Frontiers in Bioscience-Landmark (FBL) is published by IMR Press from Volume 26 Issue 5 (2021). Previous articles were published by another publisher on a subscription basis, and they are hosted by IMR Press on imrpress.com as a courtesy and upon agreement with Frontiers in Bioscience.
1 Department of Pharmacy, Jing’ an District Centre Hospital of Shanghai (Huashan Hospital Fudan University Jing’ An Branch), Shanghaii, 200040, China
2 Department of Anesthesiology, Ruijin Hospital North, Shanghai Jiaotong University School of Medicine, Shanghai, 201821, China
Abstract
Diabetic nephropathy (DN) is a major cause of chronic kidney disease characterized by insulin resistance and lipid deposition in tissues. To this end, we examined the effect of Resveratrol (RES) in streptozotocin (STZ) induced diabetic nephropathy. RES, in a dose dependent manner, decreased the insulin resistance, and improved kidney function and lipid metabolism in STZ treated rats. RES treatment increased p-AMPKα/AMPKα and p-ULK1 S777/ULK1 and the autophagy related proteins (Beclin1, LC3 II/I) and its effects on TC and improvement in insulin resistence were quenched by the inhibitor of autophagy, 3-MA. Together, these results suggest that the effect of RES in treatment of DN may involve AMPKα/mTOR-mediated autophagy.
Keywords
- Resveratrol
- lipid metabolism
- insulin resistance
- diabetic nephropathy
- autophagy
Diabetic nephropathy (DN) is one of the most common microvascular complications of diabetes mellitus and is also the leading cause of end-stage renal diseases (1, 2). Current therapies of DN include glycemic control, blood pressure control and a low-protein diet, but the established complications remain unmanageable (3). Considering that DN has brought heavy burden to both patients and government, it is important to carry out studies on the prevention and treatment of DN.
Dyslipidemia in patients with type 2 diabetes is a reversible risk factor for the progression of kidney disease (4). In uncontrolled type 2 diabetes mellitus, elevated serum lipid profiles, such as total cholesterol (TC), triglyceride (TG), and free fatty acid (FFA) cause ectopic lipid accumulation in nonadipose tissues (5). Growing evidences indicate that abnormal lipid metabolism and renal accumulation of lipids play an important role in the pathogenesis of DN (6, 7). Lipid deposition is associated with dysregulation of lipid metabolism genes. Therefore, targeting genes involved in renal lipid metabolism may serve as an effective therapy for DN treatment.
Insulin resistance is an important determinant in the initiation and progression of DN (8). It has been reported that patients with type 2 diabetes mellitus (T2DM) who develop DN are more likely to develop insulin resistance than those who do not. Insulin resistance is also correlated with microalbuminuria which is a common symptom of DN (9, 10). Hence, having a better understanding of the mechanisms underlying insulin resistance in renal dysfunction can help to find novel treatment for DN.
Autophagy is an intracellular degradation system that delivers cytoplasmic constituents to the lysosome to maintain intracellular homeostasis (11). Previous studies reported that insulin resistance was correlated with autophagy under various pathological conditions. Wei’s study indicated that the activation of autophagy could inhibit insulin resistance and played a protective role in DN (12).
Resveratrol (3, 4′, 5-trihydroxystlben; RES) is a polyphenolic compound commonly identified in various fruits including grapes, nuts and berries (13). RES exerts diverse beneficial health effects such as anti-oxidative, anti-inflammation and anti-cancer activities (14-16). Previous reports demonstrated that RES could ameliorate renal insufficiency and pathological changes in DN (17). However, studies on the molecular mechanism of RES ameliorating DN through regulating autophagy are still limited.
The present study has revealed the role of RES on the treatment of DN. Our study demonstrated that RES attenuated DN through improving lipid metabolism and alleviating insulin resistance via induction of AMPKα/mTOR-mediated autophagy.
Male, 6-week-old Sprague–Dawley (SD) rats were obtained from Animal center of Ankang City Chinese Medicine Hospital. All animal experiments were performed according to the guidelines of Institutional Animal Care and Use, with approval of Animal Research Ethics Committee of Ankang City Chinese Medicine Hospital. The rats were given free access to food and clean water and were caged individually under controlled temperature (23°C) and humidity (55%) with an artificial light cycle. Streptozotocin (STZ, Sigma-Aldrich, St Louis, MO, USA) was used to induce DN in rats. Rats were injected intraperitoneally with RES at a dose of 100 mg/kg body weight for 2 consecutive days. Then, rats with a blood glucose levels over 350 mg/dL were considered as having diabetes and were divided into 3 groups. Rats in DN + RES (5 mg/kg) group, DN + RES (20mg/kg) group and DN model group were different concentrations of RES (Sigma Chemical Co. MO, USA) or equal volume of sterile saline respectively by daily gavage for 8 weeks. Normal rats without STZ treatment were randomly divided into 2 groups and supplied with RES (20 mg/kg) or equal volume of sterile saline respectively. After 8 weeks treatment, rats were sacrificed and their weight was recorded. The kidneys, urine and blood sample were collected for the following experiments.
The body weight and kidney weight of rats were recorded respectively. Urinary albumin concentrations were measured using an ELISA Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions. Blood urea nitrogen (BUN), serum creatinine (Scr) were measured using an automatic biochemistry analyzer (Olympus AU2700, Japan).
The concentrations of total cholesterol (TC), Triglyceride (TG), low density lipoprotein (LDL-C) and high density lipoprotein (HDL-C) were detected by the automatic biochemistry analyzer (Olympus AU2700, Japan).
RIPA lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China) was used for protein extracting. The same amount of proteins were separated by 10% SDS-PAGE gel and then transferred into PVDF membranes. After blocking with nonfat milk at room temperature for 1 h, membranes were incubated with primary antibodies at 4°C overnight. The following antibodies were used (at 1:1000 dilution): PPARα, CPT-1, ACS (Abcam, Cambridge, UK); SREBP-1c (Thermo Scientific, Rockford, IL, USA); Beclin1, LC3 I/II, mTOR, p-mTOR, ULK1, p-ULK1 (Cell Signaling Technology, Beverly, MA, USA). The membrane was washed with TBST three times, followed by incubation with goat anti-rabbit or mouse IgG horseradish peroxidase (HP)-conjugated secondary antibodies (Thermo Scientific, Rockford, IL, USA), and the expression of proteins was detected with enhanced chemiluminescence reagent (ECL, PerkinElmer, Boston, MA, USA).
The homeostasis model assessment index (HOMA-IR) was calculated by the equation fasting glucose (mmol/liter) × fasting insulin (mU/liter)/22.5. The blood levels of hemoglobin A1c (HbA1c) were calculated by an IN2IT system (Bio-Rad, UK). Plasma insulin levels (P-insulin) and plasma adiponectin levels were measured using an ELISA kit (Linco RESearch, St. Charles, MO).
ITT and GTT were performed to determine insulin resistance and glucose intolerance of each group. ITT was conducted by intraperitoneally injection of 0.75 U/kg regular insulin in fasted mice with blood glucose measurements at 0, 30, 60, 90, and 120 min. GTT was performed by oral gavage of 3 g dextrose/kg after a 5-h fast, and blood samples were collected through the tail vein.
Data was expressed as the mean ± standard deviation (SD) of at least three independent experiments. All statistical analysis was performed with SPSS 19.0. Group comparisons were performed using Student’s t test or One-way ANOVA. The difference was considered statistically significant at p<0.05.
As shown in Figure 1A, compared with healthy control group, the model rats showed serious pathological injury. RES obviously reduced the pathological injury of the model rats. In addition, the immunohistochemical results showed that the TGF-β expression was high in model group, while RES treatment decreased TGF-β expressions in a dose-dependent manner (Figure 1B). Compared with healthy control group, the rats injected with STZ induced diabetes (STZ-induced Model group) and showed higher ratio of kidney weight/body weight, higher levels of 24 h urinary protein (PRO) extraction, higher levels of both BUN and Scr, respectively (Figure 1C-1F). RES treatment didn’t have significant effect on healthy rats. However, RES treatments suppressed the STZ induced higher index (kidney weight/body weight, 24 h PRO extraction, BUN and Scr) in a dose-dependent manner (Figure 1C-1F, p<0.01, p<0.05). Our results indicated that RES ameliorated renal damage in STZ-induced DN animal model.
 Figure 1
Figure 1RES ameliorates biochemical and physical indexes of DN. (A) HE staining for kidney tissues. (B) Immunohistochemistry for TGF-β. Scale bar=200μm. (C-F) Kidney weight/body weight, 24h PRO, BUN and Scr parameters were compared among different groups. The bars showed means ± SD of three independent experiments. **p<0.01 compared with healthy control group, #p<0.05 compared with DN model group.
As shown in Figure 2, STZ-induced DN rat model had higher levels of TC, TG, LDL-Chol and lowed level of HDL-Chol as compared with healthy control group. After RES treatment, the levels of TC, TG and LDL-Chol were decreased while the level of HDL-C was increased in DN + RES group dose-dependently compared with DN group (Figure 2A-2D, p<0.01, p<0.05). We then measured the expression of several key genes involved in lipid metabolism through Western blot. We observed that RES increased the low level of lipidolysis related proteins (PPARα, CPT-1) while decreased the high level of lipogenic related proteins (SREBP-1c, ACS) induced by STZ remarkably in a dose-dependent manner (Figure 2E-2F, p<0.01, p<0.05). What’s more, the mRNA levels of the PPARα, CPT-1, SREBP-1c and ACS are same to the protein levels (Figure 2G-2H). These data suggested that RES improved lipid metabolism in DN.
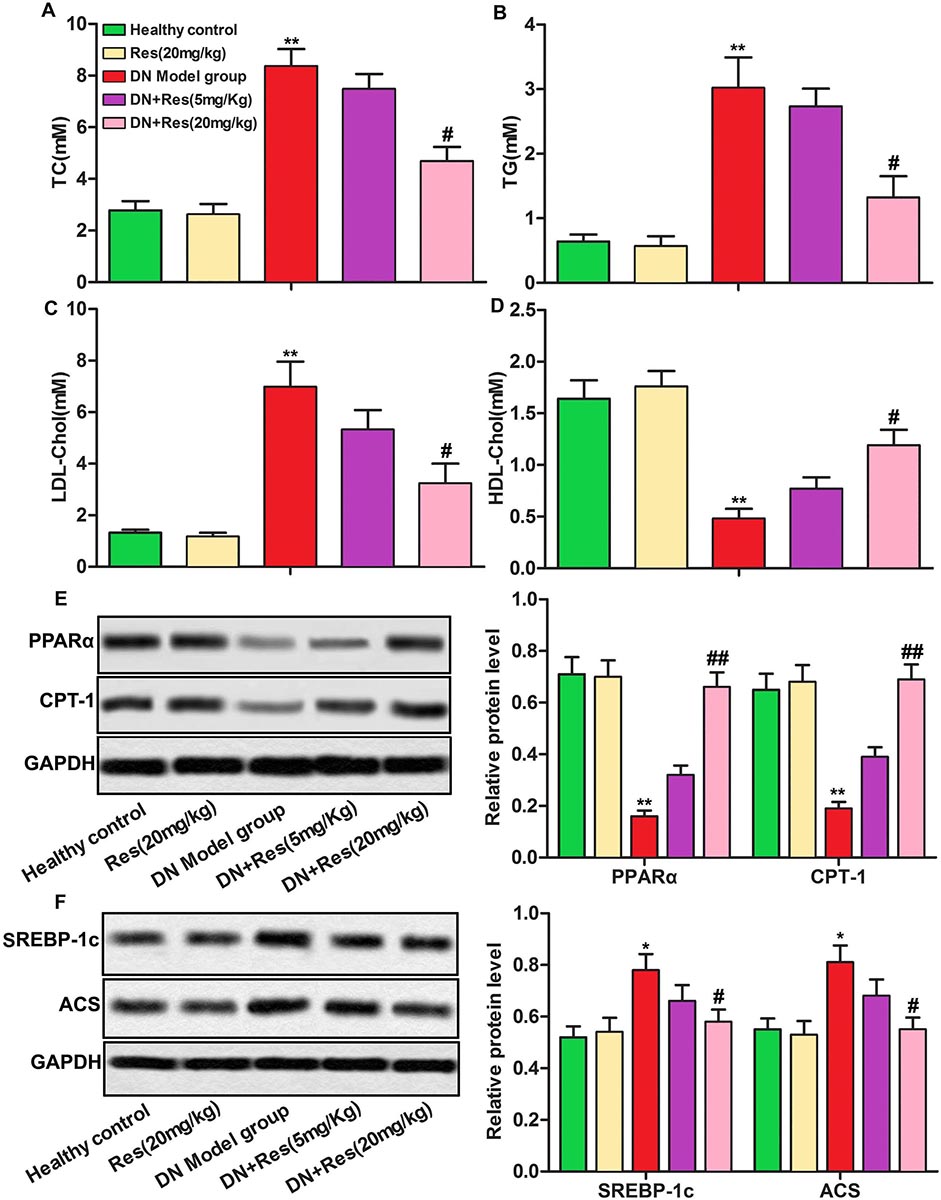 Figure 2
Figure 2RES improves lipid metabolism in DN. (A-D) The levels of TC, TG, LDL-Chol and HDL-Chol were compared among different groups. (E-F) Relative protein levels of PPARα, CPT-1, SREBP-1c and ACS were detected by Western blot. The bars showed means ± SD of three independent experiments. *p<0.05, **p<0.01 compared with healthy control group, #p<0.05, ##p<0.01 compared with DN model group.
As shown in Figure 3, the high levels of HOMA-IR and HbA1c in STZ treated rats were significantly decreased after RES treatment (Figure 3A-3B, p<0.05, p<0.01). Moreover, RES treatment up-regulated plasma adiponectin level while down-regulated P-insulin level dose-dependently compared with DN model group (Fig 3C-3D, p<0.05, p<0.01,). The improvement in insulin resistance and glucose intolerance after RES treatment was confirmed by intraperitoneal ITT and oral GTT (Figure 3E-3F, p<0.01, P < 0.001, p<0.05). These results demonstrated that RES suppressed insulin resistance in DN.
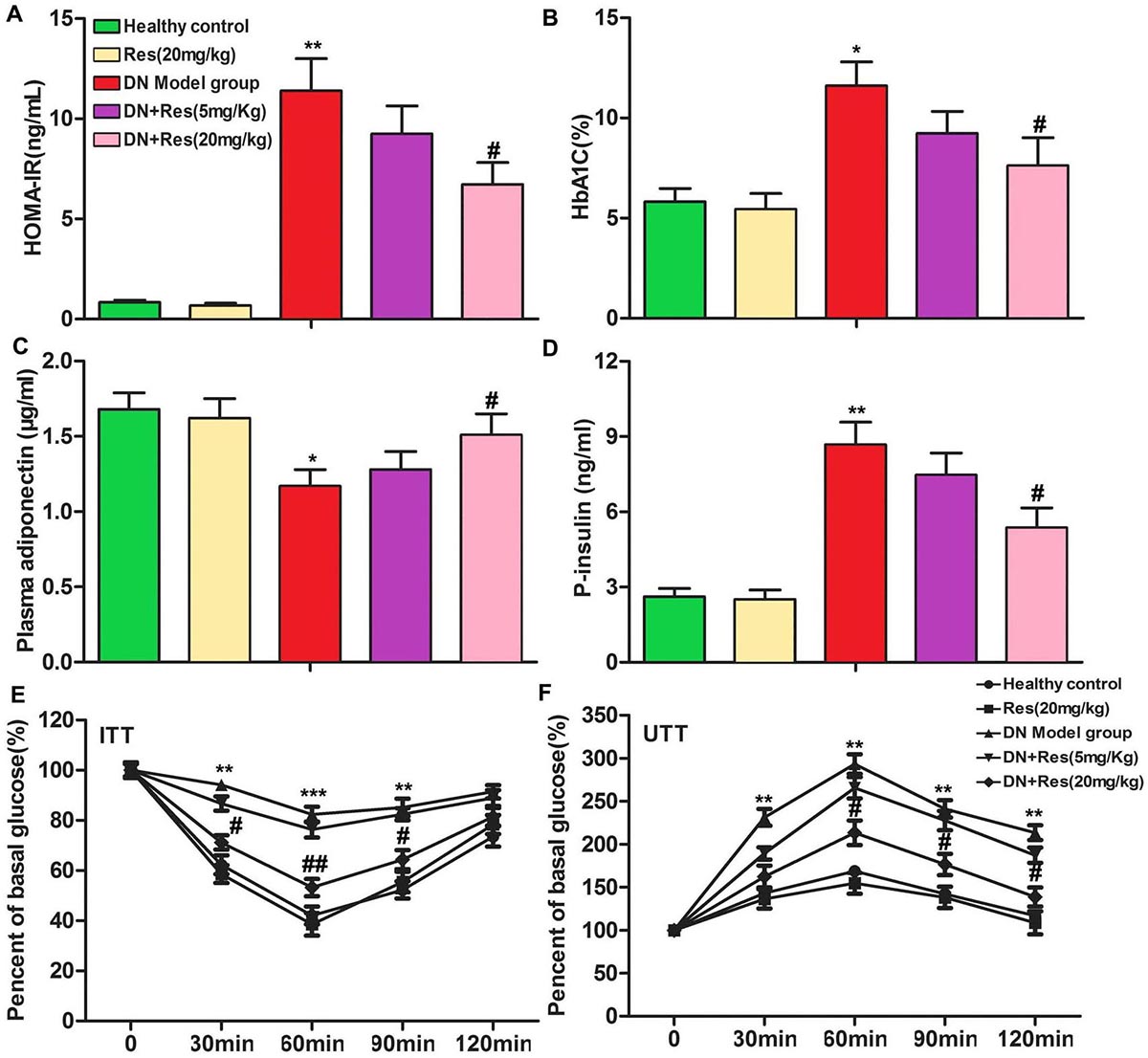 Figure 3
Figure 3RES suppresses insulin resistance in DN. (A-D) HOMA-IR, HbA1C, plasma adiponectin and plasma insulin levels were compared among different groups. (E-F) The results of ITT and UTT. The bars showed means ± SD of three independent experiments. *p<0.05, **p<0.01 compared with healthy control group, #p<0.05, ##p<0.01 compared with DN model group.
As shown in Figure 4A-D, 20 mg/kg RES significantly decreased the pathological injury of DN rats and the PRO level (p<0.01). Furtherly, RES reduced the levels of TC and improved insulin resistance. Interestingly, autophagy inhibitor 3-MA exhibited contrary effect. Expectedly, RES up-regulated the protein levels of Beclin-1, Atg5, and ratio of LC3II/LC3I obviously (p<0.01), while 3-MA down-regulated the above proteins. These results indicate that RES improve kidney function, lipid accumulation and insulin resistance via inducing autophagy.
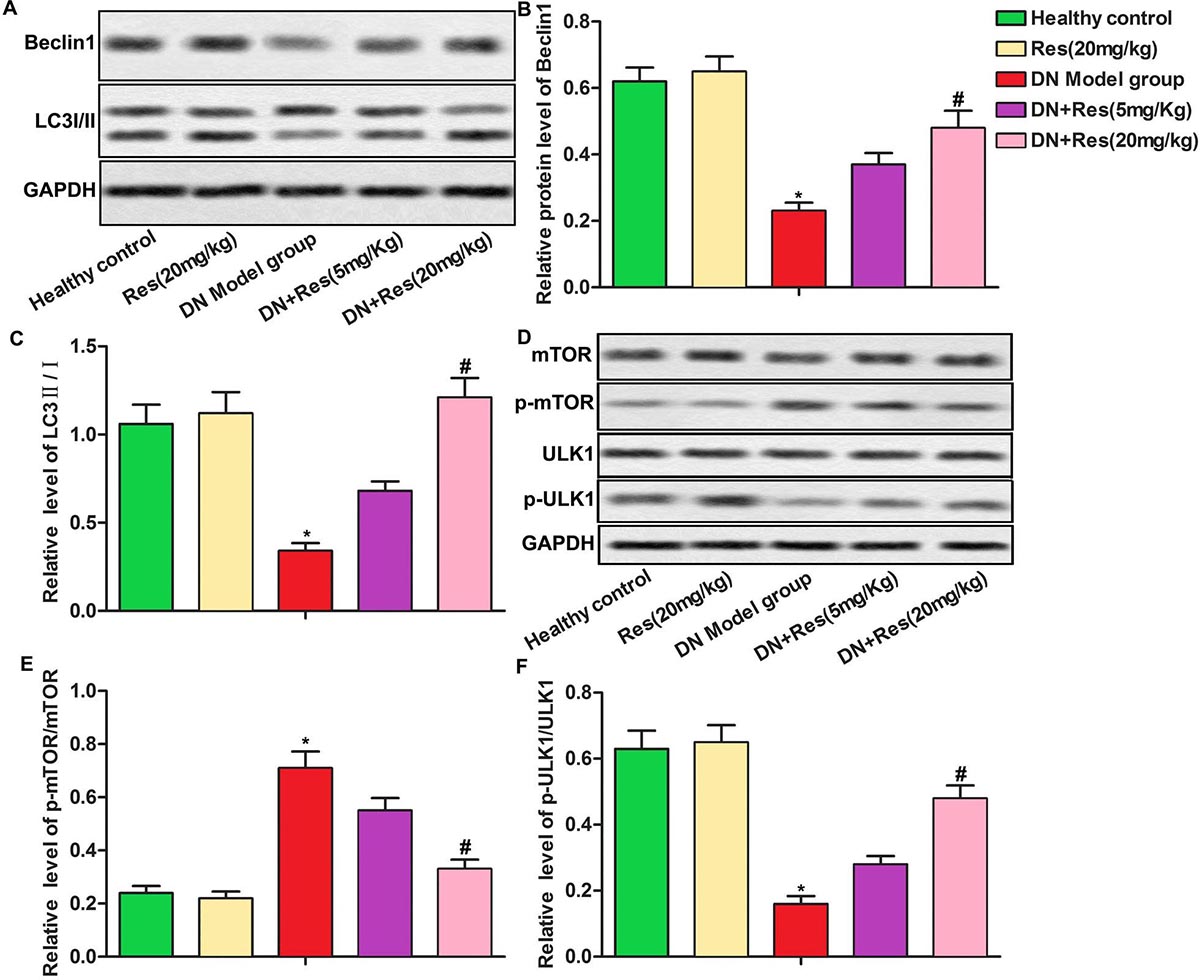 Figure 4
Figure 4RES inhibits DN via inducing autophagy. (A) HE staining for kidney tissues. Scale bar=200μm. (B) Kidney function parameter 24h PRO was detected. (C) The level of TC was detected. (D) The result of ITT. (E-F) Relative protein levels of Beclin-1, Atg5 and LC3 were detected. The bars showed means ± SD of three independent experiments. **p<0.01 compared with healthy group, ##p<0.01 compared with DN model group, $$ p<0.01 compared with DN+3-MA group.
Previous studies reported that insulin resistance was correlated with autophagy under various pathological conditions (12). Here we explored relative expression of autophagy related proteins (Beclin1, LC3 II/I). The data showed that RES treatment increased Beclin1 level and LC3II/I rate compared with DN model group (Figure 5A-5B, p<0.05). In addition, RES treatment reduced p-mTOR/mTOR and p-ULK1 S757/ULK1 rate while increased p-AMPKα/AMPKα and p-ULK1 S777/ULK1 rate significantly as compared with DN model group (Figure Fig 5C-5D, p<0.01). Taken together, our data suggested that RES induced autophagy via activating AMPKα/mTOR pathway in DN rats.
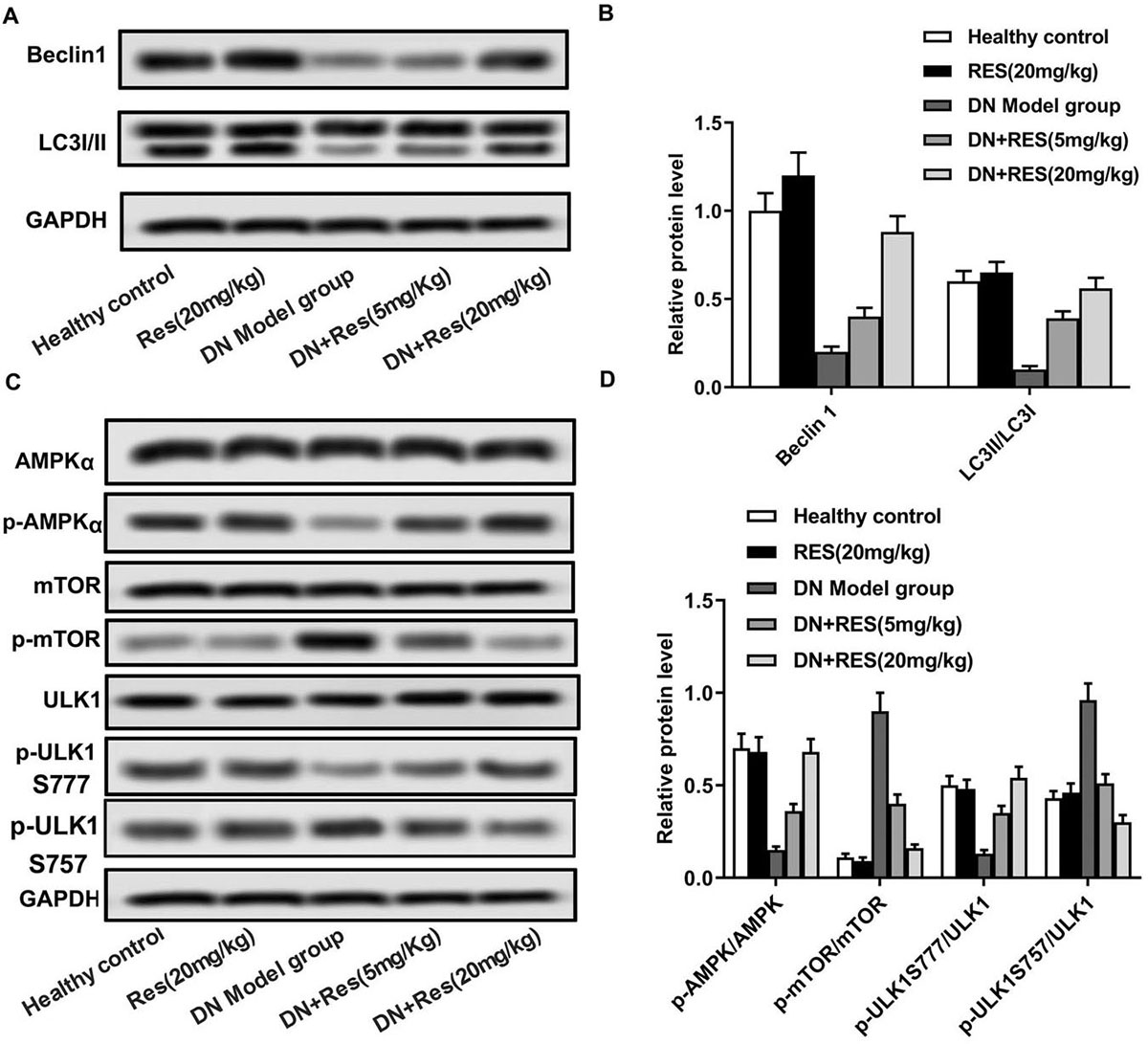 Figure 5
Figure 5RES induces autophagy via activating AMPKα/mTOR pathway in DN. (A-D) Relative protein levels of AMPKα, p-AMPKα, Beclin1, LC3 I/II, mTOR, p-mTOR, ULK1 and p-ULK1S777 and p-ULK1S757 were detected by Western blot. The bars showed means ± SD of three independent experiments. *p<0.05 compared with healthy control group, #p<0.05 compared with DN model group.
Ectopic lipid accumulation in nonadipose tissues such as liver, heart and pancreas can induce cellular dysfunction and tissue injury, which is called lipotoxicity (18, 19). Previous studies reported that renal lipid metabolism played an important role in DN pathogenesis (20, 21). Moreover, insulin resistance is also a crucial process in the initiation and progression of DN (8). RES has been reported to reduce DN in several studies (22-24), for instance, RES inhibits apoptosis via activating autophagy of podocytes in DN mouse (22); in addition, RES inhibits renal interstitial fibrosis in db/db mice (24). In this present study, we demonstrated that RES treatment significantly improved lipid metabolism and alleviated insulin resistance in STZ-induced DN rats through induction of AMPKα/mTOR-mediated autophagy. Our study revealed the protective role of RES in the pathogenesis of DN.
Development of DN is characterized by increased plasma levels of creatinine and BUN in STZ-induced diabetic rats (25). In agreement with previous studies, STZ treatment was found to increase kidney weight/body weight, 24 h PRO, BUN and Scr in rats of our study (26, 27). Kitada et al. reported that RES treatment alleviated albuminuria and reduced the increased levels of renal oxidative stress and inflammation in the kidneys of db/db mice (28). Xu also indicated that treatment with RES for 12 weeks attenuated the albuminuria (29). Similarly, our present study also showed that RES treatment attenuated the albuminuria and ameliorated biochemical and physical indexes of DN, suggesting the protective role of RES in the pathogenesis of STZ-induced DN.
Heavy lipid deposition is a common feature of human DN (7). Wang’s study demonstrated that accumulation of lipid, lipotoxicity, and lipid metabolism dysregulation were associated with renal damage in animal models of DN (30). In the present study, RES was observed to reduce the high concentration of TC, TG and LDL-Chol while increase the low concentration of HDL-Chol in DN animal models, suggesting that RES could alleviate lipid dysregulation in DN. PPARs which includes three subtype (PPARα, PPARβ, and PPARγ) is correlated with the transport of TG in the blood, cellular fatty acid uptake, and mitochondrial beta oxidation (31). PPARα is important in the regulation of mitochondrial and peroxisomal fatty acid oxidation, including modulation of downstream targets, such as CPT-1(32). RES repairs the reduced expression of PPARα, which suggests that RES helps lipids oxidation and export. SREBP-1c is a transcription factor that active many genes involved in lipid synthesis and deposition (33). ACS is an enzyme involved in metabolism of acetate. Here we found that RES up-regulated the expression of PPARα and CPT-1 while down-regulated the expression of SREBP-1c and ACS, suggesting that RES alleviated lipid synthesis and increased lipid catabolism via increasing the level of lipidolysis related proteins (PPARα, CPT-1) while decreasing the level of lipogenic related proteins (SREBP-1c, ACS) in DN.
Free fatty acids (FFA) induced by obesity is one of external factors of insulin resistance. FFA triggers insulin resistance through lipid accumulation (ectopic lipids) (34). Insulin resistance usually companied with disorder of lipid metabolism. Insulin resistance is also a crucial process in the initiation and progression of DN. A great number of researches reported that RES played a crucial role in the regulation of insulin resistance. Cheng reported that RES attenuated methylglyoxal-induced insulin resistance by up-regulating Nrf2 expression in Hep G2 cells (35). Study by Chen indicated that RES attenuated insulin resistance by influencing skeletal muscle lipid transport and subsarcolemmal mitochondrial β-oxidation (36). Consistent with these previous studies, we found that RES treatment markedly reduced HOMA-IR index scores and HbA1C levels in DN animal models. RES decreased plasma insulin level while increased the level of plasma adiponectin which is a protein hormone involved in regulating glucose levels as well as fatty acid breakdown (37). Improvements in insulin resistance were further confirmed by insulin tolerance test and glucose tolerance tests. Our study demonstrated that RES could attenuate insulin resistance during pathogenesis of DN.
Autophagy is a lysosomal degradation process with the functions to degrade long-lived proteins and damaged organelles for intracellular homeostasis (38). Diabetic kidneys are reported to be deficient in autophagy activity (39). Xu demonstrated that RES ameliorated DN via increasing autophagy mediated by miRNA-18a-5p (29). Here, in this present study, we observed that RES increased the expression of Beclin1 which is necessary for the induction of autophagy. Moreover, the rate of LC3II/LC3I was also increased by RES, reflecting the level of autophagy was more enhanced after RES treatment. In addition, AMPKα/mTOR signaling pathway was reported to play an important role in the regulation of autophagy (40, 41). Similarly, in our study, the data showed that RES induced autophagy via activating AMPKα/mTOR pathway in DN.
In conclusion, RES protected against DN via several mechanisms including improving lipid metabolism and alleviating insulin resistance through induction of AMPKα/mTOR-mediated autophagy. These findings suggest that RES may be a potential therapeutic approach in the treatment of type 2 diabetes mellitus and DN.
Yong-hong Zhao and You-Jia Fan contributed equally to this work and should be considered co-first authors.
DN
Diabetic nephropathy
Resveratrol
streptozotocin
total cholesterol: TG, triglyceride: FFA free fatty acid
Type 2 diabetes mellitus
carnitine palmityltransferase-1
low density lipoprotein
high density lipoprotein
peroxisome proliferators-activated receptor α
Acetyl-CoA synthetase
Sterol regulatory element-binding protein 1
Insulin tolerance tests
glucose tolerance tests





