2. Introduction
In the immune system, NK cells are a subset of lymphocytes derived from the
development and differentiation of hematopoietic stem cells. As a “natural
killer”, NK cells do not need to be pre-sensitized, so they are the forerunners
of the body’s anti-tumor immunity, which is essential to directly identify and
kill tumor cells. However, most tumors can evolve immune escape mechanisms. On
one hand, tumor cells change their antigenicity, immunogenicity and related
molecular proteins to avoid recognition and killing by the immune system. On the
other hand, tumor cells can also promote the production of anoxic acid
immunosuppressive microenvironment and inhibit the cancer immunosurveillance [1].
TGF-1 is one of the main accomplices of tumor immune escape,
which is able to reduce the release of killing mediators and inhibit tumor
immunity mediated by NK cells through various routes [2, 3].
TGF-1 can drive NK cells to upregulate the expression of
fructose-1,6-bisphosphatase (FBP1), inhibiting the glycolysis metabolism and
weakening the killing function of NK cells [4]. It has been well recognized that
tumor microenvironment (TME) rich in TGF- promotes immune
escape by differentiating NK cells into intrinsic lymphoid cell type 1 (ILC1)
lacking cytotoxicity [5]. In addition, high concentration of
TGF-1 in tumor patients’ plasma tend to upregulate the
expression of CD96 and dynamically change the balance of CD96, TIGIT and CD226 in
NK cells, thereby suppressing the immune function of NK cells [6]. Also, blocking
TGF- signaling pathway is beneficial to enhance the killing
effects of NK cells on breast cancer cells in vitro [7].
Our previous studies showed that the release of TGF-1 was
significantly increased in the activated platelet-tumor cell co-culture system,
and repression of TGF-1 could dramatically retard the malignant
biological progression of breast cancer cells, suggesting that
TGF-1 may act as a key molecule mediating the interactions
between tumor cells and platelets [8]. It has been documented that Salvia
miltiorrhiza exerts an important effect on enhancing immunity, which was
evidenced by the fact that it boosted the killing activities of cytotoxic T
lymphocytes (CTL) and NK cells and stimulated the phagocytosis of macrophages.
Meanwhile, it could potentiate the synthesis and release of perforin and granzyme
B of NK cells and improve the anti-tumor immune function in the rats with gastric
cancer [9]. In addition, it was reported that Cryptotanshinone and Tanshinone
IIA, two predominant fat-soluble components, could reinforce the differentiation
and maturation of NK cells induced by IL-15, and then promote their killing
effect on target cells [10, 11].
In this study, our results showed that immunosuppressive factor
TGF-1 could destroy the NKG2DL-NKG2D signaling axis and
restrict the release of anti-tumor cytotoxic killing mediators in the NK cells,
whereas Tanshinol was able to reverse the immunosuppression activity of NK cells
triggered by TGF-1, restore the degranulation function of NK
cells, enhance tumor immunity and inhibit the occurrence and development of
tumor. Our study complements the anti-tumor mechanisms of Tanshinol and explores
the role of Tanshinol in promoting tumor immunity and inhibiting tumor immune
escape.
3. Materials and methods
3.1 Mouse tumor model
Four-week-old female Balb/c nude mice were purchased from Shanghai Slac
Laboratory Co., Ltd. All animal procedures were conducted under the guidance of
the Animal Ethics Committee and approved by the Institutional Animal Committee of
Nanjing University of Chinese Medicine (Ethical Review Number: 202005A018).
Each of the Balb/c nude mouse was inoculated with 10 ZR-75-1 cells. Three
treatment groups were given 15 mg/kg, 30 mg/kg and 60 mg/kg Tanshinol powder
prepared with double distilled water by intragastric administration. Both control
group and model group were given the same volume of double distilled water. The
weight of mice was measured every week. The mice were sacrificed three weeks
following cell injection, after which the tissues and serum were collected for
further studies.
To demonstrate the effect of Tanshinol on the survival duration of tumor-bearing
mice, we took the week of all mice in the model group deaths as the experimental
end point, and made statistics on the survival rate of each group of mice.
3.2 Cell lines and drug preparation
The NK92MI cells from patients with human malignant non-Hodgkin lymphoma were
purchased from Guangzhou Saiku Biotechnology Company. The NK92MI cells were
cultured in Alpha MEM supplemented with 12.5% horse serum, 12.5% fetal bovine
serum, 0.2 mM inositol, 0.1 mM mercaptoethanol and 0.02 mM folate. ZR-75-1 human
metastatic breast cancer cells were kindly provided by Prof. Qiang Xu from
Nanjing University, ZR-75-1 cells were grown in 1640 medium containing 10% FBS.
All cells were cultured in a cell incubator at 37 C, 5% CO, and 95% air.
Tanshinol (HPLC 98%, molecular weight: 198.17, Cat. NO.: A4544) was
purchased from Shanghai Yuanye Biotechnology has a molecular weight of 198.17.
Tanshinol was dissolved in PBS, made up into 16 mM stock solution, and stored at
–20 C in the dark. The working solutions were prepared from the stock solution.
TGF-1 (Cat. No.; 100-21) was obtained from the American
PeproTech company, diluted with 10 mM citric acid solution (pH = 3) and 0.1% BSA
solution to 1 g/mL.
3.3 Histology
Liver, spleen, lung, and kidney tissues were harvested, fixed in 4%
paraformaldehyde for 24 h, embedded with paraffin, and cut into 5 m
sections. The sections were stained with H&E, and the images were acquired on
Mantra Pathology Workstation (PerkinElmer. Waltham, MA, USA).
3.4 Cell proliferation assay
The ZR-75-1 tumor cells were plated into the 96-well plate at the density of 1.2
10 cells/well. The adjustment group, the control group, and the
treated groups (5 M, 10 M, 20 M, 40 M and 80
M) were set with 6 replicates in each group. Cell proliferation assay was
performed based on the instructions of the CCK8 kit, a BioTek microplate reader
(model: 270133) was used to detect the absorbance at 450 nm and then the relative
cell proliferation rate was calculated. The CCK8 kit (Cat. No.: C0038) was
purchased from Shanghai Beyotime Biotechnology Company.
3.5 LDH cytotoxicity assay
Lactate dehydrogenase (LDH) cytotoxicity was performed according to the
manufacturer’s instructions. NK cells were seeded into 12-well plates at a
density of 1 10 cells/well. TGF-1 (10 ng/mL)
and different concentrations of Tanshinol (5 M, 10 M, 20 M,
40 M and 80 M) were added and cultured for 24 h. ZR-75-1 cells were
seeded into the 24-well plates at a density of 1 10 cells/well.
NK cells were pre-treated according to different target ratios (10:1, 20:1, and
40:1) to construct a co-incubation system. After co-cultivation for 3 h, the
prescribed ratios of LDH release reagent were added to the sample’s maximum
enzyme activity control well. LDH content detection was performed according to
the kit instructions. LDH kit (Cat. No.: C0017) was purchased from Shanghai
Beyotime Biotechnology Company.
3.6 Western blot analysis
ZR-75-1 cells were seeded in a 6-well plate at a density of 2
10 cells/well. After the cells adhered, TGF-1 (10 ng/mL),
Tanshinol (5 M 10 M and 20 M) were added and incubated with
cells for 24 h. The protein extract (RIPA lysate:phosphatase inhibitor:protease
inhibitor = 100:1:1) was used to lyse the cells, and then the BCA kit (Thermo,
Cat. No.: 23227) was employed to detect the protein concentration. The denatured
proteins were separated and transferred to PVDF membranes using SDS-PAGE
electrophoresis and wet transfer, respectively. The PVDF membranes were blocked
with blocking solution (5% skimmed milk powder/TBST) at room temperature for 2
h, and incubated with indicated primary antibodies at 4 C overnight. TBST was
eluted 4 times (5 min/time), followed by incubation with corresponding secondary
antibodies at room temperature for 1.5 h. The proteins were developed by ECL kit
(Millipore, Cat. No.: P36599). The gel imaging system (ChemiDoc™
XRS +) was used to acquire images, and protein bands were quantified using Image
J software (version 1.8.0, National Institutes of
Health, Bethesda, MD, USA).
MICA (A12622), MICB (A9802), ULBP1 (A10483), ULBP2 (A15194), KLRK1/NKG2D
(A6123), P-PLC2 (AP0785) antibodies were from ABclonal
Technology. DAP10 (sc-133173) antibody was obtained from Santa Cruz
Biotechnology. GAPDH (AP0063) was from Bioworld Technology. PI3K (4249), P-PI3K
(4228), ERK1/2 (4695), P-ERK1/2 (4370), PLC2 (3872), smad2/3
(8685), p-smad2/3 (8828) antibodies were purchased from Cell Signaling
Technology.
3.7 Immunofluorescence
ZR-75-1 cells were seeded in a 6-well plate at a density of 2
10 cells/well with a round cover glass pre-positioned at the bottom,
TGF-1 and different concentrations of Tanshinol were incubated
with the cells for 24 h. The cells were fixed with 4% paraformaldehyde (PFA),
permeabilized with 0.2% Triton X-100/PBS, blocked with 1% BSA (BSA/PBS), and
the indicated primary antibodies were incubated at 4 C overnight. The
corresponding fluorescent secondary antibodies were incubated in the dark for 2
h. Hoechst 33324 was used to stain the nuclei. Images were taken with ZEISS
fluorescence microscope (Oberkochen, Germany, model: Vert.A1).
3.8 Co-immunoprecipitation (Co-IP)
The protein samples of NK92MI cells were extracted with IP lysate (Jiangsu
Keygen Biotechnology. Cat. No.: KGP701. Nanjing, China), and the protein
concentration was quantified by BCA method. 2 g IP primary antibody was
added and incubated at 4 C overnight. 20 L of fully resuspended
Protein A/G PLUS-Agarose was added (Santa Cruz, Cat. No.: sc-2003. Dallas, TX,
USA) and mixed slowly at 4 C for 3 h. The supernatant was removed and the pellet
was washed with PBS. The immunoblot detection was performed and the protein bands
were developed with ECL reagents.
3.9 Flow cytometry
NK cells treated with TGF-1 and Tanshinol were collected,
seeded into a 6-well plate, and then ZR-75-1 cells were added at a 10:1 target
ratio to construct a co-incubation system. After 4 h of co-culture, the cell
suspension was collected, centrifuged at 1500 rpm for 5 min, washed twice with
PBS, and fixed with 4% paraformaldehyde at room temperature for 10 min. 500
L PBS was added to each tube to resuspend the sample. 5 L
PerCP-Cy5.5 CD56 and 20 L PE-CD107a flow cytometry antibodies were added
to each sample according to the instructions, and incubated on ice for 30 min. BD
C6 flow cytometry was used to detect the expression of CD107a on the surface of
NK cells.
In order to detect the levels of the intracellular factors Perforin, Granzyme B
and IFN-, a protein transport blocker BFA or monensin was
added to the co-incubation system. After cell fixation, 0.2% Triton X-100/PBS
was permeabilized for 10 min at room temperature. Flow cytometry antibodies
PerCP-Cy5.5 CD56 (Cat. No.: 560842) and PE-CD107a (Cat. No.: 555801) were from BD
PharmingenTM. Perforin (Cat. No.: 12-9994-41), Granzyme B (Cat. No.: 12-8899-41),
and IFN- (Cat. No.: 12-7319-42) antibodies were purchased from
Invitrogen.
3.10 Enzyme-linked immunosorbent assay
The serum samples of mice were agglutinated at room temperature for 30 min, and
left overnight at 4 C to completely release TGF-1. After
Centrifugation at 1000 g for 10 min, the serum samples were detected according to
the instructions. The cell suspension of the co-incubation system was collected
and centrifuged at 1500 g for 10 min to obtain a supernatant sample. The results
with 3 replicates in each group were detected at 450 nm with a microplate reader.
Human perforin ELISA kit (Cat. No.: RK00135) and human granzyme B ELISA Kit
(Cat. No.: RK00089) were from ABclonal Technology. Human IFN-
ELISA Kit (Cat. No.: 70-EK180-96) was purchased from Link Bio. Mouse
TGF-1 ELISA kit (Cat. No.: EK981-48), mouse perforin ELISA Kit
(Cat. No.: JEB-13034), mouse granzyme B ELISA Kit (Cat. No.: JEB-12517) and mouse
IFN- ELISA kit (Cat. No.: JEB-12796) were obtained from Nanjing
Jin Yibai Biological Technology Co. Ltd.
3.11 RT-PCR
TRIzol extraction kit (Invitrogen. Carlsbad, CA, USA) was used to extract the
total RNA in NK cells, and the purity was verified by the mRNA detection plate
provided with BioTek microplate reader. cDNA was synthesized by the reverse
transcription kit (Vazyme Biotech Co., Ltd, Nanjing, China) using gradient PCR
instrument (Thermo Fisher Scientific, Applied Biosystems life Veriti96. Waltham,
MA, USA). The Ct values of each cDNA were detected with a fluorescence
quantitative PCR instrument (BIO-RAD, model: iQ5. Hercules, CA, USA), and the
relative change of mRNA was calculated by 2. The
primer sequences are shown in Table 1.
Table 1.The primer sequences.
| Name |
Forward |
Reverse |
| GAPDH |
5′-GGTTGTCTCCTGCGACTTCA-3′ |
5′-TGGTCCAGGGTTTCTTACTCC-3′ |
| NKG2D |
5′-TCTCGACACAGCTGGGAGATG-3′ |
5′-GACATCTTTGCTTTTGCCATCGTG-3′ |
| DAP10 |
5′-TCCATCTGGGTCACATCCTCTTCC-3′ |
5′-GAGTGATGATCTCTCTCCTGGAGTCGTCTGAGCTG-3′ |
| Perforin |
5′-ACCAGCAATGTGCATGTGTCTGTG-3′ |
5′-GAAGGAGGCCGTCATCTTGTGCTT-3′ |
| Granzyme B |
5′-TGCAGGAAGATCGAAAGTGCG-3′ |
5′-GAGGCATGCCATTGTTTCGTC-3′ |
| IFN-γ |
5′-TCCAACGCAAAGCAATACAT-3′ |
5′-GCAGGCAGGACAACCATTAC-3′ |
3.12 Statistical analysis
GraphPad Prism 5.0 software (San Diego, CA, USA) was used for statistical
difference analysis. All data were expressed by mean standard deviation
(SD). Comparison between groups was analyzed by one-way analysis of variance.
p 0.05 was considered statistically significant.
4. Results
4.1 Tanshinol prevents the breast cancer metastasis in vivo
In order to preliminarily verify the efficacy of Tanshinol, each of the Balb/c
nude mouse was inoculated with ZR-75-1 cells to constructed an animal model of
breast cancer including treatment groups and model group. Three treatment groups
were given 15 mg/kg, 30 mg/kg and 60 mg/kg Tanshinol, and control group and model
group were given the same volume of double distilled water. Compared with the
model group, the body weights of treatment groups were significantly increased
(Fig. 1A). Meanwhile, the spleen organ index of the medium or high dose group was
significantly lower than that of model group, whereas the liver, lung and kidney
indexes showed no significant changes (Fig. 1B). Notably, H&E staining results
revealed that the mice in the model group exhibited obvious lung metastasis, and
Tanshinol could alleviate the lung metastasis in a dose-dependent manner (Fig. 1C). In order to examine the expression of TGF-1 in
tumor-bearing mice and the effect of Tanshinol on TGF-1 in the
serum of mice, ELISA assay was thus performed. The results demonstrated that the
level of TGF-1 in the model group was significantly increased
compared with that in the control group, and high-dose of Tanshinol
administration significantly reduced TGF-1 production in the
tumor-bearing mice (Fig. 1D). Given the fact that NK cells can exert anti-tumor
and anti-metastasis activities by secreting various effector molecules such as
IFN-, perforin and granzyme B [12], we further detected the
levels of IFN-, perforin and granzyme B in the tumor-bearing
mice. It was found that high-dose of Tanshinol could effectively promote the
release of above-mentioned killing mediators in the serum, which may be related
to the activation and enhanced function of NK cells mediated by Tanshinol (Fig. 1E). In addition, we demonstrate the effect of Tanshinol on the survival duration
of tumor-bearing mice, the results showed that all mice in the model group died
in the seventh week, and the high-dose Tanshinol group significantly optimized
the survival rate of tumor-bearing mice.
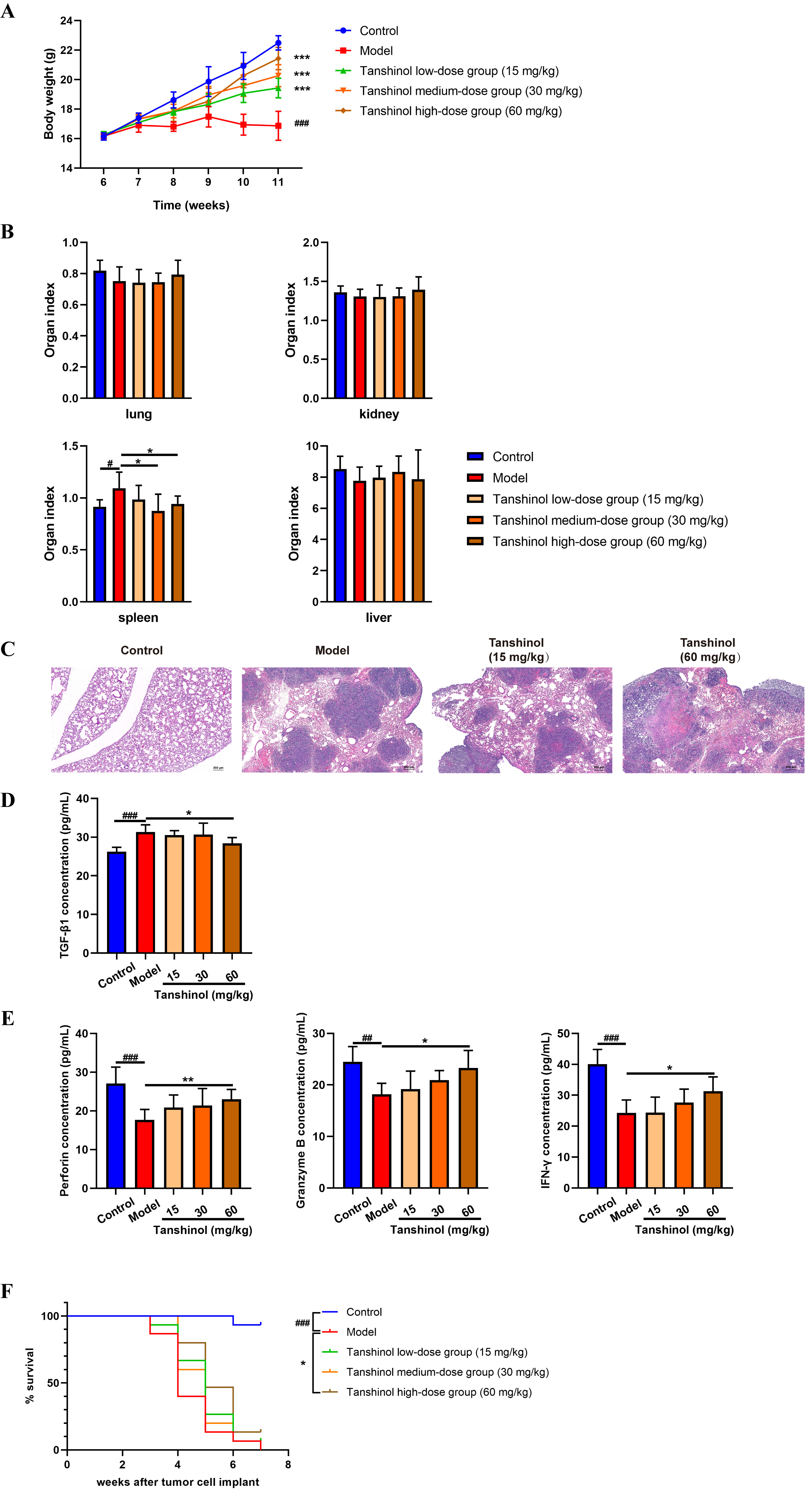 Fig. 1.
Fig. 1.
The effects of Tanshinol on breast cancer metastasis. (A) Body
weight of mice. (B) Organ index. (C) H&E staining of lung tissue sections
(50). (D) Serum TGF-1 content in mice. (E) The levels
of NK cell effector molecules. All data are presented as the means SD, n
= 8. (F) The survival rate of tumor-bearing mice, n = 15. #p 0.05,
##p 0.01, ###p 0.001 (vs. control group).
p 0.05, p 0.01,
p 0.001 (vs. model group).
4.2 Tanshinol reverses TGF-1-meidated inhibition of NK cell
functions in the breast cancer cells
To verify the role of Tanshinol in vitro, we thus evaluated its effects
on the proliferation of ZR-75-1 and NK92MI cells. As shown in Fig. 2A, there were
no significant changes in cell proliferation between control and treated groups.
To further explore the effects of Tanshinol on modulating NK cells and tumor
cells, we used the LDH experiment to detect the killing effect of NK cells on
ZR-75-1 cells. The results showed that TGF-1 could
significantly inhibit the LDH level in the supernatant of the co-culture system
under different target-effect ratios, which indicates that
TGF-1 remarkably inhibits the immune killing effect of NK92MI
cells on the ZR-75-1 cells (Fig. 2B). Together, the above results suggest that
Tanshinol may exert anti-tumor effect by improving the NK cell-mediated immune
killing function.
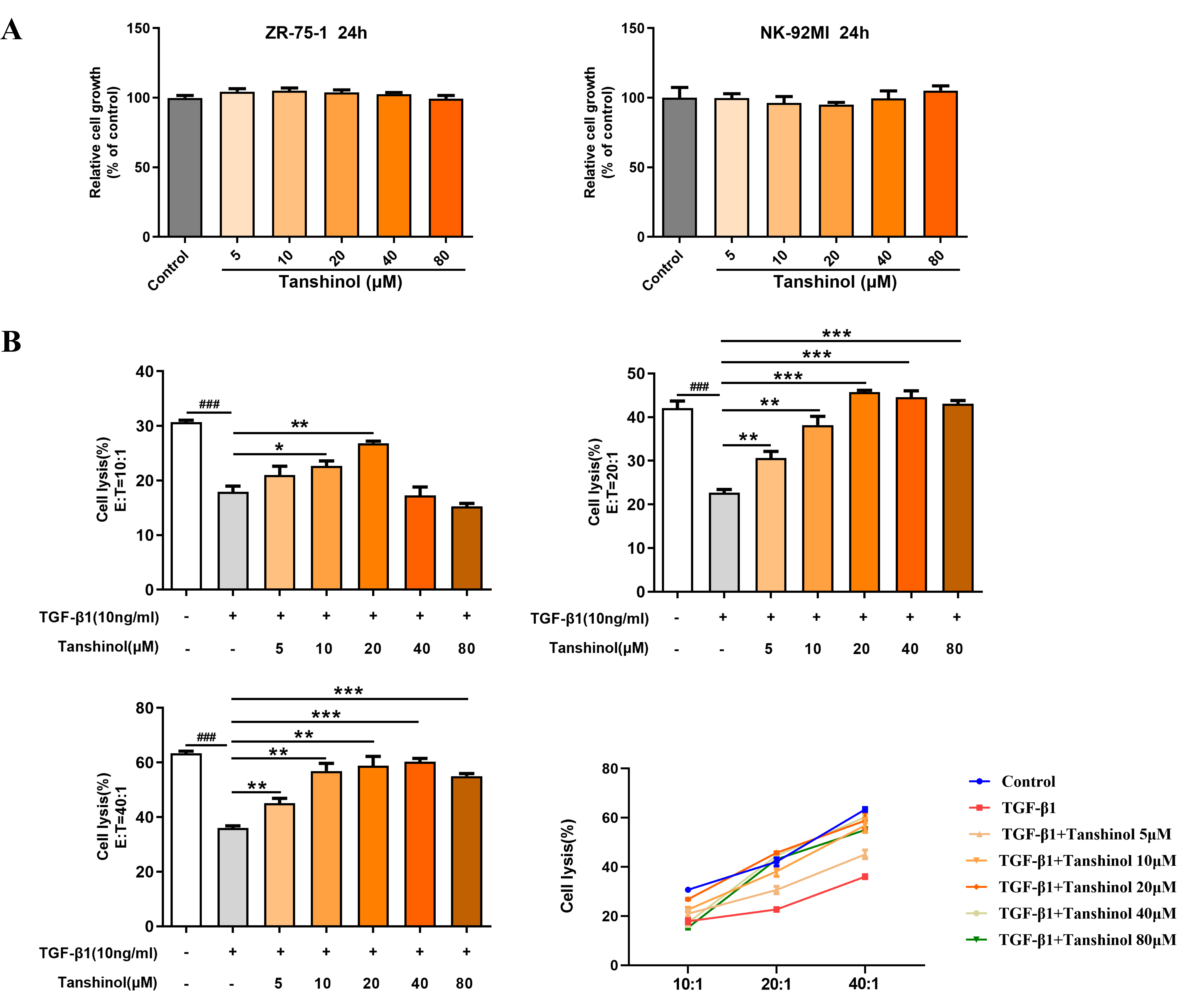 Fig. 2.
Fig. 2.
Effects of Tanshinol on ZR-75-1 and NK92MI cells. (A) The
relative growth rate of ZR-75-1 and NK92MI treated with Tanshinol (n = 6). (B)
The effect of Tanshinol on the percentage of killing tumor cells by NK cells
following the intervention of TGF-1 (n = 3). The data are
presented as the mean SD. #p 0.05, ##p 0.01,
###p 0.001 (vs. control group). p 0.05,
p 0.01, p
0.001 (vs. TGF-1 treated group).
4.3 Tanshinol rescues the inhibitory effect of TGF-1 on
degranulation of NK cells
Since degranulation of NK cells plays a pivotal role in regulating NK cell
functions, we therefore evaluated the effects of TGF-1 and
Tanshinol on influencing the degranulation function of NK cells by detecting the
expression of CD107a on the membrane surface of NK cells using flow cytometry.
Our data revealed that Tanshinol was able to significantly reverse the inhibition
of CD107a expression mediated by TGF-1 and promote the
degranulation function of NK cells (Fig. 3A). Furthermore, we also investigated
the effects of TGF-1 and Tanshinol on modulating
PI3K-ERK1/2-PLC2 signaling pathway, which emerges as a key
cascade of NK cell degranulation. The western blot results showed that
TGF-1 markedly inhibited the phosphorylation of PI3K, ERK1/2
and PLC2, which could be reversed in the presence of
Tanshinol. The activation of PI3K-ERK1/2-PLC2 by Tanshinol
contributed to the degranulation of NK cells and reduced immune escape of tumor
cells (Fig. 3B).
In addition, in order to further explore the effects of TGF-1
and Tanshinol on the synthesis and release of killing mediators during NK cells
degranulation, we thus detected the expression of Perforin, Granzyme B and
IFN-. At the transcriptional level, Tanshinol could
significantly increase the mRNA levels of Perforin and IFN- in
a concentration-dependent manner, but had no significant effect on the expression
of Granzyme B (Fig. 3C). This was also substantiated by the results of
ELISA (Fig. 3D), all of which imply that Tanshinol can prominently reverse the
inhibitory effect of TGF-1 on the synthesis and release of
cytotoxic mediators in the NK cells.
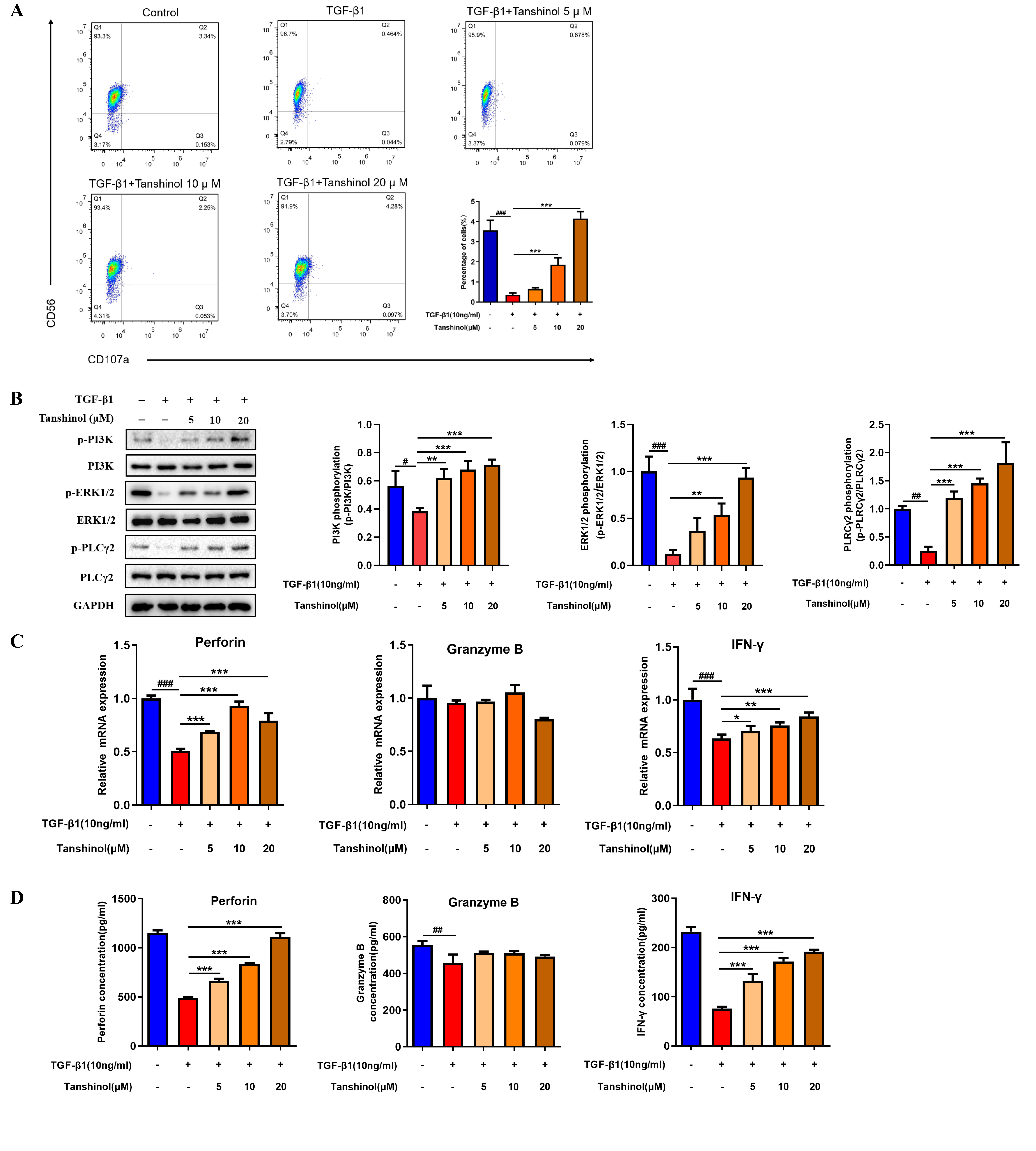 Fig. 3.
Fig. 3.
Tanshinol restores the inhibited activity of NK cells mediated
by TGF-1. (A) The expression of CD107a, a degranulation marker
on the surface of NK92MI cells. (B) The effect of Tanshinol on the
PI3K-ERK1/2-PLC2 signaling pathway that is related to
degranulation following the intervention of TGF-1. (C) The
effects of Tanshinol on Perforin, Granzyme B and IFN- mRNA
levels in NK92MI cells following TGF-1 intervention. (D) The
effects of Tanshinol on the release of Perforin, Granzyme B and
IFN- in NK92MI cells following the intervention of
TGF-1. The data are presented as the mean SD, n = 3.
#p 0.05, ##p 0.01, ###p 0.001 (vs.
control group). p 0.05, p
0.01, p 0.001 (vs.
TGF-1 treated group).
4.4 Tanshinol restores the inhibitory effect of TGF-1 on
NKG2DL-NKG2D signaling axis
It has been well accepted that the activity of NK cells depends on the
interaction of NK cell surface receptors and their associated ligands on target
cells. NKG2DLs expressed on the surface of tumor cells can bind to activated
receptor NKG2D on the surface of NK cells, thereby activating NK cells to exert
anti-tumor killing effects [13, 14, 15]. As shown in Fig. 4A, TGF-1
declined the expression of NKG2DLs, but Tanshinol alleviated the inhibitory
effects of TGF-1 on the expression levels of MICA, MICB and
ULBP2. Besides, ULBP1 was not significantly affected by TGF-1
and Tanshinol (Fig. 4A). All of these could be validated by the
immunofluorescence results (Fig. 4B), indicating that Tanshinol can reverse the
repression of NKG2DL expression mediated by TGF-1 on the
surface of tumor cells.
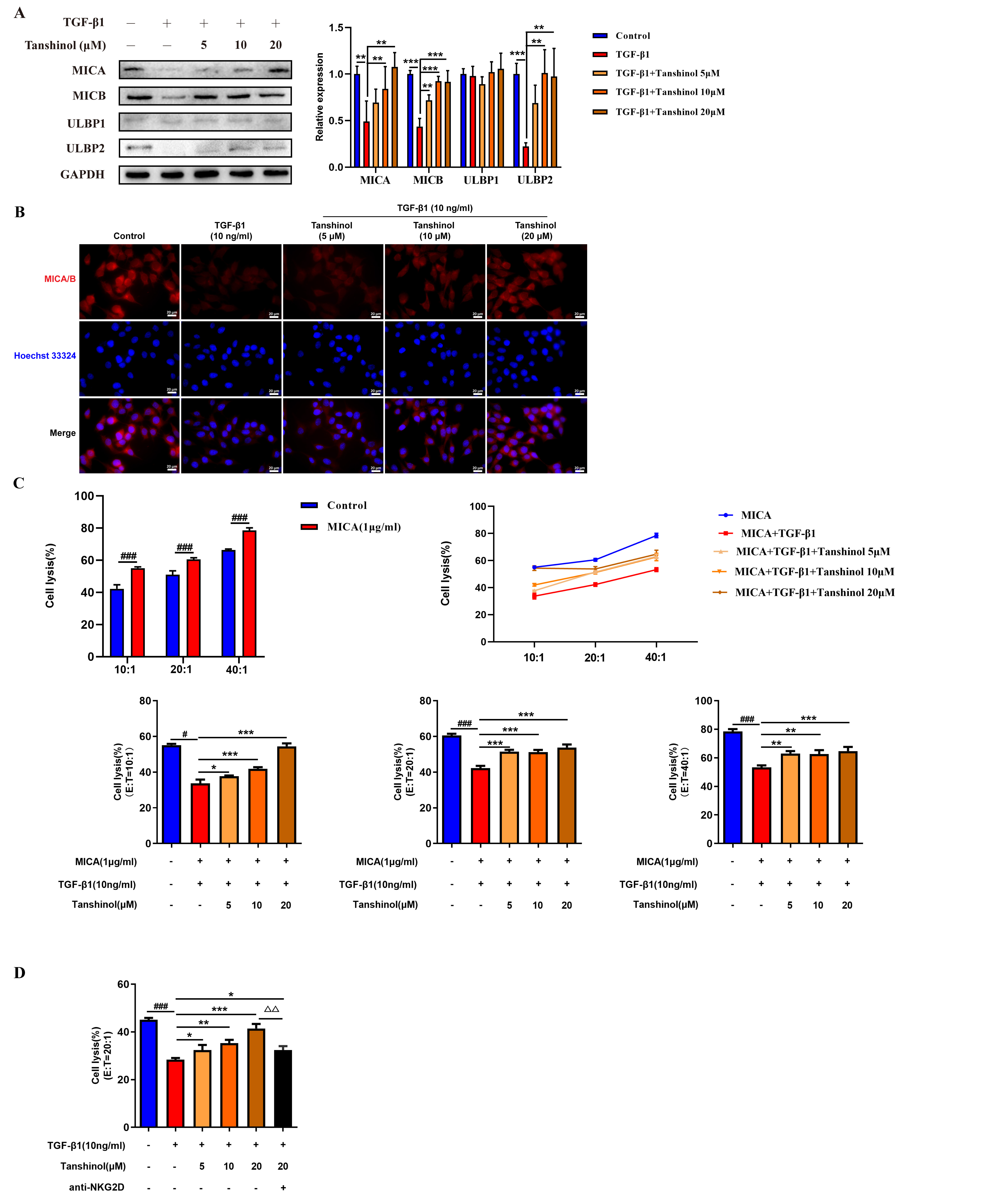 Fig. 4.
Fig. 4.
Tanshinol activates the NKG2D-NKG2DLs signaling axis and
reverses the effect of TGF-1. (A) The effect of Tanshinol on
the inhibition of NKG2DL protein expression by TGF-1. (B) The
effect of Tanshinol on the inhibition of MICA/B protein expression induced by
TGF-1 (400). (C) The activation of NK92MI by
exogenous MICA and the intervention of Tanshinol on TGF-1
inhibiting the activation of NK92MI cells by exogenous MICA. (D) Tanshinol
reverses the TGF-1-induced immunosuppression after neutralizing
NKG2D. The data are presented as the mean SD, n = 3. #p
0.05, ##p 0.01, ###p 0.001 (vs. control group).
p 0.05, p 0.01,
p 0.001 (vs. TGF-1
treated group). p 0.01 (NKG2D
neutralizing antibody vs. without antibody).
Interestingly, after exogenous administration of human MICA recombinant protein
(1 g/mL), the killing effects of NK92MI cells under different effect
target ratios were significantly enhanced, in comparison to the control group
(Fig. 4C). However, the killing effect of MICA-mediated NK92MI cells was
significantly inhibited following the stimulation of TGF-1,
which could be reversed following the treatment of Tanshinol (Fig. 4C). Further,
the restoration of NK cell killing effect disappeared following the utilization
of NKG2D neutralizing antibody to inhibit the expression of NKG2D on the surface
of NK cells, indicating that the alleviation of TGF-1-mediated
immunosuppression of NK cells by Tanshinol is closely associated with the
activation of functional receptor NKG2D (Fig. 4D). Based on the above results, it
can be concluded that Tanshinol may improve the immune killing function of NK
cells by rescuing the inhibitory effect of TGF-1 on the
NKG2D-NKG2DL signaling axis.
4.5 Tanshinol abolishes TGF-1-mediated upregulation of
p-smad2/3 level and its translocation into the nucleus, increasing the expression
of NKG2D
Numerous studies have reported that TGF-1 can promote the
phosphorylation of the key transcription factor smad2/3, which results in the
dramatic elevation of intracellular p-smad2/3 level, and in turn negatively
regulates the expression of functional receptors in NK cells [16, 17]. As shown
in Fig. 5A, the expression of p-smad2/3 was upregulated in a time-dependent
manner following the stimulation of TGF-1 (5 min, 15 min, 30
min and 60 min) in the NK92MI cells and reached the peak at 30 min compared with
those without TGF-1 treatment. However, after the intervention
of Tanshinol, the elevation of p-smad2 mediated by TGF-1 was
rescued at 15 min, 30 min and 60 min, whereas the expression levels of p-smad3
and smad2/3 remained unchanged. Furthermore, it was observed that the
fluorescence intensity of p-smad2/3 in the nucleus of NK92MI cells treated with
TGF-1 for 30 min was significantly enhanced, indicating
TGF-1 plays an essential role in promoting the nucleus
translocation of p-smad2/3. Nevertheless, it was shown that Tanshinol could
inhibit the entry of p-smad2/3 into the nucleus (Fig. 5B). These results suggest
that Tanshinol can reverse the upregulation of p-smad2/3 level and inhibit its
translocation into the nucleus mediated by TGF-1, which may be
one of the underlying mechanisms that Tanshinol modulates the expression of key
functional receptors on the surface of NK cells.
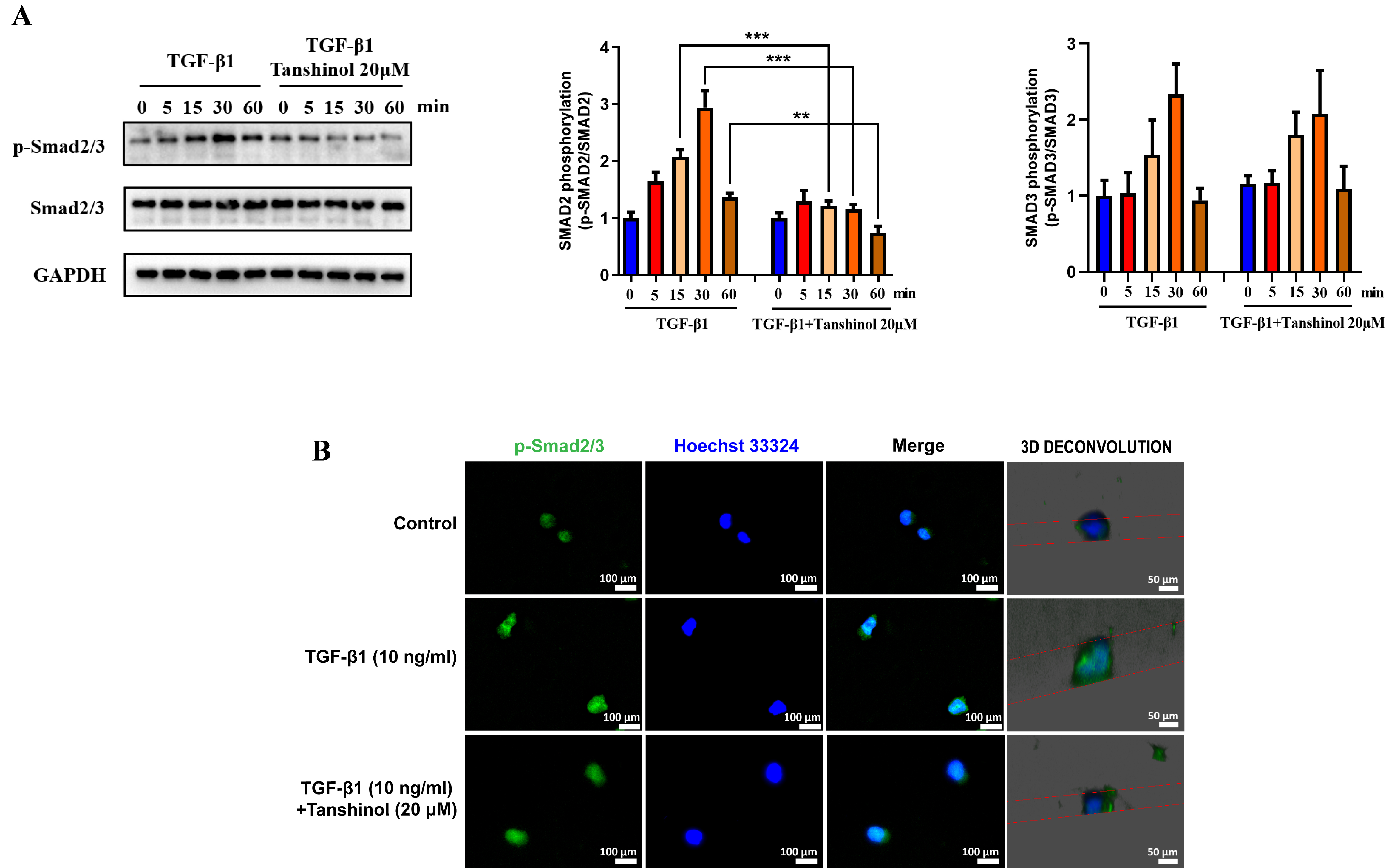 Fig. 5.
Fig. 5.
Tanshinol reverses TGF-1-mediated increased
p-smad2/3 expression and translocation into the nucleus. (A) The effect of
Tanshinol on the increased p-smad2/3 and smad2/3 protein expression mediated by
TGF-1 in the NK92MI cells. (B) Immunofluorescence images
(400) of Tanshinol affecting TGF-1-mediated p-smad2/3
nuclear translocation. The data are presented as the mean SD, n = 3.
p 0.05, p 0.01,
p 0.001.
4.6 Tanshinol antagonizes the intervention effect of TGF-1
on the formation of NKG2D-DAP10 complex
It has been known that the binding of NKG2DL to NKG2D on the tumor surface can
trigger the coupling of NKG2D with the transporter DNAX activating protein
(DAP10) to form an immune recognition receptor complex NKG2D-DAP10, which is the
central link in mediating the release of immune killing mediators from NK cells
[18, 19, 20]. Hence, we detected the expression of NKG2D and DAP10 by western blot and
immunofluorescence assays, and found that TGF-1 mitigated the
expression levels of NKG2D and DAP10, while Tanshinol could interfere with this
coupling process and restore the expression of NKG2D and DAP10 (Fig. 6A–D). The
formation of NKG2D-DAP10 complex on the surface of NK cells is the key step to
initiate the activation of NK cells. Therefore, we further investigated the
binding of NKG2D to DAP10 in NK cells by co-immunoprecipitation. It was observed
that TGF-1 could significantly promote the dissociation of
NKG2D and DAP10, but Tanshinol counteracted this process in a
concentration-dependent manner and strengthen the binding of NKG2D and DAP10 (Fig. 6E). In order to further verify the key role of Tanshinol in the formation of
NKG2D-DAP10 complex, we visualized the co-localization of NKG2D and DAP10 by
immunofluorescence, the results of which were consistent with those of
co-immunoprecipitation (Fig. 6F). Together, these data suggest that Tanshinol can
promote the interaction and binding capability of NKG2D and DAP10, thus
reconciling the inhibitory effect of TGF-1 on the formation of
NKG2D-DAP10 complex, which may be the potential driving force of Tanshinol
promoting the cytotoxicity of NK cells.
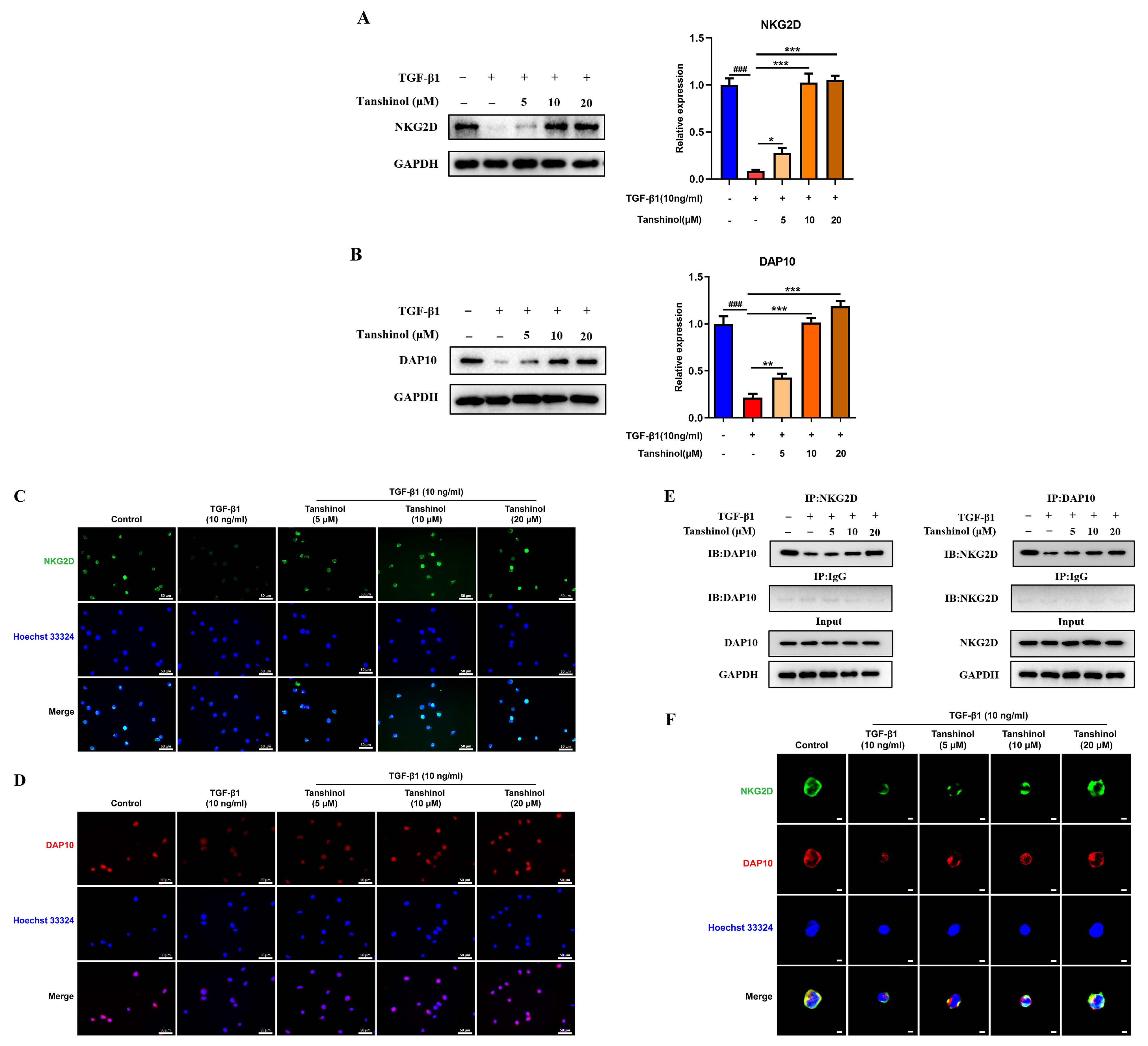 Fig. 6.
Fig. 6.
Effects of TGF-1 and Tanshinol on the
formation of NKG2D-DAP10 complex. (A) Tanshinol promoted the expression of NKG2D
protein following the intervention of TGF-1. (B) The effect of
Tanshinol on the expression level of DAP10 protein interfered by
TGF-1. (C) Fluorescence images (400) of NKG2D protein
expression level following the treatment of Tanshinol in the presence of
TGF-1. (D) Fluorescence images of Tanshinol reversing the
inhibition of DAP10 protein expression by TGF-1
(400). (E) The effect of Tanshinol on the binding ability of NKG2D and
DAP10 following the intervention of TGF-1. (F) Fluorescence
images (400) of Tanshinol on the co-localization of NKG2D-DAP10
following the intervention of TGF-1. The data are presented as
the mean SD, n = 3. #p 0.05, ##p 0.01,
###p 0.001 (vs. control group). p 0.05,
p 0.01, p
0.001 (vs. TGF-1 treated group).
5. Discussion
The International Agency for Research on Cancer (IARC) released the latest
global data on cancer burden in 2020, which indicated that breast cancer overtook
lung cancer as the leading cause of cancer-associated death worldwide.
Traditional Chinese medicine has a long history of effectively treating breast
cancer, in which Salvia miltiorrhiza plays an important role [21, 22]. Tanshinol
is one of the main active components of Salvia miltiorrhiza, but its anti-tumor
mechanism is not comprehensive. In this study, we robustly verified the role of
Tanshinol in tumor immune escape from the perspective of immunity and outlined
the potential mechanisms, which can be used as a basis supplement for Salvia
miltiorrhiza in treating breast cancer.
As the main activating receptor of NK cells, NKG2D can trigger the coupling with
the adaptor protein DAP10 after binding to various ligands on the surface of
tumor cells, thus forming an immune recognition receptor complex NKG2D-DAP10. The
complex then initiates the internalization mediated by DAP10 ubiquitin, which
propels it to translocate from the cell membrane to the cytoplasm. This suggests
that NKG2D-DAP10 endocytosis is a main route to hamper its abundance on the cell
surface and regulate the signaling transduction of NK cells and other cytotoxic
lymphocytes to kill tumor cells [23]. The recognition and binding of NKG2D and
NKG2DLs acts as a vital step in activating NK cells. In the present study, we
demonstrated that TGF-1 could interfere with NKG2D-NKG2DL
signaling axis and inhibit the expression of NKG2D and its related ligands such
as MICA/B and ULBP2, while Tanshinol was prone to overcome the immunosuppressive
effect induced by TGF-1. The formation and endocytosis of
NKG2D-DAP10 active complexes are critical in activating the downstream signaling
pathway for NK cell degranulation to exert anti-tumor activity. During the
hematogenous metastasis of tumor cells, activated platelets release a large
amount of immunosuppressive factor TGF-1. It has been
illustrated that TGF-1 can significantly downregulate the
expression levels of NKG2D and DAP10 on NK cell membranes, and inhibit the
synthesis and secretion of tumor-killing mediators from NK cells [24, 25, 26]. Our
results revealed that Tanshinol attenuated the immunosuppressive effect mediated
by TGF-1 in the NK cells, which was responsible for restoring
the expression of NKG2D and DAP10 and promoting the formation of NKG2D-DAP10
complex.
It has been widely held that TGF-/SMAD signaling pathway plays
an important role in the activation of NK cells. TGF-1 can
boost the phosphorylation of SMAD2/3, inhibit the expression of NKG2D, and
restore the cytolytic ability of NK cells [27]. Our data demonstrated that the
decreased expression of NKG2D by TGF-1 was ascribed to the
augmented phosphorylation and nuclear translocation of SMAD2/3, whereas Tanshinol
could interfere with the activation of SMAD2/3 triggered by
TGF-1, thus restoring the expression level of NKG2D. It has
been accepted that NK cells tend to promote the degranulation of NK cells when
they interact with target cells. Perforin derived from NK cells can cleave the
target cells, and granzyme can activate the apoptosis pathway of target cells
[28, 29]. Additionally, IFN- released by NK cells can also
activate macrophages, promote the synchronous activation of other immune cells,
and promote the expression of major histocompatibility complex (MHC) and antigen
presentation [30]. Our in vivo experimental results showed that
Tanshinol prominently inhibited the lung metastasis of tumor-bearing mice,
diminished the level of TGF-1 and increased the content of
effector molecules in the serum. Moreover, the in vitro results revealed
that Tanshinol could activate PI3K-ERK1/2-PLC2 signaling
cascade that is involved in NK cell degranulation. The synthesis and secretion of
Perforin and IFN- interfered by TGF-1 could
be restored though those of granzyme B remained unchanged in the presence of
Tanshinol.
 Fig. 1.
Fig. 1. Fig. 2.
Fig. 2. Fig. 3.
Fig. 3. Fig. 4.
Fig. 4. Fig. 5.
Fig. 5. Fig. 6.
Fig. 6.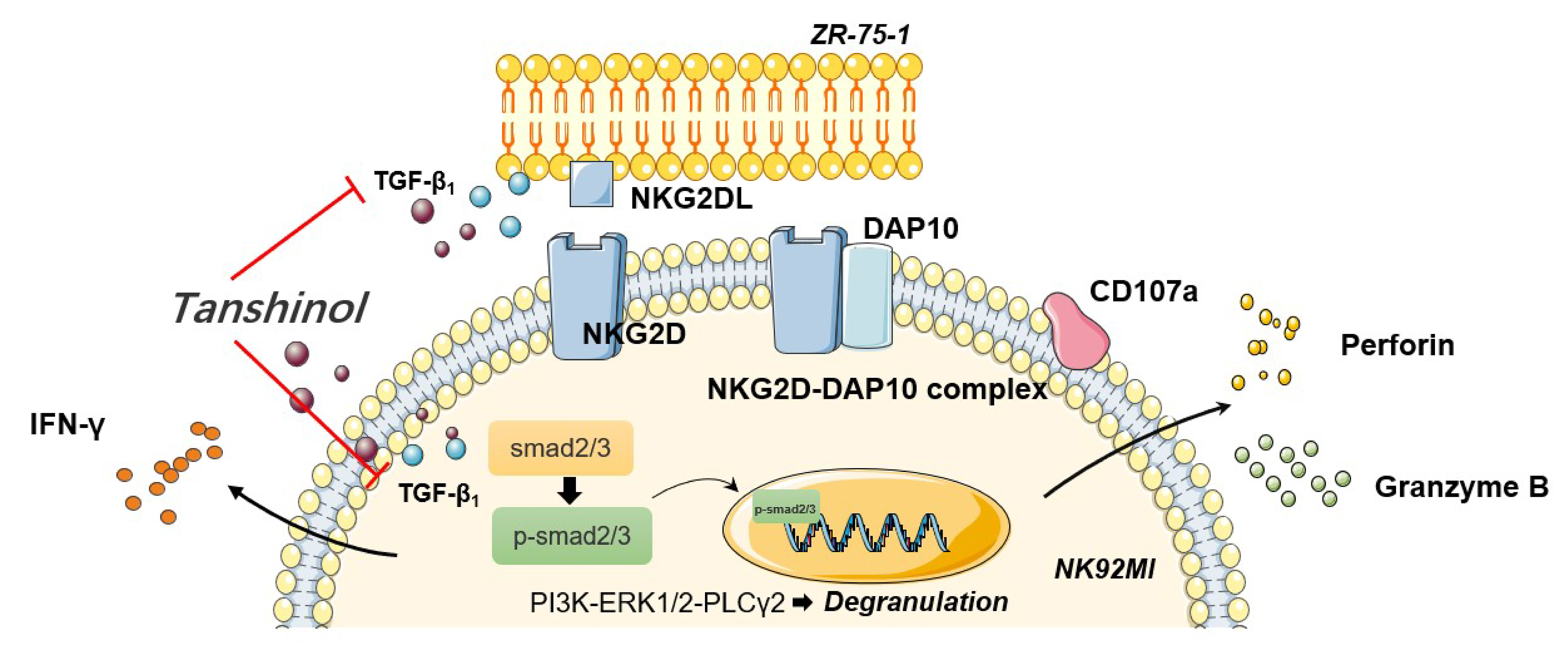 Fig. 7.
Fig. 7.






