Frontiers in Bioscience-Landmark (FBL) is published by IMR Press from Volume 26 Issue 5 (2021). Previous articles were published by another publisher on a subscription basis, and they are hosted by IMR Press on imrpress.com as a courtesy and upon agreement with Frontiers in Bioscience.
1 DBT-National Institute of Animal Biotechnology (DBT-NIAB), Hyderabad-500032, Telangana, India
Abstract
Japanese Encephalitis Virus (JEV) is the most common Flavivirus based mosquito borne viral encephalitis in the world, especially in countries of South-East Asia. The conventional methods such as Enzyme-Linked Immunosorbent Assays (ELISA), Reverse Transcriptase Polymerase Chain Reaction (RT-PCR), Plaque Reduction Neutralization Test and virus isolation are still in use today but new advances are being made to develop more efficient, inexpensive, quicker, sensitive and time-saving techniques to detect JEV. Some of these include the use of immunosensors, both lateral flow based assays and electrochemical, as well as the incorporation of nanotechnology into biosensors to develop highly sensitive detection tools. This review focuses on the recent advances that have been made to diagnose Japanese Encephalitis Virus which are critical in breaking the link to zoonotic transmission into the human population where humans are dead-end hosts.
Keywords
- Japanese Encephalitis Virus
- Diagnosis
- Sensitive
- Biosensors
- Nanoparticles
- Point of Care
- Review
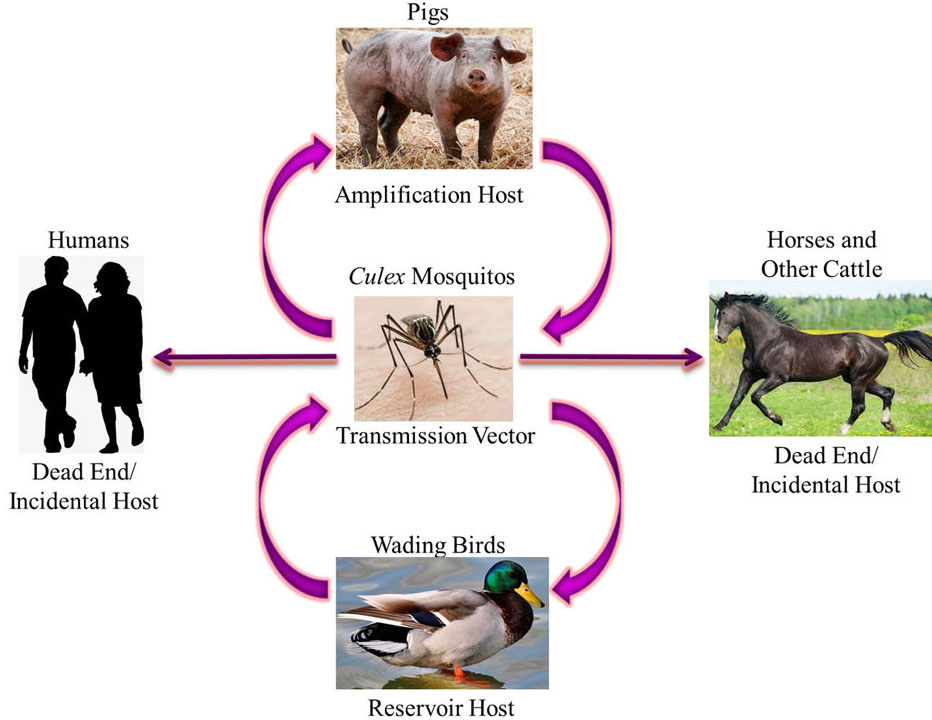
Japanese Encephalitis Virus belongs to the family Flaviviridae and genus Flavivirus (1) and its mode of infection is a zoonotic cycle (Figure 1.) between the vector i.e. Culex mosquitos, the amplifying host i.e. pigs and, the reservoir host i.e. wading birds. Other animals, including humans, horses, etc. are incidental dead end hosts due to low and short-lived viraemia of JEV and hence can get infected but are unable to transmit the virus along to another transmission vector (2–6). Due to its prevalence in South-East Asia and the Western Pacific, JEV has been described as “a plague of the orient” (7). These regions have large rural agricultural and peri-urban settings where rice farming occurs which is a breeding ground for mosquitoes and there is also a decent pig/water fowl population. Seasonal transmission occurs which varies with monsoon rains and irrigation practices. The first case of JEV was documented in Japan in 1871 (8). In 1935, the Nakayama strain prototype was isolated from the brain of a patient for the first time (9). According to the World Health Organization (WHO), major outbreaks of JEV occur every 2-15 years and transmission intensifies during monsoons as the vector population increases and is seen in agricultural areas and intensive rice cultivation. After an outbreak, measures are taken to reduce mosquito infestation and quarantine pig farms, but the virus remains dormant until the next ideal monsoon season and negligence of the people and hence the outbreak reoccurs. Since 1955, many major outbreaks have been recorded in India, for example, Bankura in 1973, and Gorakhpur in 1978, etc. (10). JEV exists as one phenotype and five distinguishable genotypes – G-I, G-II, G-III, G-IV and G-V – based on nucleotide homology in the E protein gene; three subtypes (a, b and c) are also distinguishable within G-I (11, 12). Most of the JEV strains isolated in India belong to genotype III and sometimes genotype II (13). However, JEV genotype I, has recently been reported in human patients from Gorakhpur (14).
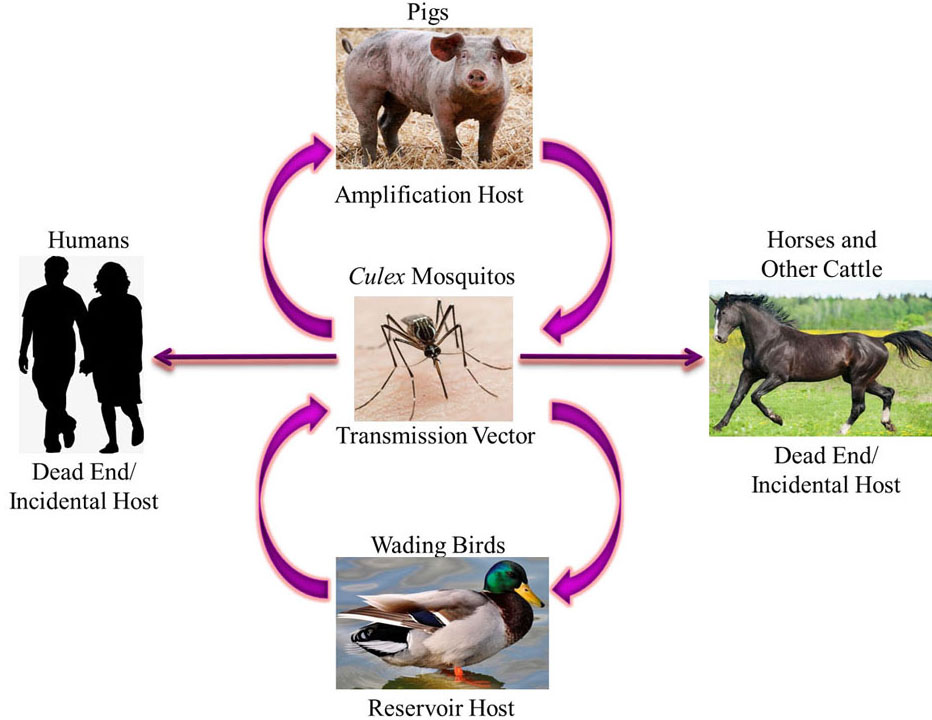 Figure 1
Figure 1Zoonotic Transmission Cycle of Japanese Encephalitis Virus where Culex mosquitos transmit the virus in a cycle between the amplification hosts i.e. pigs and reservoir hosts i.e. wading birds. Humans, horses and other cattle are incidental dead end hosts as they can get infected but cannot transmit the virus.
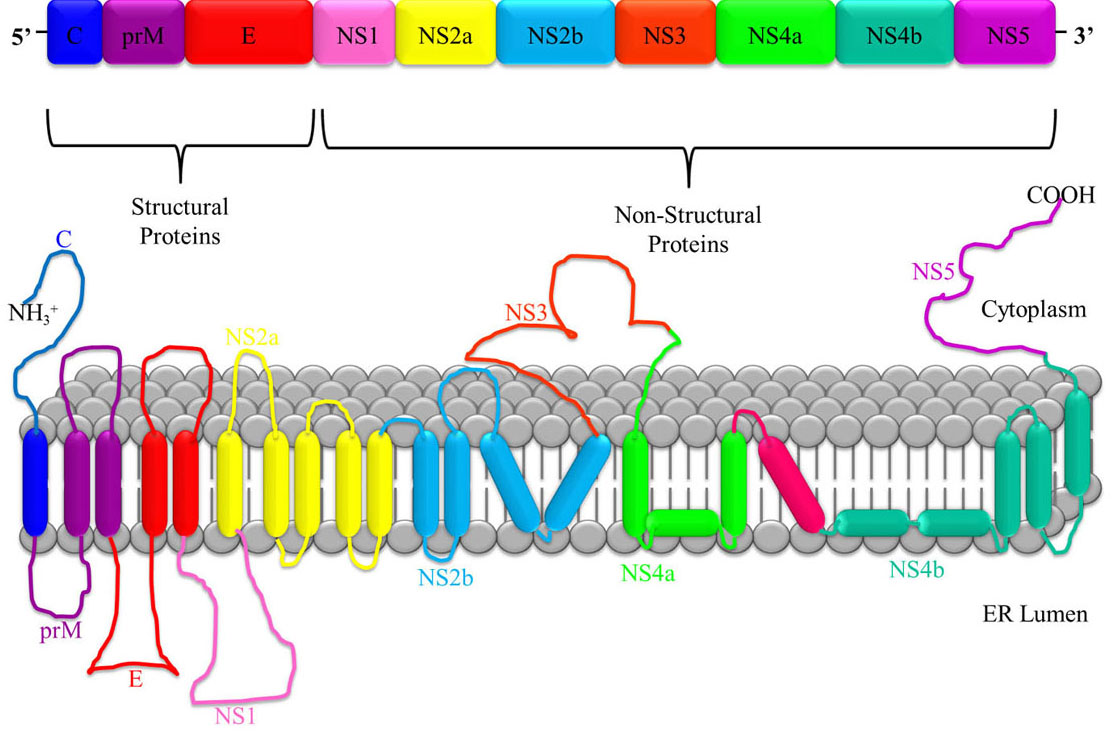
JEV is a single stranded, positive-sense polarity Ribonucleic Acid (RNA) genome of approximately 11 kb in length and contains a 5′ untranslated region (UTR), a single open reading frame (ORF) and a 3′ UTR. The ORF encodes a long polyprotein, which is co- and post-translationally processed by a combination of viral and cellular proteases into three structural proteins - nucleocapsid or capsid protein (C), non-glycosylated pre-membrane protein (prM), and glycosylated envelope protein (E), as well as seven non-structural (NS) proteins - NS1, NS2A, NS2B, NS3 (helicase), NS4A, NS4B, and NS5 (RNA directed polymerase) as shown in Figure 2. (10, 15). An NS1 protein is also produced via ribosomal frame-shifting as a consequence of an RNA pseudo knot structure (16). The virion is formed by the C protein forming an icosahedral cage encasing the genome, and being enveloped by host cell membrane studded with the E protein (17).
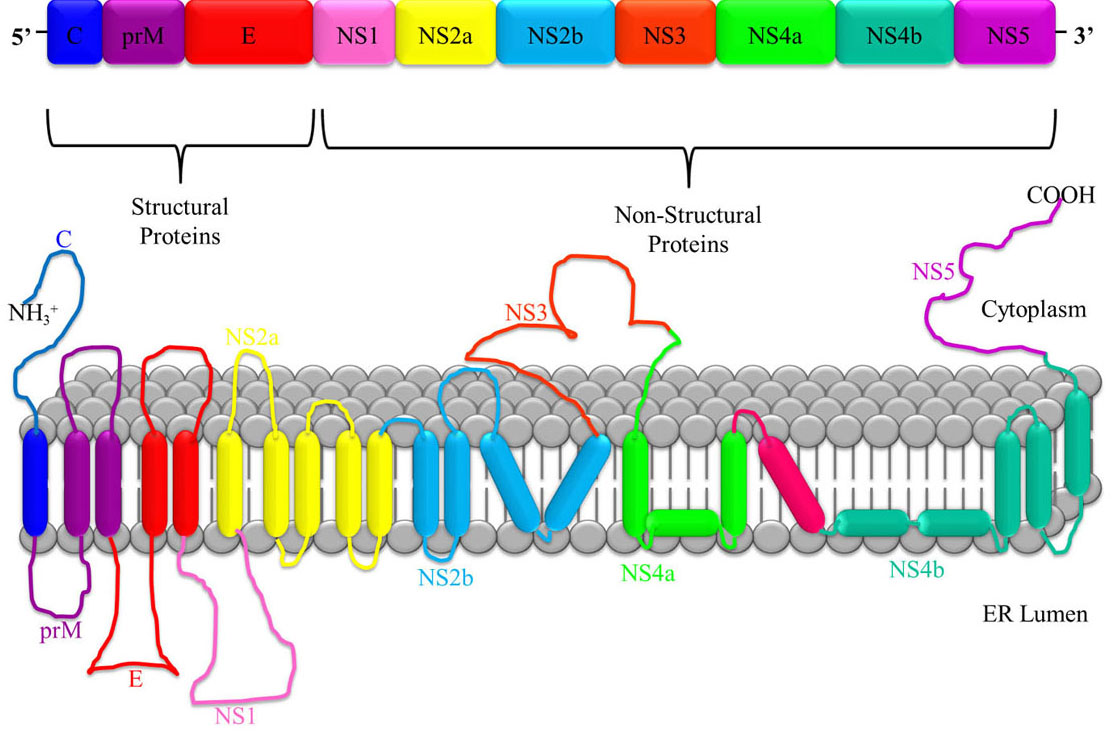 Figure 2
Figure 2JEV RNA consists of a single polyprotein sequence made up of three structural proteins i.e. capsid (C), pre membrane (prM) and envelope (E), and seven non-structural proteins i.e. NS1, NS2a, NS2b, NS3, NS4a, NS4b and NS5.
According to JEV statistics given by WHO in 2019, 24 countries in South-East Asia and Western Pacific regions have endemic JEV transmission, wich exposes 3 billion people to the risk of infection. There is an estimate of nearly 68,000 clinical cases of JEV globally each year with 13,600-20,400 deaths approximately. Most infections of JEV are asymptomatic, however, the case-fatality rate among those with encephalitis can be as high as 30%, more so in children. Permanent neurologic or psychiatric sequelae can occur in 30-50% of cases. It causes clinical symptoms in humans, including a non-specific febrile illness, meningitis, encephalitis and meningo-encephalitis. There is no specific treatment for JEV and only palliative therapy can be recommended. Currently, the vaccines available are inactivated, live-attenuated and chimeric vaccines (13) which have their own set of problems such as the inherent risk of using the live-attenuated viral vaccines, the potential for allergic reactions with the mouse brain-derived inactivated vaccine, insufficient long-term immunity to provide effective protection by inactivated vaccines and the costly 3 dose inoculation requirement. Hence, vaccination is not a fool proof solution. Also as JEV is incurable, an early diagnosis is critical in preventing an epidemic outbreak, especially since the initial symptoms are usually mistaken for dengue or malaria.
The conventional diagnostic methods for JEV such as Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) (18), Plaque Reduction Neutralization Test (19) and Virus Isolation (20, 21) are costly and time-consuming diagnostic/assay procedures which require large equipment and trained personnel. Another major concern is the health and safety of the people carrying out the diagnosis when the whole virus is detected as JEV is highly communicable. Hence, conventional methods such as virus isolation require an elaborate Biological Safety Level-3 (BSL-3) laboratory set up with Personal Protective Equipment (PPE). The added unfeasibility is that most JEV cases happen in rural parts of the country (farms and villages), where diagnostic laboratories with specialized equipment and personnel is not always available. Also, the advantage of detecting the whole virus or antigen instead of the antibodies is that the virus or the antigen is present from day 1 of the infection whereas the antibodies appear only after Day 4/5 of the infection. Hence, this will facilitate rapid detection in the early stages of the infection and treatment can be started immediately.
A portable diagnostic technique, for the detection of JEV, which can provide rapid results even with a minute amount of viral antigen available in the sample, is required (22). In recent times, immune biosensors/biochips have begun to replace the conventional methods. The basic principle involved is antigen-antibody interaction which makes these immunosensors highly specific and sensitive in the detection of narcotics, pesticides, bacteria, virus, diseases, etc. (23–30). Out of these immunosensors, electrochemical biosensors have received much attention as a reliable diagnostic tool for infectious diseases as their sensing performances are not affected by turbidity or absorbance of the sample (31). Moreover, electrochemical biosensors offer the advantages of being highly sensitive, rapid in signal generation and readout, highly amenable for miniaturization, not much sample preparation, and inexpensive for virus detection, and it requires relatively simple operational instrumentation (32). Lateral flow based immunoassays have also been developed which have a major advantage of being cheaper than other immunosensors (33).
Another advancement to increase the sensitivity and response time of the immunosensors is to incorporate nanoparticles. This research has been conducted using silver nanoparticles (34) and carbon nanoparticles (35). Since nanoparticles have a very high surface to volume ratio, there is an increase in loading of recognition elements on the electrodes and more efficient electron transfer. This enhances the sensitivity of the biosensor. Hence, in this review paper, we shall shed some light on all the recent techniques and methods that have been developed for the diagnosis of Japanese Encephalitis Virus which are critical in breaking the link to zoonotic transmission into the human population where humans are dead-end hosts.
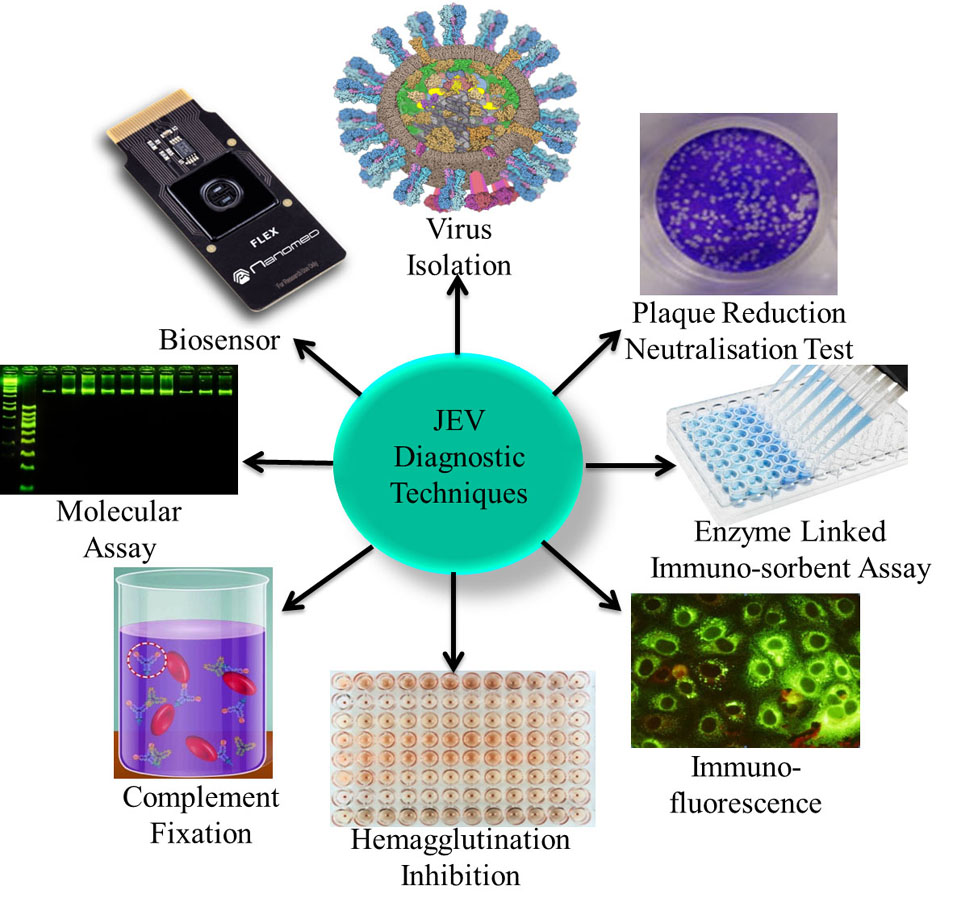
The detection of JEV infection is problematic due to the short duration of viraemia and the asymptomatic nature of infection. These factors present a challenge for obtaining a definitive diagnosis of JEV infection or for establishing prevalence levels in livestock populations. Another major challenge in choosing a diagnostic technique is the specificity of the test due to cross-reactivity between flavivirus (36). Therefore, diagnosis relies on the use of a combination of clinical, serological and pathological tests (37) as shown in Figure 3.
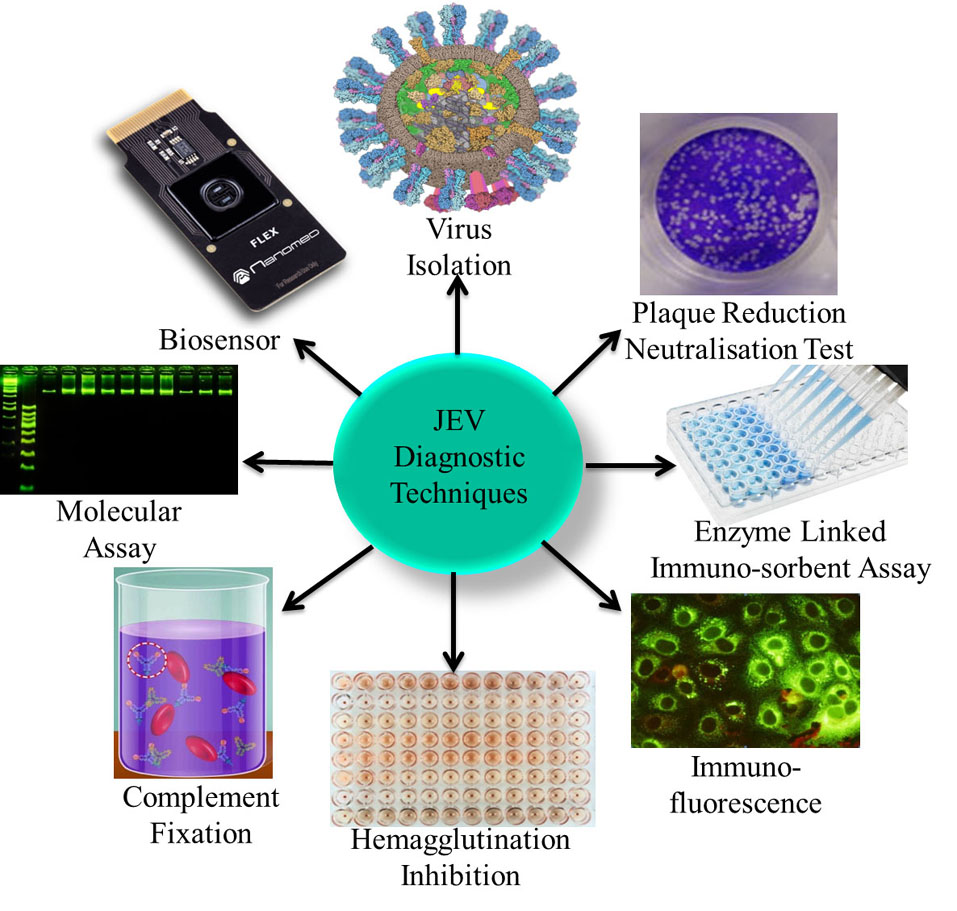 Figure 3
Figure 3Various different types of JEV diagnostic techniques including clinical, serological and pathological are used to conclusively diagnose JEV such as Virus Isolation, PRNT, ELISA, IFA, HI, CFT, Molecular Assay, and Biosensor detection.
The most common form of definitive diagnosis even today, especially in horses, relies upon the isolation of the complete virus (20, 21) from central nervous system (CNS) tissue of diseased or dead animals. This however has its limitations due to virus instability under certain conditions as well as the effect of host antibodies. The World Health Organization for Animal Health has provided a list of different techniques which may be used for the conclusive diagnosis of JEV (Office International des Epizooties – OIE) (OIE, 2012).
Isolation of the complete Japanese Encephalitis (JE) virus can be done both using in vitro techniques or by the inoculation of mice (38). BSL-3 protection is a must when dealing with isolation of JEV virus to avoid the risk of human infection. JEV can be isolated from different sections of the brain such the corpus striatum, cortex or thalamus as well as from the spinal cord and blood. Since JEV infects the CNS, brain sections are the most preferred source for virus isolation followed by spinal cord and blood.
For in-vitro isolation of the virus, primary cell cultures can be prepared from chicken embryos, porcine or hamster kidney cells, or tissue culture can also be performed on established cell lines such as C6/36 (mosquito – Aedes albopictus), BHK (baby hamster kidney) or Vero (African green monkey kidney). On incubation with JEV, the mammalian cells get lysed by JEV infection and a gel overlay can be used to restrict the virus from spreading in a cell sheet. Using a cell dye such as crystal violet, individual plaques formed can be visualized.
For in vivo isolation of the virus, 2–4 day old mice are inoculated with a homogenate of CNS tissue taken from an infected animal intracerebrally. If the diagnostic sample is positive for JEV, the mice will exhibit neurological signs and die within 14 days, usually with convulsions just before death. Mice showing severe infection should be euthanized. Once dead, the brain of the mice is removed and JEV can be isolated and identified using cell culture techniques. Further confirmation of the isolated JE virus is done using subsequent serological and molecular tests.
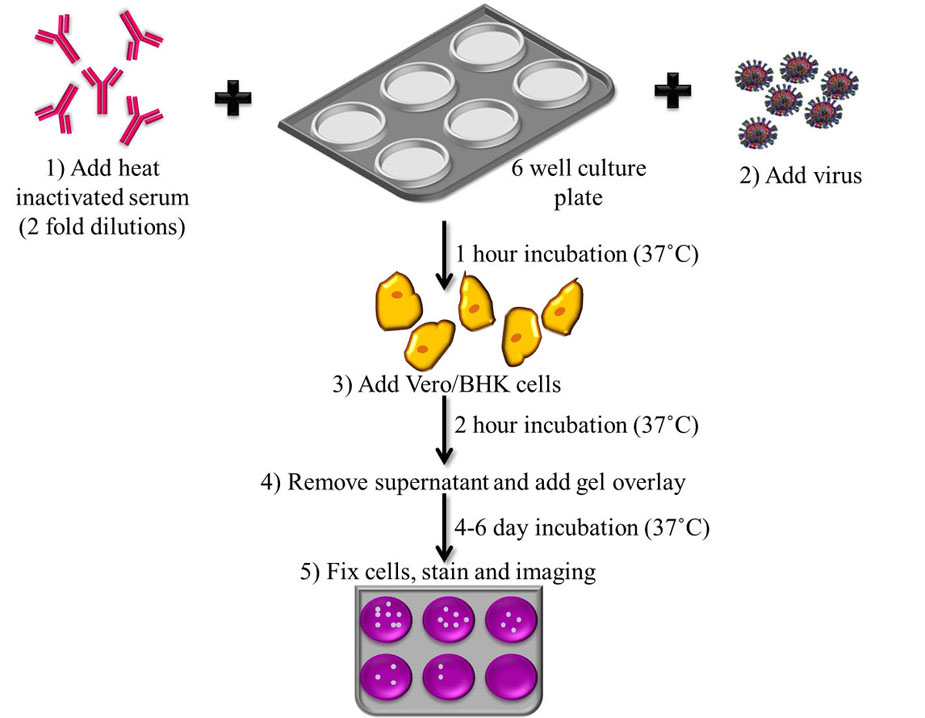
The plaque reduction neutralization test (PRNT) is the most specific of the serological assays, as it shows minimal cross-reactivity with other flaviviruses (OIE, 2012) (39). This assay is therefore considered the ‘gold-standard’ assay for JEV diagnosis and is widely used (19, 40, 41). This test is used to quantify the neutralizing antibodies for a virus. The OIE-described assay which is accepted for diagnosis worldwide, follows a 2-fold dilution series of heat inactivated serum, which is incubated with JEV strains such as the Nakayama strain to allow the antibodies present in the serum to react with the virus. After an hour of incubation at 37 ˚C, the serum along with the JEV strain is plated onto 6-well culture plates in monolayers of BHK cells, Vero cells or chicken embryo primary cultures and allowed to incubate for 2 h at 37 ˚C. After incubation, supernatant is removed, a gel overlay is added to restrict the virus from spreading indiscriminately in the cell sheet, and the plates are incubated at 37 ˚C for 4-6 days, which has been standardized as the minimum number of days for plaque formation to occur in the case of JEV. Following incubation, the plates undergo fixing and staining for the determination of titre in plaque forming units (PFU) (OIE, 2012). Flowchart of the protocol for PRNT has been shown in Figure 4.
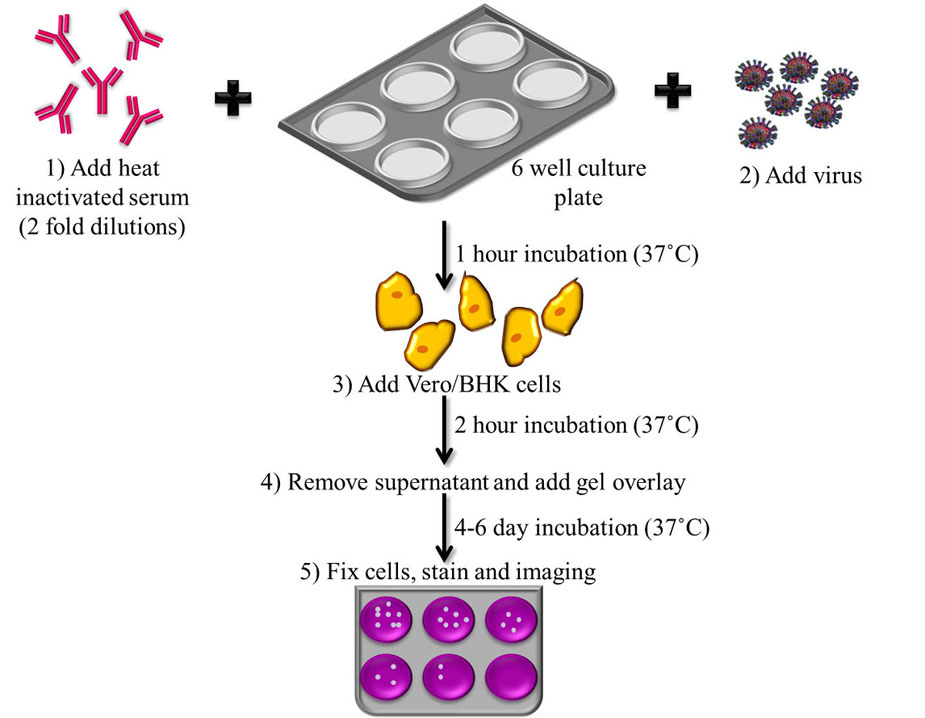 Figure 4
Figure 4Flowchart depicting the steps involved in Plaque Reduction Neutralization Test. 1) Incubation of JE virus with heat inactivated serum at 37 ˚C, 2) Addition of virus, 3) Plating onto monolayers of cell lines with 2 h incubation at 37 ˚C, 4) Gel overlay is added and 5) Plaques are allowed to form over 4-6 days by incubating at 37 ˚C which are then fixed, stained and titer determined by Plaque forming unit (PFU).
The nucleic acids of various viruses encode surface proteins that agglutinate red blood cells (RBCs) of a variety of species. The reaction of RBCs with viral hemagglutinins forms a lattice of agglutinated cells which settle down irregularly whereas unagglutinated cells settle into a compact button. This process is called hemagglutination. The basis of this assay is that in the presence of antibodies, the virus will be unable to attach to the RBCs and hence inhibit hemagglutination. This test is widely used, but can be non-specific due to antibody cross-reactivity with other flaviviruses and also may lack sensitivity (34, 42). For identification of viruses, a second passage in mice is undertaken, and sucrose/acetone-extracted antigen is prepared from the infected brains, which can then be checked for agglutination of goose/chicken RBCs (43). If hemagglutination is observed, the antigen can then be used in a Hemagglutination Inhibition (HI) test using JEV antiserum. When there is a four-fold difference in titre between acute and convalescent serum samples the patient is diagnosed with JEV (or a virus antigenically-related to that used in the assay eg. other flavivirus such as dengue).
The complement fixation test (CFT) is occasionally used for diagnosis by detecting presence of antibody in patient serum, but can also demonstrate a lack of sensitivity (42). The complement fixation test consists of two components. The first component is an indicator system that uses combination of sheep red blood cells, complement-fixing antibody such as immunoglobulin G produced against the sheep red blood cells and an exogenous source of complement usually guinea pig serum. When these elements are mixed in optimum conditions, the anti-sheep antibody binds on the surface of red blood cells. Complement subsequently binds to this antigen -antibody complex formed and will cause the red blood cells to lyse. In case of JEV, the antigen for use in the test is extracted with acetone/ether from the brains of inoculated mice. Test sera are combined with antigen and complement (guinea pig serum), with haemolysis assessed using sheep red blood cells. The titre of a serum sample is the highest dilution showing no haemolysis (OIE, 2012).
Immunofluorescence assay (IFA), is a standard virological method to detect a virus, by identifying the presence of antibodies by their specific ability to react with viral antigens expressed in infected cells (44). In this method, biochemically characterized substances are used as antigen substrates. In positive samples, specific antibodies in the diluted serum sample attach to the antigens immobilized on a solid phase. The attached antibodies are stained with fluorescein-labeled anti-human antibodies and visualized using a fluorescence microscope. Commercially-available indirect immunofluorescence tests (IIFT) which detect either anti-JEV Immunoglobulin G (IgG) or Immunoglobulin M (IgM) have been developed for human serum application (Euroimmun, Germany). IFA has been compared to other JEV detection techniques and the IgG IIFT was found to be comparable to the PRNT, with sensitivity and specificity of 93.8% and 100% respectively (40). When compared with a commercially available anti-JEV IgM ELISA kit, the IgM IIFT showed sensitivity and specificity of 83.9% and 100% respectively (40). Hence, the IgM IIFT may be a useful addition to the cohort of JEV diagnostic tools used.
A number of molecular methods have been developed for JEV nucleic acid detection by reverse transcription polymerase chain reaction (RT-PCR) (18, 38, 42, 45–49). These include a reverse transcription loop-mediated isothermal amplification (RT-LAMP) assay (50, 51), which can be used for on field testing in areas with limited laboratory equipment. To identify the geographical origin of a particular JEV strain, full genome sequencing techniques can be applied for a more thorough analysis of genetic identity (9, 52–54). Genotype I is the predominant JEV genotype in most parts of the world (55), which needs to be taken into account when developing primer sets for nucleic acid detection.
Recently there has been increasing interest in multiplex molecular assays which can be used to differentiate between different viruses including JEV (56, 57). A recent study compared the diagnostic efficiency of RT-PCR, real-time RT-PCR and RT-LAMP assays JEV nucleic acid detection of JEV which showed they were all 100% specific (58). However, RT-LAMP and real-time RT-PCR were observed to be more sensitive than RT-PCR, which shows standard RT-PCR assays may cause misdiagnosis of JEV. A further study assessed human CSF samples (n = 45) taken from patients suspected to have JEV in Vietnam, and compared molecular and serological diagnostics. Although real-time RT-PCR used was highly sensitive and generated a 4 % positive rate, the IgM capture ELISA gave a positive rate of 23.1%; which proved serological assays are equally important and multiple tests need to be performed for confirmed diagnosis of JEV (59). More recently, Luminex technology has contributed to serological diagnostic methods of JEV and a range of other arboviruses, and is highly sensitive (60).
One of the most common kit based diagnostic techniques to detect JEV is by the detection of IgM and IgG virus-specific antibodies using Enzyme-Linked Immunosorbent Assay (ELISA) techniques. Both commercially patented ELISA kits as well as ‘In-house’ ELISA assays are widely used for JE diagnosis (40). IgM antibodies are detectable in cerebrospinal fluid (CSF) as well as serum within 7 days of the onset of the disease (WHO, 2006) and hence are most commonly used for JEV diagnosis as they can be used for the identification of recent acute cases of JEV (49). A number of published in-house ELISAs have been described, including ELISAs for the detection of virus-specific IgM and IgG antibodies in CSF (61), an indirect IgG ELISA to study the seasonal sero prevalence of JEV (62), and an indirect ELISA (basic schematic shown in Figure 5.) for prevalence studies of JE-specific antibodies in pigs (63).
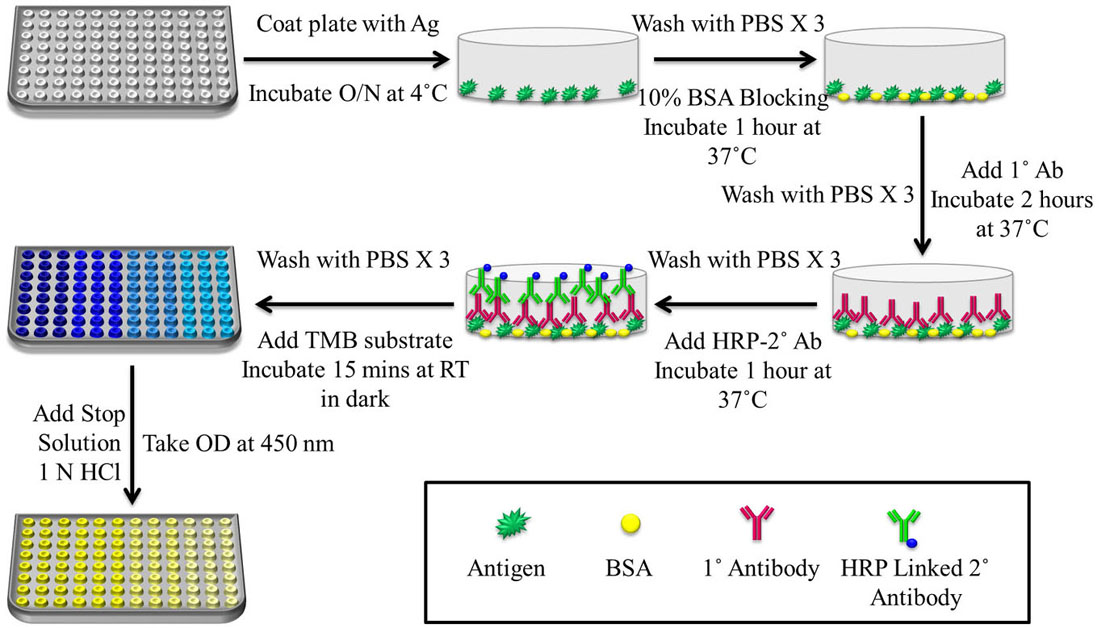
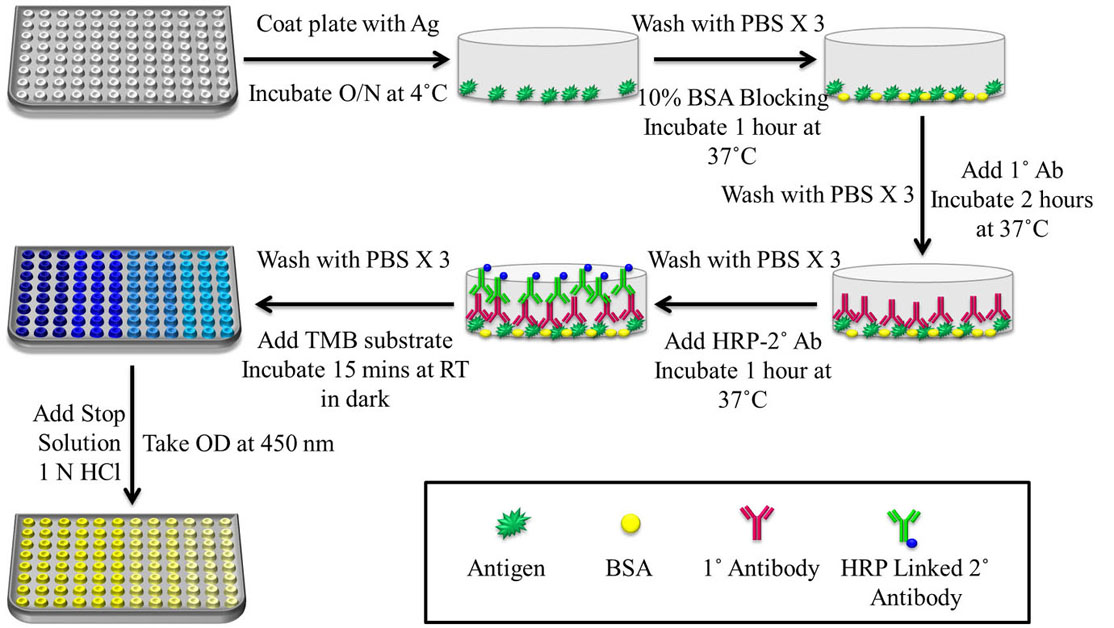 Figure 5
Figure 5Schematic representation of a basic protocol for indirect ELISA. The steps include coating of plate with Ag for overnight incubation at 4 ˚C, followed by washing with 0.02% Phosphate Buffer Saline-Tween 20 (PBS-T) and blocking for 1 h at 37 ˚C with 2% Skimmed Milk in Phosphate Buffer Saline-Skimmed Milk (PBS-M) or Bovine Serum Albumin (BSA). After washing with 0.02% PBS-T, Primary antibody (1˚ Ab) was allowed to incubate for 2 h at 37 ˚C, followed by washing with 0.02% PBS-T and Secondary antibody (2˚ Ab)-HRP incubation for 1 h at 37 ˚C. 3,3’,5,5’-Tetramethylbenzidine (TMB) substrate was added and after 15 min of incubation in the dark, 1 N Sulfuric Acid (H2SO4) was added as stop solution and absorbance was taken at 450 nm since the yellow colour formed after addition of stop solution gives a UV peak reading at 450 nm.
Additionally, a number of commercially sold ELISA kits are now available in the market for the detection of JEV both in humans as well as pigs, including the JE Detect IgM Capture ELISA (InBios, USA) and the Japanese Encephalitis-Dengue IgM Combo ELISA (Panbio, Australia) where both assays make use of the JE recombinant antigen (JERA), a serological marker made up of different parts of JEV antigens. It has also been shown that antibodies induced by natural infection and antibodies induced by vaccination can be differentiated based on a previously-published ELISA for detection of antibodies specific for the JEV non-structural protein 1 (NS1) which from a veterinary perspective, enables differentiation between infected and vaccinated animals (DIVA) (64). One of the most recent advances in terms of commercial ELISA kits is the JE IgM ELISA kit for the control of Swine and Detection of Antigen developed by the Indian Council of Agricultural Research (ICAR)-Indian Veterinary Research Institute (IVRI) which is really helpful for assessing the active infection of JE virus in the swine population.
Biosensors are the most recent development in the field of diagnostics as they helped to get rid of cumbersome laboratory detection techniques as well as reduce time and skilled labour. Biosensors are optical, chemical, mechanical or a combination of these sensors which can be used to detect the presence of chemical or biological species (antibody (Ab), antigen (Ag), Deoxyribonucleic Acid (DNA), enzyme), physical parameters (temperature, pressure, force, mass), or particles in nanoscale and convert it to analyzable microscopic and macroscopic level for easier detection and interpretation (65–69). The device usually has three simple units-Biorecognition elements, a signal amplifier and a transducer element. Biosensors, when modified with nano-sized material such as, metallic nanoparticles (NPs), nanopolymers, etc. mainly target to achieve higher sensitivity as NPs possess the property of signal amplification (70–74). Because of their high surface to volume ratio, a certain amount of biorecognition molecules (oligonucleotide detection probes as well as capture probes and antibodies) against target analytes can be conjugated with NPs. The main advantage of biosensors is that they can be used in Point of Care (PoC) diagnostics in the field for a rapid, sensitive and specific detection of JEV. Especially since the outbreak of JEV occurs mostly in rural areas, it is not feasible to have an elaborate laboratory set up and skilled technicians to carry out JEV diagnosis. Hence biosensors combined with NPs are the most recent advancement in the detection of JEV. Different types of biosensors along with their properties have been shown in Table 1.
| Type of Biosensor | LOD | Range of Detection | Reference |
|---|---|---|---|
| Silanized interdigitated sensor | 0.75 μg/mL | 1 to 10 μg/mL | (81) |
| FRET based virus-MIP fluorescent sensor | 9.6 pM | 24 to 960 pM | (82) |
| MIP silica microspheres based fluorescence sensor | 0.11 pM | 2.4 to 24 pM | (83) |
| Magnetic MIP based resonance light scattering sensor | 1.3 pM | - | (84) |
| Electrochemical Immunosensor using APTES-glutaraldehyde-serum | 10 ng/mL | 25 ng/mL to 1 μg/mL | (85) |
| Polyaniline nanowires-based interdigitated platinum sensor | <10 ng/mL | 10 to 500 ng/mL | (86) |
| Gold coated magnetic bead based sensor with MWCNT | 0.56 ng/mL | 0.84 to 11,200 ng/mL | (87) |
| AuNP based SPCE electrochemical impedimetric sensor | 167 pfu/mL | 500 to 5 × 105 pfu/mL | (88) |
| AgNP based silanized glass slide sensor | 12.8 ng/mL | 14 to 100 ng/mL | (34) |
| CNP (from starch NP) based SPCE electrochemical sensor | 2 ng/mL | 5 to 20 ng/mL | (89) |
| CNP (from chitosan NP) based SPCE electrochemical sensor | 0.36 ng/mL | 1 to 20 ng/mL | (35) |
A novel silver nanoparticles (AgNPs) based optical sensing probe was developed for the detection of Japanese Encephalitis virus (JEV) (34). Optical Surface Plasmon Resonance (SPR) based biosensors make use of the unique optical properties of novel metal NPs i.e. Surface Plasmon Polariton (SPP) waves. SPR-based optical biosensors are very sensitive and respond to changes in refractive index (RI) of the analyte, so they can be used to detect the presence of biomolecules with the biorecognition element which is used as the material of the analyte (75). In this biosensor, AgNPs were initially synthesized using the Turkevich method (76) and deposited onto amine functionalized glass slides using (3-Aminopropyl)triethoxysilane (APTES). They were characterized by Transmission Electron Microscope (TEM) as well as Ultraviolet-Visible (UV-Vis) Spectroscopy. JEV antibodies were self-assembled onto the surface of AgNPs to form optical sensing probes. After blocking by BSA, JEV Ag was allowed to bind to the immobilized JEV Abs on the sensor, causing a change in the light absorbance of the AgNPs which could be detected using optical sensors. Every step of the fabrication of the biosensor, as shown in Figure 6., was characterized using different methods such as Field Emission Scanning Electron Microscopy (FESEM), Fourier-Transform Infrared (FTIR) Spectroscopy and UV-Vis Spectroscopy. This work led to the development of a highly sensitive and rapid optical sensing probe for JEV antigen with a limit of detection (LOD) of 12.8 ng/mL (for Signal to Noise (S/N) ratio = 3) and an analysis assay time of 1 h. The device could be stored for up to 6 months in a dry environment at 4 ˚C (34).
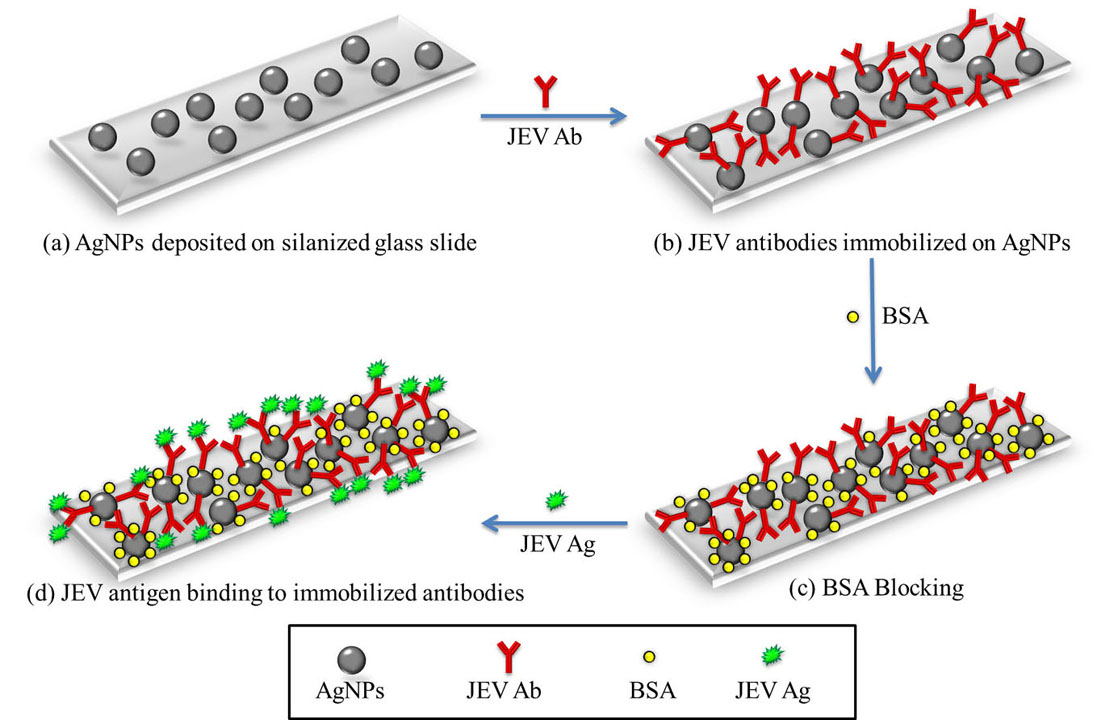
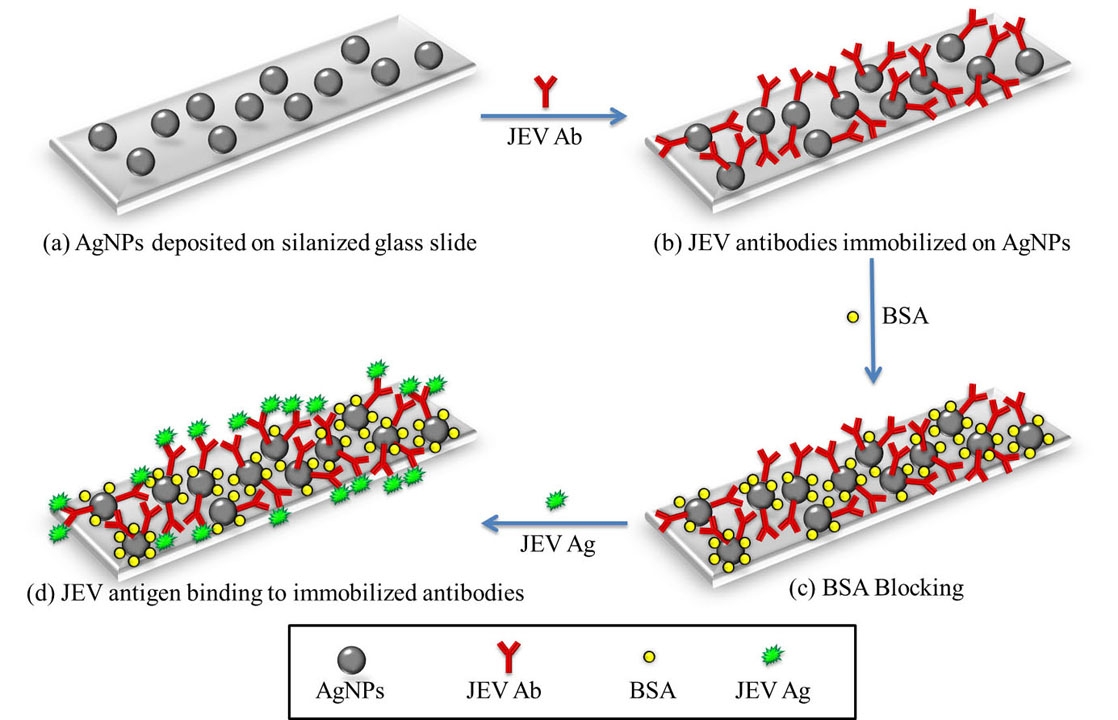 Figure 6
Figure 6(a) AgNPs were first synthesized and deposited onto a silanized glass slide using APTES. (b) JEV antibodies were immobilized onto the AgNPs. (c) The unbound surface of the AgNPs were blocked by BSA to avoid non-specific binding of the antigen. (d) The JEV antigen bind to the antibodies and were detected by checking absorbance at 427 nm since AgNPs show a UV absorbance peak at 427 nm and as the AgNPs conjugate with different analytes, a shift in this peak is observed.
Another biosensor developed was a disposable and sensitive electrochemical biosensor strip based on carbon nanoparticles modified screen-printed carbon electrode (SPCE) for rapid and sensitive detection of JEV. Screen printed technology has led to the development of highly sensitive and selective electrochemical sensors (77) and the emergence of several carbon materials such as graphene, there has been a considerable advancement in the scope of carbon based electrodes such as SPCEs (78). SPCEs are able to detect changes in current in the presence of analytes, biomolecules and biorecognition elements. In this research work, amino group functionalized carbon nanoparticles (CNPs) were prepared from preformed chitosan nanoparticles. JEV antibodies were immobilized onto the surface of CNPs through amide bond formation, i.e. 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide-N-Hydroxysuccinimide (EDC-NHS) coupling, between amino groups of CNPs and carboxylic groups of JEV antibody. This was incubated on the working electrode of an SPCE for 48 h at 37 ˚C and then the non-specific binding sites were blocking using 2% BSA. The fabrication of this biosensor has been shown in Figure 7. This biosensor was used to detect JEV antigen in serum by incubating the sample onto the SPCE at 37˚C for. Scanning Electron Microscope (SEM) and FTIR spectroscopy were used to characterize the fabrication steps of the biosensor and the analytical performance of SPCE electrochemical biosensor strip was characterized using Cyclic Voltammetry (CV) and Electrochemical Impedance Spectroscopy (EIS). Changes in the electrical signals were used to detect the presence or absence of JEV. SPCE electrochemical biosensor strip exhibited a linear detection range of 1–20 ng/mL with a low detection limit of 0.36 ng/mL (at S/N = 3) for JEV, detection sensitivity was 0.024 ng/mL for JEV, and analysis results were obtained within 10 min (35).
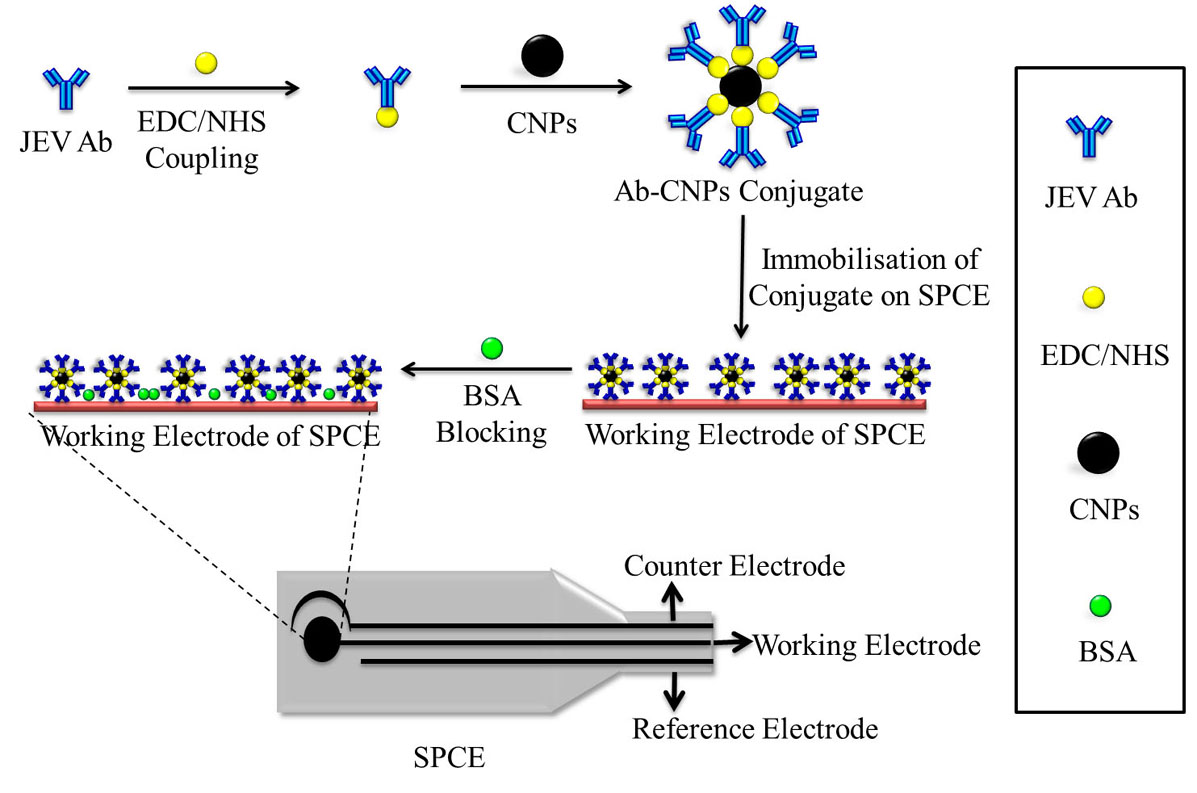
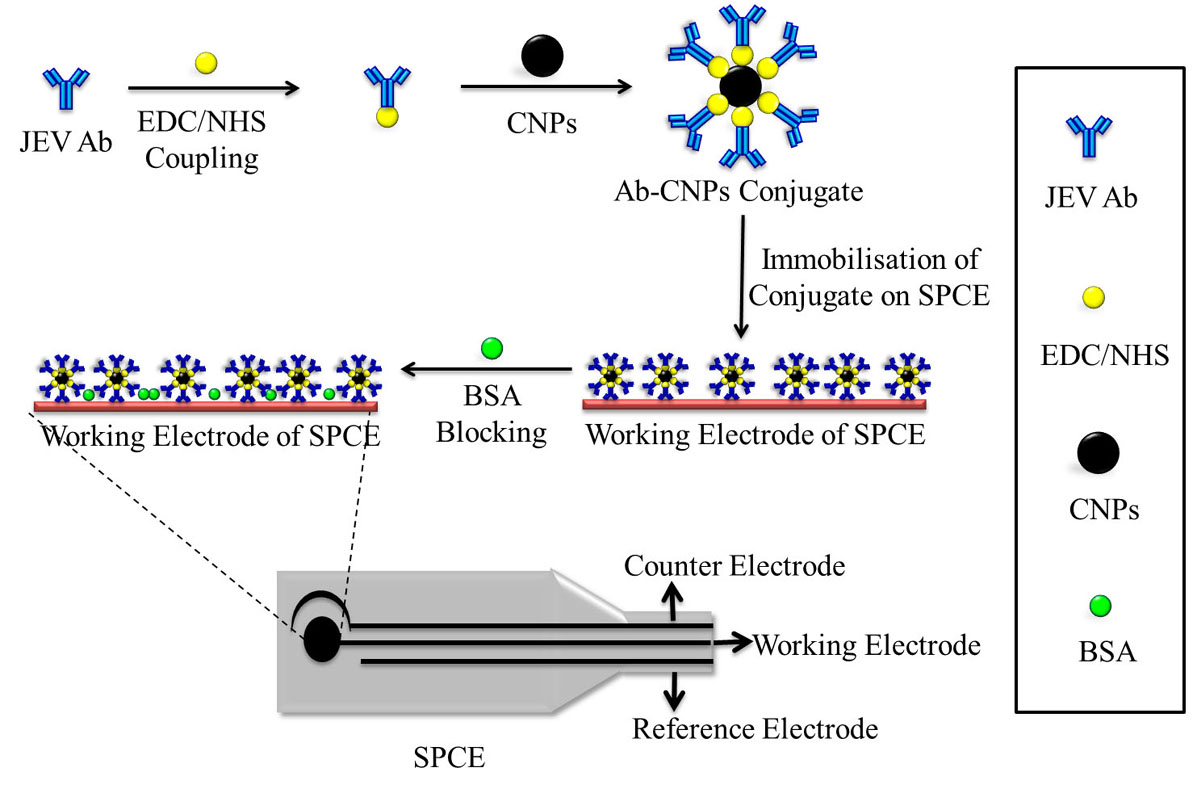 Figure 7
Figure 7The schematic representation of the fabrication of CNPs based SPCE biosensor for the detection of JEV. The antibody is bound to the CNPs by EDC/NHS coupling and then immobilized on the working electrode of the SPCE. The unspecific binding sites on the electrode are blocked using BSA. All steps of the fabrication process are characterized using CV and EIS.
Also a Lateral Flow Assay (LFA) has been developed for detecting JEV antibodies in swine sera (33). Over the years, LFA has generated both scientific and industrial interest, due to its attractive ability of rapid, one-step, in-situ analysis (79). The advantage of LFA is that it can be used to combine a number of variants such as formats, labels, biorecognition molecules, detection systems and applications (80). In this study, three different formats of lateral flow assays were tried using recombinant NS1 protein as antigen in order to select the best format as shown in Figure 8. The property of the gold nanoparticles (AuNPs) to help develop a red colour has been made use of in this experiment. In format I, gold nanoparticles were conjugated with antigen followed by spotting of antigen on Nitrocellulose Membrane (NCM) as test line and anti-antigen IgG on NCM as control line. This format worked with the rabbit hyper immune serum but not on the positive field serum samples from pigs. Control line was formed for both positive and negative samples but no test line formed for positive samples. Therefore, the format I was not found suitable for screening of JEV antibodies. In format II, gold nanoparticles were conjugated with antigen followed by spotting of staphylococcal protein A as test line and anti-antigen IgG as control line. Format III used gold nanoparticles conjugated with goat anti-pig IgG followed by spotting of antigen as test line and pig IgG as control line. Both LFA II and LFA III showed positive results and the comparative diagnostic sensitivity of format III with respect to format II was 25.64% (20/78) while comparative diagnostic specificity was 100% (100/100). Hence, amongst the three formats, format II was found to be superior with 100% relative diagnostic sensitivity and 100% relative diagnostic specificity during monsoon and post-monsoon period. A panel of 500 field swine serum samples was tested using format II which revealed sero-positivity of 15.6%, and the format was found suitable to screen swine serum samples during monsoon and post-monsoon period.
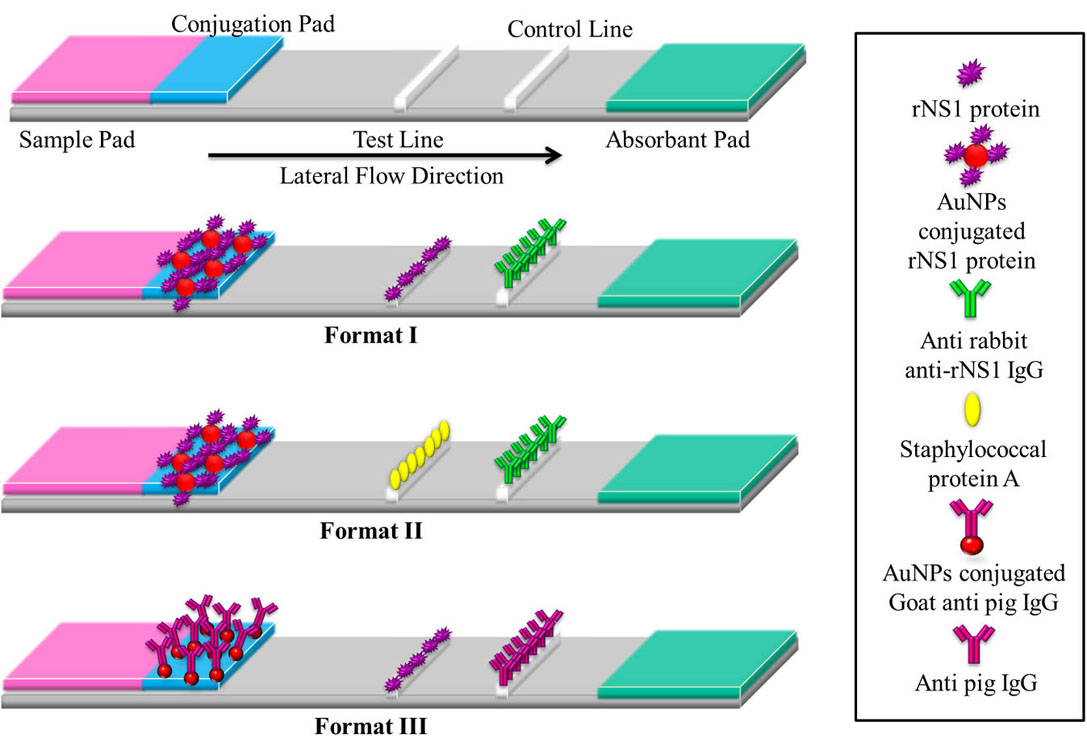
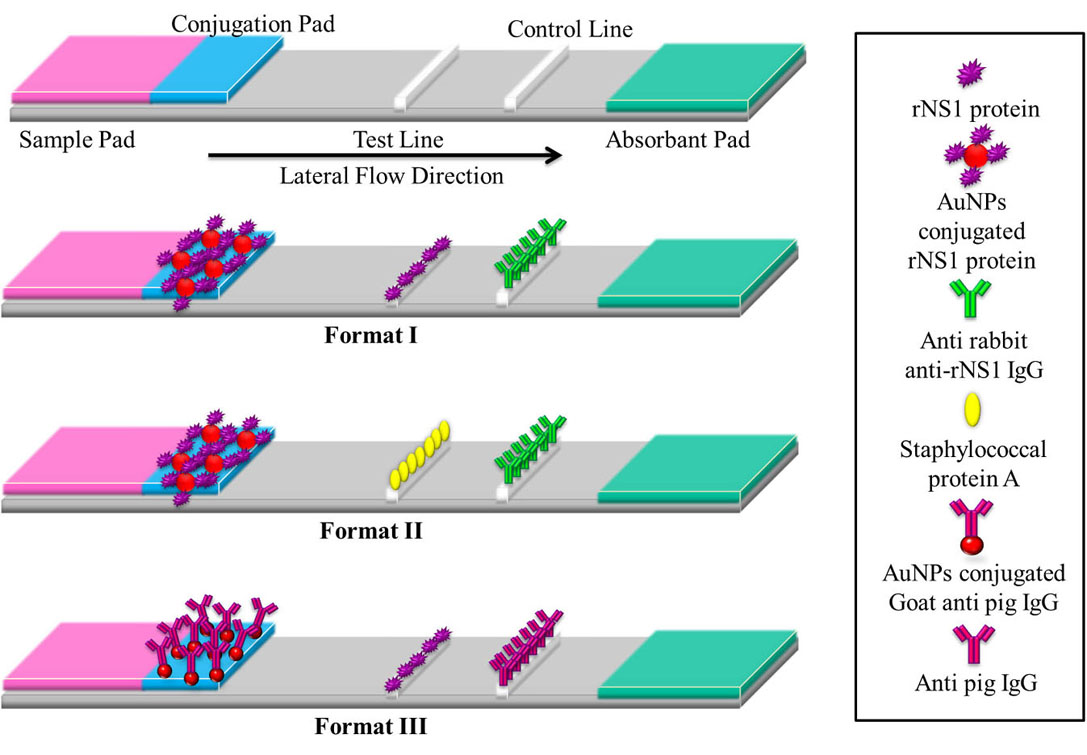 Figure 8
Figure 8The basic layout of a lateral flow assay strip comprises of a base nitrocellulose membrane, a sample pad for loading of sample, a conjugation pad for immobilization of bio recognition element, a test line for detection of analyte and a control line for confirmation of flow. Format I- AuNPs conjugated rNS1 protein immobilized on conjugation pad, rNS1 protein on test line and anti-rabbit anti-rNS1 IgG on control line. Format II- AuNPs conjugated rNS1 protein immobilized on conjugation pad, staphylococcal protein A on test line and anti-rabbit anti-rNS1 IgG on control line. Format III- AuNPs conjugated Goat anti-pig IgG on conjugation pad, rNS1 protein on test line and Anti-pig IgG on control line.
Despite the advances and research being done in the field of biosensors, there are still a number of limitations that need to be addressed and minimized to improve these detection techniques. Electrochemical, piezoelectric and mechanical biosensors may be slightly expensive to fabricate and require instrumentation for analysis which does not make them portable for on field detection unlike LFA and paper based assays. However, LFAs and paper based assays are usually qualitative on field but require instrumentation for quantitative analysis. Hence, research is required to develop on field portable instruments, that will allow qualitative as well as quantitative analysis of biosensors. Also, in certain cases, sample preparation may be required before application on the biosensor which requires skilled personnel. Biosensors also require to be standardized under different environmental, pH and temperature conditions as slight changes may result in false detection. All these limitations need to be overcome to make biosensors the most preferred method of detection.
Due to the various constraints involved in the detection of JEV is difficult and often misdiagnosed as Dengue in earlier stages. Hence, a combination of clinical, serological and pathological tests is necessary to positively determine JEV. This includes older cumbersome tests such as virus isolation, plaque reduction neutralization test, hemagglutination inhibition test, complement fixation test, immunofluorescence test, molecular assays as well as newer, quicker detection tests such as ELISA and immunosensors. In this review, we have discussed in detail the older methods of diagnosis and their shortcomings which have led to the advancement in diagnostic techniques and their advantages. While the older methods were more cumbersome, time-consuming and required a laboratory set-up, advanced techniques give quicker sensitive and specific results and are often portable and do not require elaborate instrumentation. Also newer methods such as ELISA and biosensor detection pose less risk to the person carrying out the diagnosis as there is no requirement for BSL-3 protection. Biosensors such as LFAs and paper based colorimetric assays are inexpensive, stable and easy to fabricate and do not need expensive instrumentation and laboratory set ups. This is a major advantage in making disposable sensors that are cheap for use in rural areas. Hence, development of biosensors making use of NPs shows a lot of future prospect in making portable diagnostic kits for quick, sensitive, early and specific detection of JEV. Currently, very few biosensors exist for the detection JEV and most of them are electrochemical in nature and not portable. Development of novel biosensors based on paper assays, piezoelectric biosensors, optical biosensors, etc. for JEV detection is the need of the hour and a lot of research is required in this field.
We acknowledge the Department of Biotechnology (DBT), New Delhi, for the grant BT/AAQ/01/NIAB-Flagship/2019 and C0038 as internal core research support from DBT-National Institute of Animal Biotechnology (DBT-NIAB), Hyderabad, India to support JEV research work. A.R. is grateful for the DST-INSPIRE fellowship IF180729, sponsored by the Department of Science and Technology (DST), New Delhi.
Ab
Antibody
Antigen
Silver Nanoparticles
Gold Nanoparticles
(3-Aminopropyl)triethoxysilane
Baby Hamster Kidney
Bovine Serum Albumin
Biological Safety Level-3
Capsid
Complement Fixation Test
Carbon Nanoparticles
Central Nervous System
Cyclic Voltammetry
Envelope
1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide
Electrochemical Impedance Spectroscopy
Field Emission Scanning Electron Microscope
Fluorescence Resonance Energy Transfer
Fourier-Transform Infrared
Enzyme-Linked Immunosorbent Assay
Hydrochloric Acid
Hemagglutination Inhibition
Immunofluorescence Assay
Immunoglobulin G
Immunoglobulin M
Indian Council of Agricultural Research
Indirect Immunofluorescence Test
Indian Veterinary Research Institute
Japanese Encephalitis Recombinant Antigen
Japanese Encephalitis Virus
Lateral Flow Assay
Limit of Detection
Molecularly Imprinted Polymers
Multi-walled Carbon Nanotubes
Nitrocellulose Membrane
N- Hydroxysuccinimide
Nanoparticles
Non Structural
Office International des Epizooties
Open Reading Frame
Phosphate Buffer Saline
Polymerase Chain Reaction
Plaque Forming Units
Point of Care
Personal Protective Equipment
Pre Membrane
Plaque Reduction Neutralization Test
Refractive Index
Ribonucleic Acid
Reverse Transcription Loop-Mediated Isothermal Amplification
Reverse Transcriptase Polymerase Chain Reaction
Scanning Electron Microscope
Signal to Noise Ratio
Screen-Printed Carbon Electrode
Surface Plasmon Polariton
Surface Plasmon Resonance
Transmission Electron Microscope
3,3’,5,5’- Tetramethylbenzidine
Untranslated Region
Ultra Violet-Visible
World Health Orgnaization