Frontiers in Bioscience-Landmark (FBL) is published by IMR Press from Volume 26 Issue 5 (2021). Previous articles were published by another publisher on a subscription basis, and they are hosted by IMR Press on imrpress.com as a courtesy and upon agreement with Frontiers in Bioscience.
1 Department of Functional Chemistry, Kyoto Institute of Technology, Kyoto, 606-8585, Japan
2 Department of Biology, Cantho University, Cantho City, 900000, Vietnam
Abstract
Developmental processes are cascades of biological changes linked with information transfer, growth, and differentiation during the life cycle of an organism. Lipid metabolism plays a vital role in the life cycle of organisms. Drosophila models grant numerous advantages in investigating the underlying mechanisms of each process as well as their connections. In each section of this review, we will discuss multiple studies revealing the function of lipid-related genes in different stages of early development: spermatogenesis, oogenesis, embryogenesis along with late development in life cycle of Drosophila.
Keywords
- Lipid Metabolism
- Development
- Spermatogenesis
- Oogenesis
- Embryogenesis
- Review
‘Lipid’ is a general term used for substances that are non-polar and insoluble in water. It comprises a wide range of compounds with differing chemical structures. Lipid function varies from storing energy to acting as structural components of cell membranes, to participating in various biological processes. Lipid metabolism is a balance between lipid synthesis and degradation that determines the fat mass (2). The synthesis of lipids in tissues has been considered to be essential for component and energy metabolism during cell transformation. Therefore, lipid metabolism is crucial in the development of organisms. Drosophila melanogaster, the fruit fly, is one of the most commonly used model organisms in biomedical science (3). Most of the metabolism-related genes and gene families are conserved between Drosophila and humans (4). Additionally, many analogous organ systems involved in nutrient uptake, storage and metabolism are common in humans and fruit flies. Moreover, in Drosophila, lipids are stored in the form of triacylglycerol (TAG) in lipid droplets, which are similar to adipocyte cells in mammals. Lipid droplets are omnipresent and dynamically regulated organelles, which are found in various cell types throughout the complex life cycle of the flies (5). These features make Drosophila a versatile model for studying the mechanisms of developmental and processes of lipid metabolism. Previous studies have revealed the function of several lipid-related genes involved in the development, however the overall connection remains to be unveiled. In this article, we selectively review several studies in regard of the link between lipid metabolism and development to generate consistent understanding, as well as encourage further investigations.
Lipid metabolism in Drosophila is divided into two main distinct processes, one is the formation of lipid in the form of TAG called lipogenesis and the other is lipolysis or mobilization of TAG from lipid droplet. The lipogenesis, TAG synthesis follows Kennedy pathway consisting of four enzymatic reactions. Three of four reactions are catalyzed by acyltransferases using fatty acid Coenzyme A (FA-CoA) (6). The initial TAG synthesis step is the acylation of glycerol-3-phosphate (G-3-P) to lysophosphatidic acid (LPA) catalyzed by glycerol-3-phosphate acyltransferase (GPAT) (7). Next, the acylation of LPA to produce phosphatidic acid (PA) catalyzed by 1-acylglycerol-3-phosphate O-acyltransferase (AGPAT) (8). In the third step, the dephosphorylation of PA to diacylglycerol (DAG) at the endoplasmic reticulum (ER) membrane is catalyzed by Mg2+-dependent PA phosphatase, Lipin (9). Lipin is a multi-functional protein that acts as enzyme in TAG synthesis. It is also present in nucleus as a transcriptional co-activator in a complex with peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1alpha) and peroxisome proliferator-activated receptor alpha (PPARalpha), which are master regulators of genes related to mitochondrial biogenesis and fatty acid oxidation (10, 11). The final step from DAG to TAG is catalyzed by diacylglycerol O-acyltransferase (DGAT), encoded by CG1941, CG1942, or CG1943, which remains relatively unexplored (5, 12).
Lipid droplets are made up of neutral lipid core encapsulated with a lipid monolayer with proteins, the best known of which is a family of protein named, PAT domain proteins. The PAT domain proteins include adipose differentiation-related protein (ADRP) and TIP47, and collectively named Perilipin (PLIN) (13). In Drosophila, there are two types of PLIN, called lipid storage droplet (LSD)-1 and 2. The interaction between LSD-1/2 and lipases in lipid droplet varies in associated with different stages of lipolysis according to the body`s requirement of lipid. In basal lipolysis, LSD-1 prevents the access of lipases to lipids droplets, and suppresses activation of Brummer lipase (Bmm), a homolog of human adipocyte triglyceride lipase. This is done by composing a complex with comparative gene identification-58 (CGI-58) which is an activator of Bmm. In stimulated lipolysis, LSD-1 is phosphorylated by protein kinase A (PKA) in response to hormonal signals, and phosphorylated LSD-1 facilitates maximal lipolysis by recruiting hormone-sensitive lipase (HSL) and allowing Bmm to access the lipid droplet (14, 15), while LSD-2 protect lipid droplet from Bmm and HSL-mediated lipolysis
We are able to establish versatile research models for understanding lipid metabolism. An in vivo high throughput obesity study screening more than 500 candidates identified numerous genes which may cause obesity, most of which are related to lipid metabolism. However, several exceptions were shown, including interferon-responsive genes ARID2; the interleukin binding factor ILF2; some ubiquitin enzymes UBE2N, UBR2, HERC4, and FBWX5; and lastly, eight members of Hedgehog signaling pathway which in turn were revealed to be regulators of brown/white adipose cell fate in mice (16). Quantification of TAG content in individuals is a conventional method to identify obesity in Drosophila. Triacylglycerol can be measured using various techniques such as, thin layer chromatography (17), mass spectrometry (18), colorimetric sulfo-phospho-vanillin (19), or indirectly estimation with enzymatic assay post-lipolysis (20). Obesity can also be characterized at the cellular level via quantification of size and number of lipid droplets in the body (21). Similar to mammals, feeding a high-sugar diet to Drosophila model produces hyperglycemia, insulin resistance, and obesity, which imitate type 2 diabetes. Several transcriptional alterations were found, suggesting that Drosophila fed with high-sugar diet could be a potential model for screening genes and pathways contributing to insulin resistance (22). Likewise, high-fat diet can also produce obesity phenotype and promotes insulin resistance in Drosophila (23), as well as enhances the synthesis of cardiomyocyte-derived apoB-lipoproteins (24). In our recent study, we generated a Drosophila model for screening anti-obesity substances (25). We introduced the fusion gene of bmm promoter and enhanced green fluorescent protein (EGFP) gene with nuclear localization sequence in Drosophila. The GFP intensity in nucleus of salivary gland showed good negative correlation with obesity, suggesting that the transgenic fly is useful for screening anti-obesity candidates. By oral administration of various substances to 3rd-instar larvae, we found that histone deacetylases (HDAC) 8 and 9 inhibitors as well as several natural substances, including mulberry leaf, cabbage, and red paprika have potential anti-obesity. In contrary, flies fed with dried tomato showed a slightly decrease in GFP signal, suggesting an increase in lipid storage (25).
Spermatogenesis in Drosophila is a process to produce mature spermatozoa from germ-line stem cells (GSCs). The GSCs will be divided by mitosis to form spermatocytes, then undergo meiosis and cytokinesis to form spermatids, followed by steps of elongation, individualization and coiling to emerge as mature sperms (26). Since Drosophila testes is an organ rich of lipids, multiple membranes remodeling processes occur there, including cytokinesis and differentiation of sperm. The first evidence of a relation between fatty acid and spermatogenesis dated back to the characterization of scully (scu), a homolog of mammalian mitochondrial type II L-3-hydroxyacyl-CoA dehydrogenase, which is involved in beta-oxidation of short chain fatty acids (27). Scu mutants showed phenotypes with significant reductions in size of testes and degeneration of spermatocytes which was caused by abnormal accumulations of lipids (27). On the other hand, very long chain fatty acids (VLCFAs) with over 20 carbon chain-length are components of cellular lipid and also precursors of lipid regulators (28). Cyst cell-specific RNAi of noa (also known as Baldspot which encodes for ELOVL6, a member of elongases for synthesizing VLCFA) resulted in defects in the individualization process during spermatogenesis. Also, the noa gene activity seems to require the communication between cyst cells and germ cells, indicating that cyst cell-specific NOA plays an important role in germ cells development (29). In germ cells, a study showed the necessity of VLCFAs for successful cleavage-furrow ingression during cell division in spermatocytes, since a loss-of-function mutant in Drosophila bond gene, which encodes another member of ELOVL enzyme family, causes male-sterile phenotype (30). Bond also plays a central role in the production of Drosophila sex pheromone CH503, thus controlling the male fertility and rivalry of fertility (31). Therefore, elongation of VLCFAs on both cyst cell and germ cell are crucial for successful spermatogenesis. Moreover, in mammals, beta-oxidation of VLCFAs is performed in peroxisomes and peroxin (pex), an exclusive protein family in peroxisomes, participates in maintaining Drosophila male fertility. Mutants in pex2, pex10, pex13 show elevated levels of VLCFAs in spermatocytes, which lead to defected cytokinesis and misshapen elongated spermatid (32). Besides, lysophospholipid acyltransferase (LPLAT) also contributes in proper spermatogenesis as three Drosophila homologs of membrane-bound O-acyltransferase domain containing 1 (MBOAT1), Oys, Nes, and Frj were found to have combined effect on male fertility. Males with Oys-nes double mutant and oys-nes-frj triple mutant produce defective spermatid individualization phenotype, which can be explained by an elevated level of the saturated fatty acid content of several phospholipids (33).
Phosphatidylinositol (PI) metabolism pathway has been well characterized in Drosophila, which is a cycle between PI, PI-4-phosphate (PI4P), PI-4, 5-bisphosphate (PIP2), and PI 3, 4, 5-triphosphate (PIP3) under the regulation of multiple kinases and phosphatases. By regulating the level of these components, the PI pathway-related genes show their function in controlling sperm development. Reduced level of PIP2 by low-level expression of SigD or null mutation of Sktl (PI 5-kinase, PI5K) results in formation of abnormal bipolar spermatid cysts (34). Contrastingly, overexpression of SigD in testes leads to decrease in number of elongated spermatids, interfering with the ability of docking the basal body to the nuclear envelope, as well as disrupting normal development of flagellar axoneme (35). Notably, co-expression of Sktl, which promotes PIP2 production, suppress the phenotype of SigD overexpression (35). Besides PIP2-related gene, other genes like four wheel drive (fwd) (encodes for kinase PI4Kbeta) or giotto (also called vibrator) also cause cytokinesis defects in spermatids meiosis due to furrow instability (36–38). Further study showed that rab11, a small GTPase, is the link that mediated by both, Fwd and Giotto in the same cytokinetic pathway, responsible for proper cytokinesis (39).
Two cholesterol trafficking proteins, Niemann-Pick type C (NPC) and Oxysterol binding protein (OSBP), have been well studied for their link to Drosophila spermatogenesis. The npc1 (one of two genes encoding NPC) null mutants are male sterile due to the dysfunction in spermatids individualization (40). This study also found the phenotypes are independent of ecdysone, which suggests the requirement of cholesterol transport into testes by NPC to perform individualization (40). In vivo loss-of-function mutants of osbp exhibits spermatids individualization defective phenotype, cooperated by FAN (a member of testes-specific vesicle-associated membrane protein-associated protein, VAP protein). Furthermore, the sterility phenotype of osbp mutant can be rescued by feeding cholesterol, confirming the relation between cholesterol and spermatogenesis (41). Taken together, fatty acid, PI and cholesterol plays essential roles in multiple biological processes of Drosophila spermatogenesis. The fact that the level of these lipids needs to be adequately regulated in spermatocytes for right functions strengthen the contribution of lipid metabolism-related genes for spermatogenesis.
Early studies had revealed the crucial roles of lipid droplet in Drosophila embryo development by investigating its impact and kinetics of embryo vesicle transport (42) or analyzing its associated proteins (43). One of the first lipid metabolism-related genes which had been characterized in embryo development was midway (mdy) gene. The mdy encodes for Drosophila homolog of diglyceride O-acyltransferase (DGAT), which converts DAG into TAG. Mutants of mdy showed the diminished accumulation of neutral lipid, and subsequently induced apoptosis of egg chamber during mid-oogenesis (44). Wun and wun-2 act as lipid phosphate phosphatases and are necessary for germline development in Drosophila embryo. Wun/wun-2 mutations affect the polarity of primordial germ cells (PGCs) as well as prevent them from migrating laterally to the middle of the embryo. The PGCs that fails to relocate would undergo Wun/Wun-2-dependent manner cell death pathway (45). Furthermore, it is suggested that Wun and Wun-2 may participate in the same process with Oys and Nes functions of which were mentioned in spermatogenesis. In female oys and nes double mutant, migration of embryo germ cells is disrupted. These two genes seem to work together since the phenotype is not observed in single mutant of oys, nes or frj. Surprisingly, triple mutants of these genes produce no stronger phenotypes indicating that Frj may not need for this process (33). The effect of Nes expression, however, can be significantly increased by zygotic expression of Wun-2 (33).
The components of the PI pathway also contribute in keeping embryogenesis intact. The PI 4-kinase alpha (PI4KIIIa) is required for enrichment of PI4P and PIP2, which is essential for actin formation, membrane trafficking, and cell polarity. Null mutant of PI4KIIIa, exhibits the effects on egg chamber formation that differ from those of null mutants of fwd (encodes PI4Kβ) and PI4KII (also encodes synthesis enzymes of PI4P and PIP2) (37). Further investigation suggested that the phenotype of PI4KIIIa mutants are more likely to affect Golgi rather than the plasma membrane (46). In contrary, PI4KIIIa gives a similar effect with Sktl (PIP5K), another PIP2-regulating enzyme, in the process of maintaining egg chamber polarity (46, 47). On a side note, another member of PI kinases family, PI3K has been shown to be involved in regulating cell migration, which is crucial for embryonic development, in many different cell types via directly binding their kinase products to proteins (48, 49). Akt, a downstream kinase of PI3K, is similarly, required for normal embryo development, as reduced levels of Akt leads to incomplete centrosome migration, corrupted mitotic spindles, and loss of nuclei trafficking into the embryos (50).
Clearance of histones is necessary in most cells of Drosophila since free histones are toxic, yet in Drosophila embryo, high level of extranuclear histones are accumulated, suggesting its necessity in early development. In this stage, histones are bounded to lipid droplets for its safe storage and delivery, thus, indicating another role of lipid in embryogenesis (43). Jabba is a lipid droplet protein that required for docking of histones to the adipocyte-like organelles. In jabba mutants’ embryos, histones levels of H2A, H2B, and H2Av reduces significantly, despite the mutants develops normally due to the immediate biosynthesis of new histones to compensate for the lacking (51). However, further investigation pointed that jabba mutants’ embryos under conditions of increased temperature induced to hasten the development (from 21°C to 25°C) cannot recruit new histones fast enough to deal with this challenge. This leads to nuclear falling and reduced hatching of eggs (52). Recently, Jabba was characterized as a substrate of casein kinase 2 (CK2) and essential component for an earlier developmental process, oogenesis, as shRNA targeting jabba in female exhibits reduced egg production (53). In addition to roles of lipids in oogenesis, two lipid-related genes, fatty acid synthases named, Bad egg and a homolog of PGC-1 called, Spargel which regulates the formation of eggshells in Drosophila ovary were identified in a screening study of patterning-defect lines. Mutants of bad egg exhibited thin shell phenotype while mutants of spargel caused long pair of dorsal appendages, which act as a respiratory organelle of Drosophila egg (54). It is worth to mention that lipid-related genes can affect early development in yet another manner; two enzymes, Minotaur and Zucchini, which conventionally act in the biosynthesis pathway of phosphatidic acid (PA), also revealed to have critical roles in piRNA biosynthesis (55, 56). piRNA, in turn, guides Piwi proteins to form a molecular code that separates transposons from endogenous genes and prevents germ cell genomes against the activity of those genetic elements (56). All those evidences collectively exhibit that lipid metabolism – related genes regulate oogenesis and embryogenesis in various manners.
Lipid droplets function through all development stages of Drosophila. They are not only detected in adipose tissue but also present in other tissues such as imaginal discs, which give rise to adult body structures like eyes, legs, wing after metamorphosis, salivary glands, gut, the Malpighian tubules, etc. (5). In the fat body of Drosophila, ectopically expressed GFP-tagged lipid storage droplet 1 or 2 (LSD-1 or LSD-2) reside in the lipid droplets (57). Interestingly, our studies showed that the expression of LSD-1 and LSD-2 is not only essential for lipid metabolism but also plays a crucial function in development. The dysfunction of Lsd-1, a Drosophila homolog of perilipin 1 (PLIN1), on the wing disc by genetic knockdown leads to disruption of normal wing development. Further investigation suggested that the loss of LSD-1 function release distress signals in mitochondria, which decrease ATP production while increasing ROS generation and eventually result in cell death (58). On the other hand, while LSD-2 does not show any noticeable effects in eye, hemocytes, nervous system, or thorax; we found that genetic knockdown of lsd-2 also interrupts Drosophila wing formation via inducing cell death. However, unlike LSD-1, we did not find an increase in ROS generation in LSD-2 knockdown flies, but instead, the expression of a highly anticipated transcription factors participating in development, dFoxO and its target in caspase-dependent apoptotic pathway Reaper (Rpr) are significantly up-regulated. Moreover, loss-of-function dFoxO in LSD-2 knockdown flies can rescue the curly wing phenotype and suppress the cell death signal, while overexpression of dFoxO worsens the outcomes (59). Additionally, several studies in other models showed the role of PLIN1in inflammatory responses in lean adipose tissue through lipid dysregulation (60), and that PLIN2 associates with the progression of the age-related disease, such as fatty liver, type 2 diabetes, sarcopenia, and cancer (61).
Furthermore, there are plenty of evidences suggesting the expression of lipid-related proteins in non-adipocyte tissues may link to development. The enzyme of lipogenesis processes, Drosophila 1-acyl-sn-glycerol-3-phosphate acyltransferase 1/2 (dAGPAT1/2, CG3812) expresses exclusively in the nervous system, testes, and ovaries (62). One of the phosphatidate phosphatases, dLipin, is considered as a strong link in development. The decreased expression of this gene not only affect the healthy development of fat body, but also involves in that down-regulation of the insulin-receptor-controlled PI3K-Akt pathway and increased hemolymph sugar levels (63). Schmitt et al. indicated that insulin signaling pathways and a well-known development pathway, target of rapamycin complex 1 (TORC-1), independently regulates the nuclear translocation function of dLipin (63). In mammals, blocking of TORC1 dephosphorylates Lipin 1, leads to its translocation from cytoplasm into the nucleus, where it affects nuclear protein levels, but not mRNA levels, of the transcription factor sterol regulatory element-binding protein 1 (SREBP1). The SREBP1 controls the production of cholesterol, fatty acid, TAG and phospholipid (64). DGAT1 encoded by mdy expresses during all stages of Drosophila development in widely specific tissues such as fat body, ovaries, embryonic, salivary gland, etc. (44). Last but not least, the expression of crucial lipase, Bmm was observed in the multiple post-embryonic organs or tissues including midgut, hindgut, heart, fat body, and salivary gland (62). These findings, taken together, motivate us to investigate the function of lipid metabolism-related genes on the development of specific tissues in Drosophila.
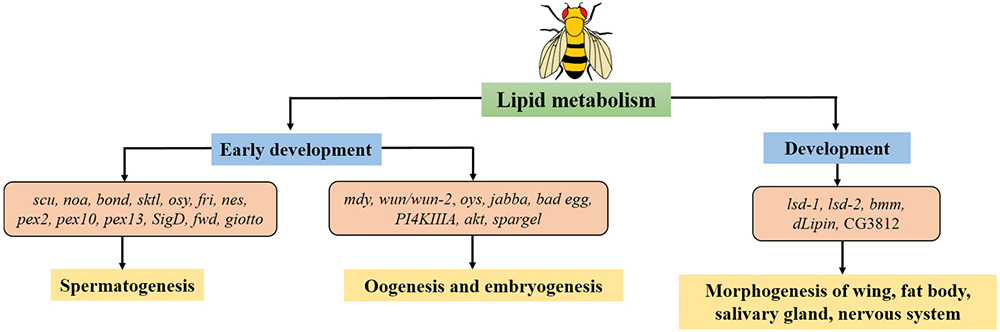
Drosophila has always been considered as a powerful model not only for study in development, but also in lipid metabolism, because of their high resemblance to human genome. It thus provides us with homogenous mechanisms in related disorders. In this review, our goal was to gather the connection between those two aspects: lipid metabolism and development. Various lipid-related genes act together to regulate specific lipid levels which is required to perform crucial biological processes in the membrane during spermatogenesis, or forming eggshells during oogenesis, as well as guiding cell migration during embryogenesis. Beyond that, in recent studies, lipid-related genes prove themselves as participants in multiple developmental processes suggesting their deeper involvement regardless of energy supply. Collectively, these findings not only provide a better understanding of the link between lipid metabolism and development, but also reassure the efficiency of Drosophila model in tackling this matter (Figure 1). In the future, more comprehensive studies on the role of lipid regulation in development and related phenotypes using Drosophila models will be necessary to identify the principle of various associated disorders.
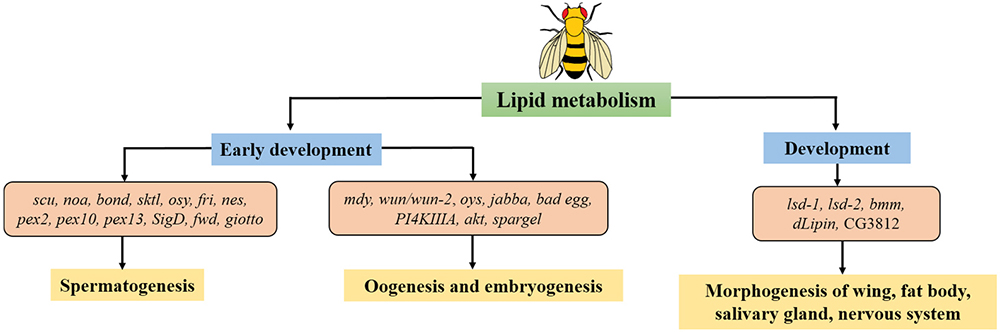 Figure 1
Figure 1Lipid metabolism relates to different stages of development in Drosophila. There are lipid-related genes function in early development: spermatogenesis, oogenesis, embryogenesis along with late development in life cycle of Drosophila. Genes described in this review are shown.
This work was partially supported by Grants-in-Aid from JSPS Core-to-Core program, B. Asia-Africa Science Platforms.