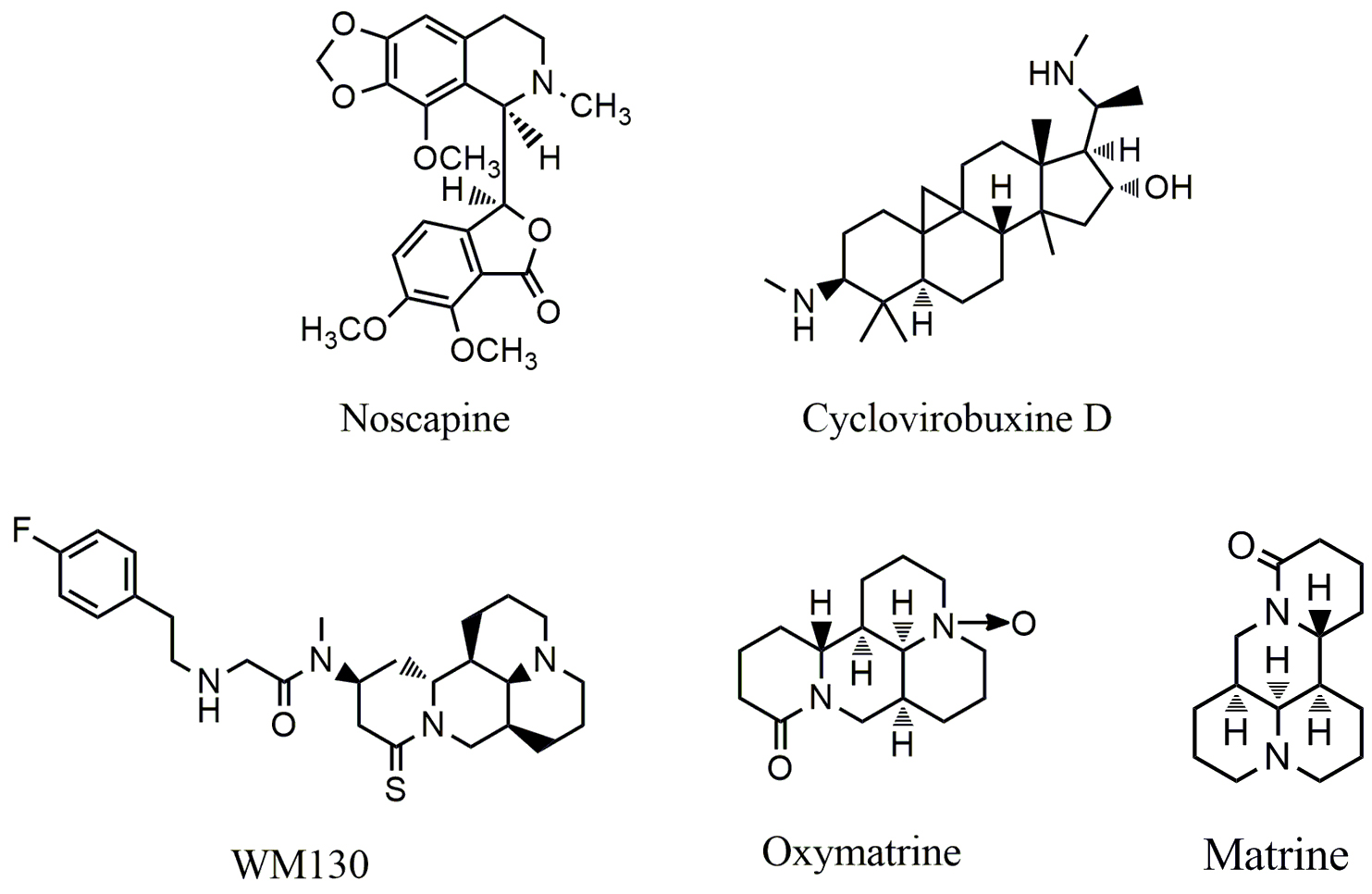
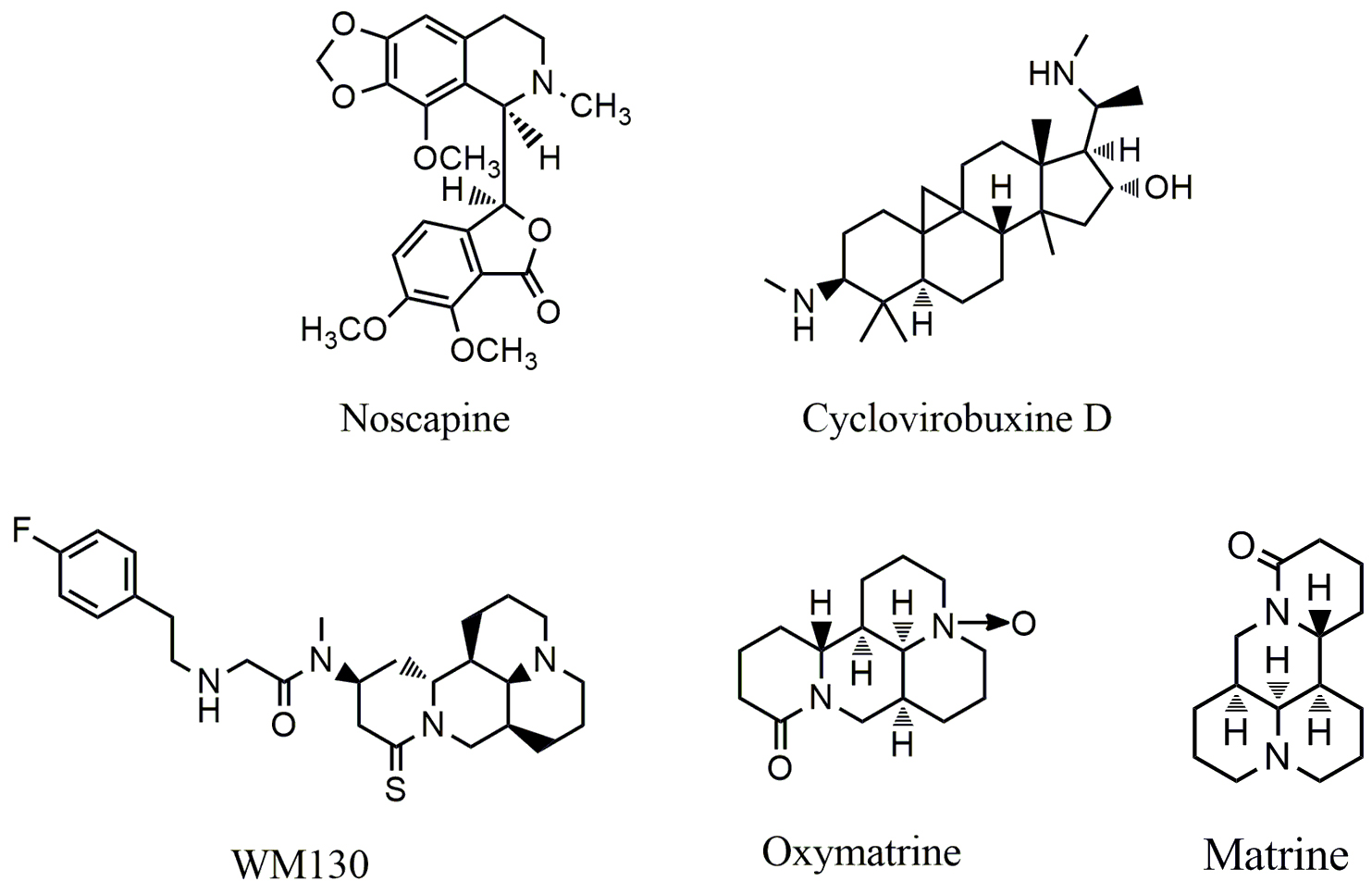 Fig. 4.
Fig. 4.Chemical structures of alkaloids that target epidermal growth factor receptor (EGFR).
Matrine (Fig. 4) and oxymatrine (Fig. 4) are the main active components of
Sophora flavescens [84]. Matrine exhibits cytotoxicity in several cancer
cells including hepatocellular carcinoma [85], gastric cancer [86], breast cancer
[87] and lung cancer [88]. Matrine displays anticancer activity through targeting
different cellular pathways in cancer cells; matrine could inhibit cancer cell
proliferation, invasion, metastasis, and angiogenesis, induce differentiation and
apoptosis, reverse multidrug resistance [89]. In order to increase the anticancer
activity, a derivative of matrine, WM130 (C
Noscapine (Fig. 4), a benzylisoquinoline alkaloid derived from the opium poppy Papaver somniferum, is widely used as a cough suppressant in Chinese folk medicine. Studies have shown that noscapine displays anticancer activity both in vitro and in vivo [92, 93, 94, 95]. Noscapine induced growth inhibition of cancer cells is dependent on EGFR-related signal pathway; treatment with noscapine resulted in inactivation of EGFR and AKT, and the reduction of the expression of cyclin D1 and CDK4/6, leading to G1 arrest in osteosarcoma MG63 cells [96]. Treatment with noscapine also led to down-regulation of VEGF in hypoxic human glioma U87 cells [97, 98].
JAK/STAT signaling pathway plays an essential role in numerous cellular functions including cell proliferation, differentiation, apoptosis, immunomodulation and hematopoiesis [99]. There are four members of JAK family; JAK1, JAK2, JAK3 and TYK2. STAT families are composed of seven members; STAT1-4, STAT5A/5B and STAT6 [100]. The activation of JAK/STAT pathway depends on a variety of cytokines and growth factors including interleukin, interferon and EGF family members [101]. The binding of cytokines and other ligands to the receptor leads to the activation of janus kinase (JAK), and the activated JAK phosphorylates the tyrosine residues in the tail of the cytoplasm receptors [11]. In the process of tumorigenesis and development, over-activated JAK/STAT signals contribute to cancer cells proliferation, survival, invasion and neovascularization [102]. Therefore, this pathway has attracted more and more attention as a new target for anticancer drugs.
Dentatin (Fig. 5), a carbazole alkaloid isolated from Murraya koenigii,
has been found to exhibit a variety of anticancer activities [103, 104]. Dentatin
can induce apoptosis in prostate cancer PC-3 and LNCaP cells [105], and breast
cancer MCF-7 cells [106]. Recent study showed that JAK/STAT signaling plays a
critical role in dentatin induced apoptosis and autophagy in cancer cells [107].
Dentatin was able to down-regulate the phosphorylation of JAK1, JAK2, STAT1 and
STAT3, and the compound is also capable of increasing the expression of Beclin-1
and LC3II, leading to autophygic cell death in HT-29 cancer cells. Dentatin can
also up-regulate the expression of cyclin D1, down-regulate the expression of
cyclin A/B1, induce S-phase arrest in HT-29 cells. Lycorine (Fig. 5), a
isoquinoline alkaloid extracted from Amaryl lidaceae displays broad
spectrum of pharmacological activities such as anti-inflammatory, emetic,
anti-malaria, anti-viral and so on [108, 109]. Lycorine can inhibit the growth of
cancer cells through different mechanisms. Lycorine inhibited the growth and
metastasis of prostate cancer [110]. The compound significantly inhibited the
proliferation, migration and invasion of prostate cancer PC-3M, DU145, LNCaP and
22RV1 cells with the IC
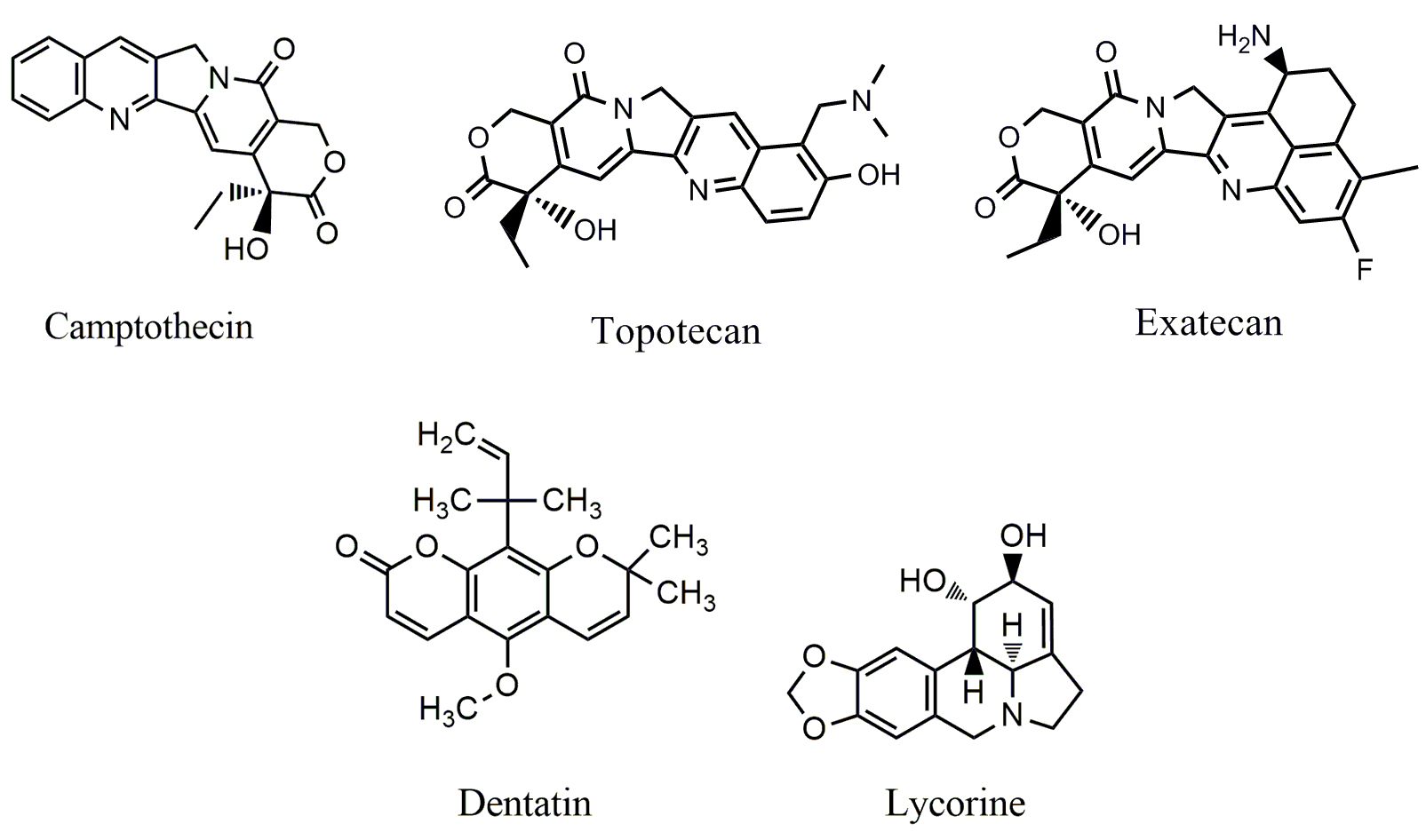 Fig. 5.
Fig. 5.Chemical structures of alkaloids targeting JAK/STAT pathway.
Camptothecin (CPT) (Fig. 5), a quinoline alkaloid, was isolated from Camptotheca acuminate. Its analogs such as irinotecan and topotecan are used clinically for the treatment of ovarian, colorectal and other cancers by inhibiting topoisomerase-I (Top-I) [113]. Moreover, CPT is also able to inhibit the phosphorylation of JAK2 and STAT3; the level of JKA2 is closely associated with the sensitivity of CPT in colon cancer cells [114].
Table 1 (Ref. [25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 90, 91, 92, 93, 94, 95, 96, 97, 98, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114]) summerizes the natural alkaloids and their main targets in protein kinase pathways. However, it should be kept in mind that some of the alkaloids are able to target multiple pathways related to protein kinases. EGFR plays critical roles in WM130 induced growth inhibition of cancer cells. However, PI3K/Akt is also involved in WM130 induced growth inhibition of cancer cells [91]. NC treatment lead to down-regulation of AKT phosphorylation and inhibition of metastasis in several cancer cells [63]. NC also inhibited the activation of STAT3 and ERK pathways in human hepatocellular carcinoma HepG2, HCCLM3, and Huh7 cells [64]. Both EGFR and PI3K/AKT were involved in CVB -D induced inhibitory effect on cancer cells [111].
| Name of Alkaloids | Sources | Targets and cell lines | References |
| Dehydrocorydaline | Corydalis yanhusuo | MEK1/2 and ERK1/2 | [25, 26, 27, 28, 29] |
| A375, MV3 cells | |||
| Aconitum szechenyianumGay | Aconitum pendulum Busch | p38 MAPK | [30, 31, 32] |
| SMMC-7721, SGC-7901, Eca-109, HepG2, Hela and A549 cells | |||
| 3 |
Melodinus suaveolens | p38 MAPK | [33, 34, 35] |
| HL60, SMMC-7721, A549, MCF-7 and SW480 cells | |||
| Isoliensinine, liensinine and neferine | Nelumbo nucifera Gaertn | p38 MAPK | [36, 37, 38] |
| MDA-MB-231, MDA-MB-436, MDA-MB-468 and MCF-7 cells | |||
| Berberine | Coptis chinensis | p38 MAPK | [39, 40, 41] |
| A549, PC-9 cells | |||
| Tetrandrine | Stephania tetrandra S.Moore | PI3K/AKT | [46, 47, 48, 49] |
| MCF-7, MDA-MB-231 and HGC-27 cells | |||
| Cepharanthine | Stephania cepharantha Hayata | PI3K/AKT/mTOR | [50, 51] |
| Jurkat T cells | |||
| Piperlongumine | Piper longum | PI3K/AKT/mTOR | [52, 53, 54, 55, 56, 57, 58, 59] |
| A549, U937, HT29, DU145, MDA-MB-231 and K562/A02 cells | |||
| Veratramine | Plants of the lily family | PI3K/AKT/mTOR | [60, 61, 62] |
| HepG2 cells | |||
| Nitidine chloride | Zanthoxylum nitidum (Roxb)DC | PI3K/AKT/mTOR | [63, 64, 65, 66] |
| U87, U251, 786-O and A498 cells | |||
| Magnoflorine | Coptidis Rhizoma | AKT/mTOR | [67, 68, 69, 70] |
| MDA-MB-231, MDA-MB-453 and MCF-7 cells | |||
| Cyclovirobuxine D | Buxus microphylla | EGFR | [72, 73, 74, 75, 76] |
| MGC-803, MKN28, HepG2 and HCCLM3 cells | |||
| Oxymatrine | Sophora flavescens. | EGFR and VEGF | [77, 78, 79, 80, 81, 82, 83] |
| PANC-1 and U251MG cells | |||
| WM130 | Sophora flavescens. | EGFR and ERK | [90, 91] |
| Huh-7 cells | |||
| Noscapine | Papaver somniferum | EGFR | [92, 93, 94, 95, 96, 97, 98] |
| MG63 and U87 cells | |||
| Dentatin | Murraya koenigii | JAK/STAT | [103, 104, 105, 106, 107] |
| HT29 cells | |||
| Lycorine | Amaryllidaceae | JAK/STAT | [108, 109, 110, 111, 112] |
| PC-3M, DU145, LNCaP and 22RV1, U266, RPMI8226 and KM3 cells | |||
| Camptothecin | Camptotheca acuminata | JAK/STAT | [113, 114] |
| Lovo, SW48, HCT116, HCT8, HT29 and WiDr cells |
Although the pathways of PI3K/Akt/mTOR, JAK/STAT, EGFR and p38 MAPK account for
most of the protein kinase enzymes, there are still many other protein kinases
responsible for the occurrence, development, proliferation, invasion, metastasis
and mitosis of cancer cells. A ser/thr protein kinase, protein kinase C (PKC)
plays very important roles in regulating a variety of cellular functions in cell
development and proliferation. Several pathophysiological conditions including
cancers are development by the dysregulation of PKC [115]. A benzophenanthridine
alkaloid, chelerythrine isolated from Chelidonium majus (L), acting as a PKC
inhibitor, was able to induce apoptosis significantly in Dalton’s lymphoma cells
[116]. The combination of berberine (10
Isoquinoline represents one of the most important class of alkaloids in cancer therapy. The structure/activity relationship (SAR) of isoquinoline has been investigated and the result revealed that the substitutions of 11H-benzothieno [3, 2-b]quinoline-4-carboxamides and indolo[1,2-b]quinoline-6-carboxamides at the pseudo-peri position to the carboxamide side chain is crucial for its cytotoxicity against several cancer cells [126]. Moreover, in protoberberine alkaloids, substitution of methoxyl to hydroxy group at the C-9 position was very important for their anticancer efficacy [127]. DHC and BBR share similar structure as that of isoquinoline and both of the compounds inhibited the cancer cell growth by inhibiting p38MAPK pathway significantly. CPT and its analogs as TOP-1 inhibitor display similar anticancer effect.
The anticancer alkaloids reviewed here are found from different kinds of
natural organisms and display diverse mechanisms for their anticancer activities.
Several anticancer agents developed from alkaloids exhibit unique anticancer
mechanism. For example, 3
XL and SC designed the work. HY, LW, LM, MI, GQ, JH, LC, YZ, XY, SC and XL collected and reviewed the references. HY wrote the first draft. XL and SC wrote and reviewed the final version of the manuscript. All authors discussed and contributed to the manuscript.
Not applicable.
Not applicable.
The study was supported the International Collaborative Project of the MOST of China (No#: 2017YFE0195000). We are also grateful for the support by the Taishan Talents project of Shandong province and the Natural Sci. Foundation in Shandong Province of China (No#: ZR2020MH421, ZR2020MH420, ZR2020QH360).
The authors declare no conflict of interest.