Frontiers in Bioscience-Landmark (FBL) is published by IMR Press from Volume 26 Issue 5 (2021). Previous articles were published by another publisher on a subscription basis, and they are hosted by IMR Press on imrpress.com as a courtesy and upon agreement with Frontiers in Bioscience.
1 Department of Physiology and Biophysics, Dalhousie University, Halifax, Nova Scotia, Canada
2 Departments of Pathology, Microbiology and Immunology, and Surgery, Dalhousie University, Halifax, Nova Scotia, Canada
Abstract
Because of their highly reactive nature and potentially toxic characteristic, reactive oxygen species (ROS) have had a bad reputation for years. However, under certain conditions, ROS generation has shown positive outcomes. It is ROS imbalance that causes toxic effects. ROS play an important role in physiological processes such as cell signaling, senescence, inflammation, and the immune response to infection. An increasing number of studies highlight the importance of ROS for the inflammatory response, whether sterile or due to infection or cancer. The purpose of this paper is to present evidence of the essential role of ROS in the inflammatory response.
Keywords
- Reactive Oxygen Species
- Inflammation
- Infection
- Cancer
- Review
Free radicals are unstable and highly reactive atoms or molecules due to unpaired electrons in their outermost shell. In order to pair these electrons and become stable, free radicals tend to "attack" the nearest stable molecules by redox reactions. Because most reactive oxygen species (ROS) are free radicals, these two terms are often used in an interchangeable fashion. However, free radicals also include reactive nitrogen species (e.g., nitric oxide - NO·), reactive lipid species (e.g., lipid alkoxyl - LO·, lipid peroxyl - LOO·). In addition, ROS include other molecules such as hydrogen peroxide (H2O2) that are not free radicals. This review will focus on ROS.
ROS are naturally produced in aerobic species, in which mitochondria are the main source of free radicals. ROS are produced during cellular respiration, a vital function that generates energy for the metabolism of the cells. The process starts in the cytoplasm with the conversion of glucose into pyruvate, which is then oxidized in the mitochondrial matrix and will produce nicotinamide adenine dinucleotide (NADH, electron transporter), flavin adenine dinucleotide (FADH2, electron transporter) and adenosine triphosphate (ATP, energy). The NADH and FADH2 previously produced, and the electrons that they transport, move to the electron transport chain located in the mitochondrial inner membrane. The electron transport chain is formed by four complexes: complex I or NADH dehydrogenase, complex II or succinate-ubiquinone oxidoreductase, complex III or cytochrome c reductase, and complex IV or cytochrome c oxidase (1). The electrons are transmitted to these 4 complexes which eject protons, leading to a membrane potential across the mitochondria that triggers ATP synthesis by ATP-synthase. During electron transport, complexes I and III generate O2·−. The produced O2·− can then be converted: first into H2O2 by superoxide dismutase 2 (SOD2); second, O2·− leads to the highly reactive HO· (hydroxyl radical) through the Fenton reaction in the presence of free iron; and third, the O2·− can also react with NO· to form the highly reactive peroxynitrite (ONOO·) molecule. Mitochondrial ROS is released as a result of oxidative stress-induced opening of the mitochondrial permeability transition pore (MPTP). ROS can also be generated by other sources, including the endoplasmic reticulum, peroxisome, prostaglandin synthesis and cytochrome P450 (2). ROS, which are very unstable and hyper-reactive molecules, are produced continuously in living beings; hence, to maintain redox homeostasis and avoid undesirable damaging effects, ROS levels are controlled by antioxidants (e.g., superoxide dismutase, glutathione and catalases; vitamins A, E and C).
For a very long time, ROS were considered to be detrimental, dangerous, toxic or even deadly molecules. Therefore, the goal of many studies was to identify strategies to reduce the production of cellular ROS and/or increase the level of antioxidants within cells (3–7). However, it is increasingly apparent that ROS are important signaling molecules, as well as mediators of inflammation. ROS play a critical role in physiological processes such as cell growth and signaling, senescence, differentiation, inflammation, apoptosis, and the immune response to infection (1, 8). During inflammation, ROS production increases to participate in the resolution of inflammation and promotion of wound healing. This increase is the result of an incompletely known process involving alteration of iron homeostasis, stimulation of the nuclear factor-kappa B transcription factor (NF-κB) and the reduction of certain antioxidants. The purpose of this review is to highlight how essential ROS are throughout the inflammatory response. We will focus on the role of ROS in clearance of damaged or infected cells, and cancer development.
ROS have a beneficial role in sterile inflammation (i.e., inflammation without pathogens), which is triggered by cellular damage under conditions such as ischemia-reperfusion injury, exposure to toxins, and arthritis (9, 10). During sterile inflammation, ROS are involved in the clearance of damaged cells, as well as in the differentiation of macrophages (11–13).
To maintain homeostasis, damaged, stressed or dying cells trigger specific mechanisms that result in their elimination. In the absence of those mechanisms, cell death occurs via necrosis, while in the presence of regulation cell death is described as apoptosis or autophagy (14, 15). Dying cells express molecules that act as danger signals in order to attract phagocytic cells (e.g. macrophages) that phagocytose and eliminate the cell. This process happens in 3 main stages (16): stage 1, expression by dying cells of “find me” signals that attract macrophages; stage 2, expression of “eat me” signals that stimulate macrophages to engulf the cell, and the disintegration of “do not eat me” signals that would otherwise prevent macrophages from attacking host cells; finally, stage 3, the engulfment of damaged or dying cells by the macrophage. ROS play an important role in all of these stages. Dying cells expose their components to the extracellular environment as a damaging-associated molecular pattern (DAMPs) in order to activate macrophages. DAMPs are cellular components - such as high-mobility group box 1 (HMGB1), mitochondrial DNA, ATP, uric acid, and many others - that are externalized (17), and recognized by pattern recognition receptors (PRRs) of phagocytic cells. Pattern recognition receptors include Toll-like receptors (TLRs), NOD receptors (NLRs) and RIG-I receptors (RLRs) (18). Despite the fact that the relationship between ROS and DAMPs remains incompletely understood, it seems that ROS play a key role in this stage since DAMPs must be oxidized prior to being externalized as danger signals. Studies by Chang et al. (19, 20) corroborated the idea by using mouse monoclonal antibodies to block oxidized low-density lipoprotein (OxLDL), a molecule that competes with DAMPs to bind to macrophages. Chang et al. found that these antibodies bind efficiently to OxLDL, but also bind to DAMPs of apoptotic cells and prevent phagocytosis of target cells by peritoneal macrophages. The results of these studies strongly suggest that DAMPs can be oxidized (21, 22). Studies show the importance of ROS for the recognition and the engulfment of damaged cells (12, 23). The last step in the clearance of damaged cells is phagocytosis. ROS are essential for dying cells (apoptotic and necrotic) that need to be phagocytized. After engulfing damaged cells, macrophages initiate a respiratory burst, i.e., the quick increase in intracellular ROS that gradually degrades damaged cells (Figure 1). The process begins by an increase in oxygen (O2) uptake by phagocytes. Then a considerable amount of ROS is produced by the conversion of O2 to superoxide followed by the conversion of superoxide into hydrogen peroxide, respectively, by the enzymes NADPH oxidase and superoxide dismutase. Hydrogen peroxide and superoxide damage the engulfed molecules but mainly participate in the production of a third molecule called hypochlorite (HOCl) by the effect of the myeloperoxidase on hydrogen peroxide (24). ROS are also critical to trigger autophagy or "self-eating", which is a mechanism by which a cell engulfs and breaks down its own components in the lysosome and recycles them for new biosynthesis (23).
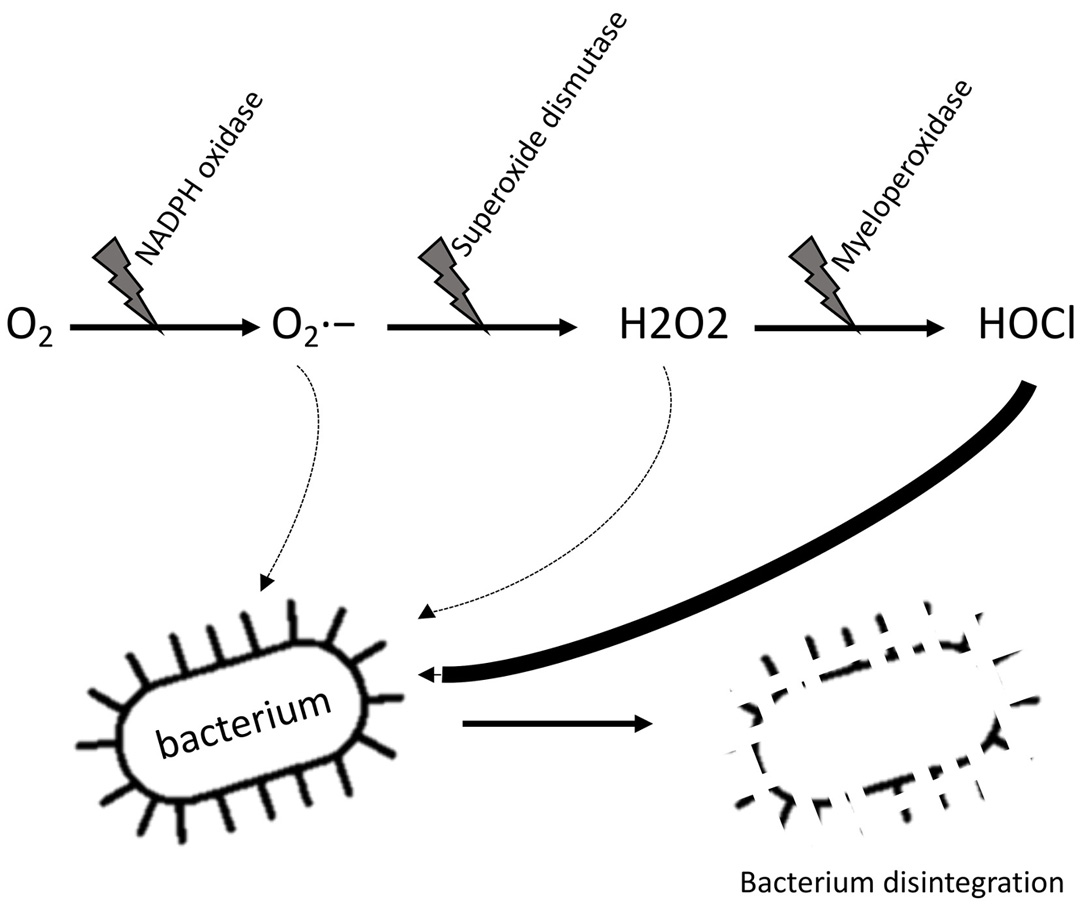 Figure 1
Figure 1. Respiratory burst in phagocytic cells. Phagocytic cells (neutrophils, macrophages, etc.) are able to initiate respiratory burst to gradually degrade engulfed elements such as bacteria or damaged cells. The process starts by an increase in oxygen (O2) uptake by phagocytes. The oxygen is converted in superoxide (O2·−) by the enzyme NADPH oxidase. Some superoxide can already damage the engulfed particle but undergo another reaction by the superoxide dismutase to produce hydrogen peroxide (H2O2). This last molecule is converted in hypochlorite (HOCl) by the myeloperoxidase.
As previously mentioned, phagocytic cells such as macrophages are important in inflammation. Two types of macrophages have been described. The first type are the classical activated macrophages, designated M1 macrophages. They have a strong microbicidal function and are able to secrete large amounts of proinflammatory molecules. The second type of macrophages are the alternative activated macrophages, designated M2 macrophages, that are involved in wound healing and tissue repair, and produce high levels of anti-inflammatory mediators (25). The process by which macrophage differentiation occurs is complex and not yet fully understood; nevertheless, studies have shown that ROS are instrumental in this process (10, 12, 26, 27). Zhang et al. found that ROS are produced during macrophage differentiation and that the use of butylated hydroxyanisole or other ROS inhibitors blocks the process (26). It seems that a "high level" of ROS leads to the differentiation of M2 macrophages while a “low level” of ROS results in M1 macrophages since only the development of M2 macrophages is compromised when ROS are inhibited.
In summary, ROS play a critical role in the immune response to sterile inflammation by participating in the differentiation of macrophages, promoting macrophage attraction by damaged cells, and contributing to the phagocytic process by aiding in the degradation of engulfed cells. Blocking or inhibiting ROS compromises these natural processes. ROS are also useful during the inflammatory response to foreign agents (such as bacteria, viruses, fungi, and parasites,) that are not normally present within the body.
The process by which the body eliminates foreign invaders and the cells that these invaders have infected is similar to sterile inflammation, in that ROS play a critical role in this process as they actively participate in the elimination of pathogens (28). When pathogenic microbes invade the body, they activate vascular endothelial cells, which then produce chemoattractants and express adhesion molecules to promote recruitment and migration of leukocytes to the site of infection. Pathogens possess specific molecular motifs called pathogen-associated molecular pattern (PAMPs) that are recognized by the PRRs of phagocytes (29). The eradication of pathogens by phagocytosis in a fashion similar to the one described in sterile inflammation then occurs, in which pathogens are engulfed by phagocytes and the subsequent respiratory burst leads to their disintegration. The detailed process by which ROS seems to lead to the death of pathogens is still not fully understood, yet a study suggest that ROS can damage pathogens by reacting directly or indirectly with key components of microbes (30). ROS can alter the DNA, RNA, proteins and lipids of pathogens or change the microenvironment surrounding the foreign invader in order to stimulate enzymes called proteases that can break down proteins (31).
Another mechanism by which ROS cause the death of microbes is NETosis. This is the ultimate defense system of neutrophils. When the number of pathogens is very high and/or pathogens are very large and therefore difficult to phagocytose, neutrophils are able to condense their chromatin and eject their DNA and antimicrobial agents in the direction of pathogens. These structures are called neutrophil extracellular traps (NETs) and the process by which they are produced is called NETosis (32, 33). The purpose of the NETosis is to provide elevated concentration of antimicrobial components and also physically snare pathogens. The entire process begins with the recognition of PAMPs by neutrophil PPRs, followed by activation of a Raf / MEK / ERK kinase pathway responsible for activation of the NADPH complex and production of ROS that seems to be the leading cause of chromatin condensation, as well as neutrophil plasma membrane degradation followed by extracellular DNA release with antimicrobial agents to trap microbes (34). However, in situations where the immune system is overactivated and the cellular respiration impaired, the redox equilibrium is broken, causing a considerable rise in the levels of intracellular ROS. Such a situation may result in damage to the host, including the death of surrounding healthy cells, damage to the vascular system, multiorgan dysfunction and even death (35–37).
It has been suggested that excessive ROS produced by immune cells during inflammation may lead to genetic mutations that can trigger carcinogenesis (38). The relationship between ROS and cancer cells is ambiguous; whereas, certain levels of ROS seem to promote carcinogenesis, high ROS levels can also eradicate cancer cells by triggering death pathways (39, 40). Different types of cancers (e.g., breast, pancreas, bladder, colon) generate ROS to promote growth, angiogenesis, chemoresistance and the ability to invade other organs or tissues (41). At the same time, cancer cells also express high concentrations of antioxidants to prevent triggering death pathways, i.e., necrosis, apoptosis or autophagy (Figure 2). Therefore, targeting the balance that exists between ROS production and antioxidant production represents a potential target for cancer treatment.
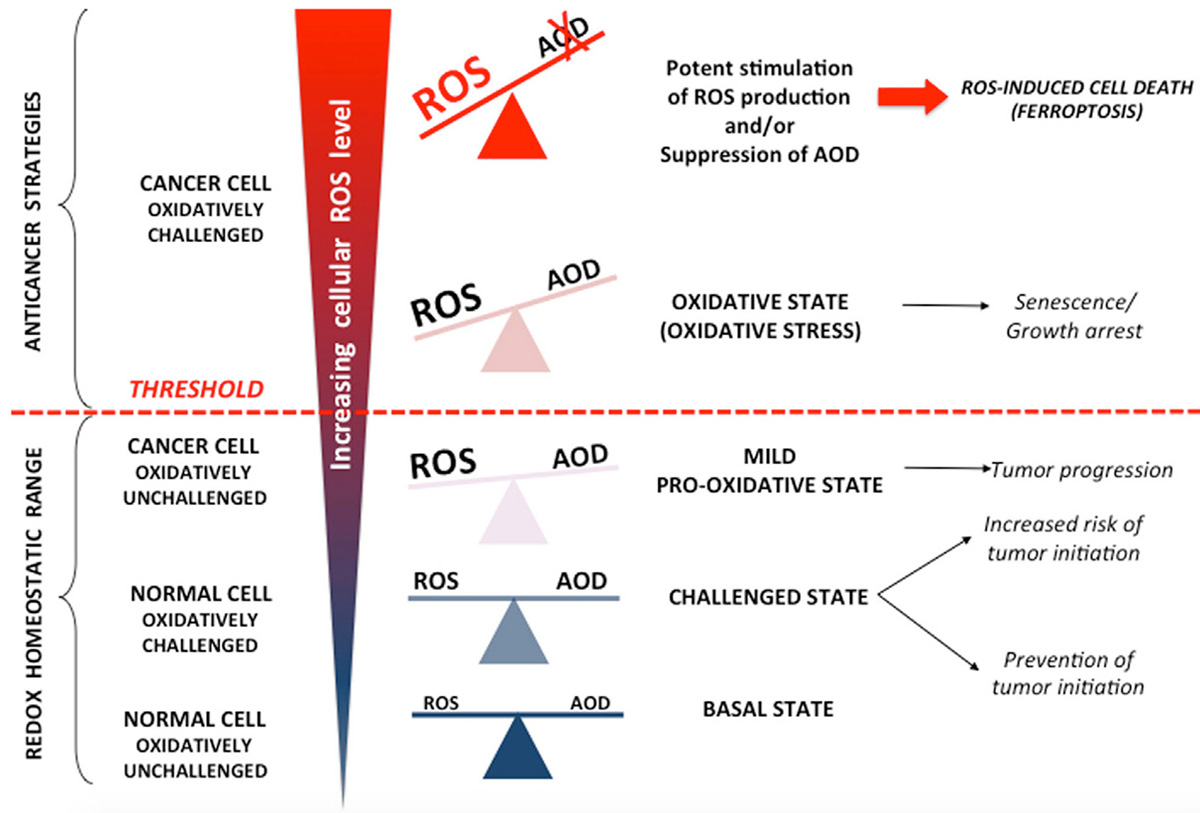 Figure 2
Figure 2(40): Cellular effect of ROS increase and antioxidant balance. A balance between ROS (reactive oxygen species) and antioxidant defense (AOD) is very important for healthy cells. An increase in oxidative pressure engenders upregulation of antioxidant to prevent cancerogenesis. An imbalance between ROS and antioxidants can promote the development and survival of cancer. Cancer cells tip the scales in favor of the ROS to maintain their increased metabolic activity. At the same time, to prevent ROS from reaching threshold that trigger cell death mechanisms, cancer cells increase antioxidant levels. When ROS level increase further (physiologically or therapeutically for example), the growth of cancer cells slow down then death pathways like necrosis, apoptosis or autophagy are trigger.
Upadhyaya et al. showed that the nontoxic dietetic component phenethyl isothiocyanate (PEITC) promotes the reduction of the antioxidant glutathione, leading to suppression of proliferation and early apoptosis of cancer cells due to a rise in intracellular ROS levels (42). Another study by Chen et al. suggested that the ROS inducer EF24 improves the efficacy of anti-neoplastic chemotherapy with rapamycin in human gastric cancer cells in vitro and in mouse models of cancer in vivo (43). Other studies have shown similar anti-neoplastic effects of PEITC and EF24 on different types of cancer cells (44–47).
Whereas strategies to induce excessive ROS production seem to be promising overall as a therapeutic approach against cancer, the potential benefits of ROS inhibition in carcinogenesis are controversial and less well studied. On one hand, molecules with antioxidative properties such as vitamin C and N-acetylcysteine show inhibitory effects on cancer progression, but on the other hand, these same molecules plus vitamin E and beta-carotene increase the rate of cancer development under certain conditions (48). The effect of antioxidants appears to be dependent on the stage of carcinogenesis (49). Early during the carcinogenesis, antioxidant expression by cancer cells is reduced because high levels of ROS are required to induce and maintain genetic mutations. Later on, cancer cells produce high levels of antioxidants in order to prevent intracellular ROS from reaching a cytotoxic level (40). Therefore, administration of antioxidants in the early stages of cancer development should most likely be protective whereas at a later stage, antioxidant could promote cancer progression.
There is an increasing body of evidence that ROS are beneficial under certain pathological conditions. During inflammation, ROS participate in cell signaling and the elimination of damaged or infected cells. ROS oxidize PAMPs and DAMPs to allow the phagocytes to recognize and eliminate these cells. ROS also regulate the process of phagocytosis, from the engulfment to the digestion of foreign particles and pathogens. The role of ROS in cancer is complex. On one hand, ROS can promote carcinogenesis but, on the other hand, ROS are promising targets for cancer treatment. The duality of ROS-related harm and benefits highlights the importance of maintaining homeostatic ROS production by eukaryotic cells.
This work received no external funding. The authors declare no conflict of interest.
ATP
adenosine triphosphate
damaging-associated molecular pattern
flavin adenine dinucleotide 2
hydrogen peroxide
high-mobility group box 1
hypochlorite
lipid alkoxyl
lipid peroxyl
mitochondrial permeability transition pore
nicotinamide adenine dinucleotide
nuclear factor-kappa B transcription factor
NOD like receptors
nitric oxide
peroxynitrite
oxidized low-density lipoprotein
pathogen-associated molecular pattern
phenethyl isothiocyanate
pattern recognition receptors
RIG-I like receptors
reactive oxygen species
superoxide dismutase 2
Toll-like receptors


