Frontiers in Bioscience-Landmark (FBL) is published by IMR Press from Volume 26 Issue 5 (2021). Previous articles were published by another publisher on a subscription basis, and they are hosted by IMR Press on imrpress.com as a courtesy and upon agreement with Frontiers in Bioscience.
Retraction of Frontiers in Bioscience-Landmark 2024, 29(11)
1 Department of General Surgery, Guangdong General Hospital, Guangdong Academy of Medical Science, Guangzhou, Guangdong 510000, China
2 Department of general Surgery, Nanfang Hospital, Southern Medical University, Guangzhou 510515, China
3 Department of General Surgery, The Second Affiliated Hospital of Shantou University, 69 Dongxia North Road, Shantou, Guangdong 515100, China
4 State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, Guangzhou Institute of Respiratory Health, the First Affiliated Hospital of Guangzhou Medical University, Guangzhou 510120, China
5 Department of Surgery, Ruijin hospital Luwan Branch, Shanghai Jiao Tong University, School of Medicine, Shanghai 200020, China
Abstract
Sophocarpine is one of the major ingredients of Sophorae flavescentis which could inhibits many kinds of cancers. However, the effect of sophocarpine on gastric cancer (GC) and the mechanism involved remain unknown. The present study aims to explore the effects of the sophocarpine on the proliferation and apoptosis of GC cells and elucidates the relevant molecular mechanisms. After treatment with sophocarpine, GC cells were evaluated on their proliferation, autophagy, cell cycle progress and apoptosis. The protein levels of LC3-I, LC3-II, Beclin, p62, PTEN, PI3K, p53, Bax, Bcl-2, AKT and p-AKT were detected by western blot. Sophocarpine inhibited the proliferation of GC cells both in vitro and in vivo dose-dependently. Sophocarpine not only caused cell apoptosis and cell cycle arrest in G0/G1 phase but also induced cell autophagy. Moreover, sophocarpine dose-dependently suppressed PI3K/AKT signaling pathway and activated apoptosis in gastric cancer cells. Thus, sophocarpine significantly inhibited the growth of GC cells through multiple mechanisms such as induction of autophagy, activation of cell apoptosis and down-regulation of cell survival signaling pathway.
Keywords
- Gastric cancer
- Autophage
- Apotosis
- Caspase 3
- PI3K/AKT
Gastric cancer (GC) was emphasized for its substantial morbidity and mortality. Thus, there is an immense need to identify novel and promising agents for the cure and treatment of GC. In the last few decades, numerous phytochemicals compounds and active alkaloids have been reported to have cancer preventive activity (1-6). Sophocarpine is one of the significant alkaloid extracted from the traditional herb medicine (TCM) Sophora flavescens which has many pharmacological properties such as anti-virus, anti-tumor, anti-inflammatory and immune regulations (6, 7) and has been used clinically in the treatment of gastric cancer. Recently, sophocarpine has been demonstrated to have anti-tumor activity in various cancer cells, including hepatocellular carcinoma, prostate cancer and colorectal cancer (8-13). Some studies have suggested that sophocarpine inhibits the proliferation and transfer of tumor cells, inducing apoptosis and differentiation. But so far, there are few researches exploring the mechanisms of anti-gastric cancer of sophocarpine which extracted from Sophora flavescens (14-16). Based on our previous research results, the current study was conducted to analyze the effect of sophocarpine on the viability and apoptosis of human GC cells (BGC-823 and MKN45). In addition, we sought to reveal the possible signaling pathways involved in the antitumor effects of the sophocarpine, which would lay a foundation for the development of new antitumor TCM agent.
Sophocarpine (93.3.%, lot#: 110715-201318) was provided by the National Institutes for Food and Drug Control (Beijing, China). MKN45 and BGC-823 cells were purchased from Cellbio (Shanghai, China). The cells were cultured in high-sugar DMEM (HyClone, Logan, UT) with 10% foetal bovine serum (FBS; Gibco, Brazil), 8mg/mL penicillin and 8000 U/mL streptomycin at 37 °C in a humidified 5% CO2 incubator. Sophocarpine was dissolved in DMEM, stored at -20 °C. 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfonic acid benzene)-2H-tetrazole monosodium (CCK-8) and Annexin V-fluorescein isothiocyanate (FITC) Detection kit were purchased from Beijing BIOSS biological technology Ltd. Rabbit polyclonal anti-human LC3-I, LC3-II, Beclin, p62, PTEN, PI3K, p53, Bax, Bcl-2, AKT and p-AKT antibodies were purchased from Wuhan Boster Biological Technology Co., Ltd. Mouse anti-β-actin and anti-rabbit IgG antibodies were purchased from Santa Cruz Biotechnology.
Female nude mice aged 5 to 6 weeks were purchased from the Experimental Animal Centre of Southern Medical University, which is certified by the Guangdong Provincial Bureau of Science. The rats had been raised in a clean environment, and all animal experiments were ethical practices.
The 50% cytotoxic concentrations (CC50) of the sophocarpine for MNK -45 and BGC-823 cell lines were determined using a 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfonic acid benzene)-2H-tetrazole monosodium (CCK-8) assay. The serial dilutions of the sophocarpine for the cytotoxicity assay were 0 mg/ml, 0.1.25 mg/ml, 0.25 mg/ml, 0.5 mg/ml, 1 mg/ml, 2 mg/ml, 4 mg/ml, 8 mg/ml. CC50 values were calculated using regression analysis.
MKN45 and BGC-823 cell lines were seeded into 6-well plates at a density of 106 cells per well and cultured for 24 h, followed by respectively being treated with 2 ml fresh DMEM (as a control), or sophocarpine (the concentration of sophocarpine was 2.4.5 mg/ml and 1.23 mg/ml in the MKN45 cells, 2.07 mg/ml and 1.03 mg/ml in the BGC-823 cells). After incubation for 48 h, the cells were washed with PBS three times, trypsinized, and harvested in Eppendorf tubes for staning with an Annexin V-FITC/PI apoptosis kit. The samples were assayed using flow cytometry, while the FITC-positive cells were considered as apoptosis cells according to the Flowjo software.
MKN45 and BGC-823 cell lines were seeded into 6-well plates at a density of 106 cells per well and cultured for 24 h, followed by respectively being treated with 2ml fresh DMEM (as a control), or sophocarpine (the concentration of sophocarpine was 2.45 mg/ml and 1.23 mg/ml in the MKN45 cells, 2.07 mg/ml and 1.03 mg/ml in the BGC-823 cells). After incubation for 48 h, the cells were washed with PBS three times, trypsinized, and harvested in Eppendorf tubes for staning with 75% ethyl alcoholan overnight. Then the samples were treated with Cell cycle propidium iodide (PI) kit. The samples were assayed using flow cytometry and further analyzed using Flowjo software.
MKN45 and BGC-823 cell lines were seeded into 6-well plates at a density of 106 cells per well and cultured for 24 h, followed by respectively being treated with 2 ml fresh DMEM (as a control), or sophocarpine (the concentration of sophocarpine was 2.45 mg/ml in the MKN45 cells and 2.07 mg/ml in the BGC-823 cells). After incubation for another 48 h, the cells were harvested and fixed with glutaraldehyde (2.5%), embedded in resin, sliced, stained by osmic acids, and finally viewed using the electron microscopic.
Total protein was extracted from treated cell samples. Equal volumes of protein were loaded onto a 10% sodium dodecyl sulfate polyacrylamide gel and separated by electrophoresis. The resulting protein bands were transferred to polyvinylidene difluoride membranes (Bio-Rad). The membranes were blocked for 1 hour in tris-buffered saline (TBST) buffer containing 5% non-fat milk. Immunoblotting was carried out by incubating the membranes in 5% milk-TBST at 4 °C overnight with primary antibodies against LC3-I, LC3-II, Beclin, p62, PTEN, PI3K, p53, Bax, Bcl-2, AKT and p-AKT (1:1000). The membranes were washed three times with TBST and incubated with a secondary antibody HRP Goat Anti-Rabbit IgG (1:1000) for 1 hour. Then, the membranes were again washed three times with TBST, and the antibodies were detected by enhanced chemiluminescence reagents (Millipore). Densitometric analysis using Quantity One software (Bio-Rad) was used for quantification, and the results were normalized to β-actin.
The nude mice were randomly divided into three groups (PBS, sophocarpine, n=3). To develop the tumor model, MKN45 and BGC-823 cells were respectively harvested at a density of 1x107 cell/mL and suspended in PBS. Then 100 uL suspension was injected into the right flank of male mice. After 10 days, sophocarpine (50 mg/kg) or saline solution was applied on these tumor-bearing mice by oral taken every day. The weight of the mice was recorded every three days for 16 days. Each tumor tissue was excised and weighed when the experiment was completed, and the tumor sizes were measured and calculated using the following formula: 1/2×L×W2, where L denotes the longest surface length (mm) and W denotes the width (mm). In addition, the main organs (heart, liver, lungs, kidneys, and brain) of the mice were harvested, fixed in 10% phosphate-buffered formalin and then embedded in paraffin, sectioned, and stained with haematoxylin and eosin (H&E).
Data were expressed as mean±standard deviation (SD), and were analyzed by SPSS 19.0. statistic software. All experiments were repeated three times. Statistical evaluation of the data was performed by using the unpaired Student’s t-test and analysis of variance (ANOVA) followed by Scheffe’s post-hoc test. Significant differences were considered when p<0.05.
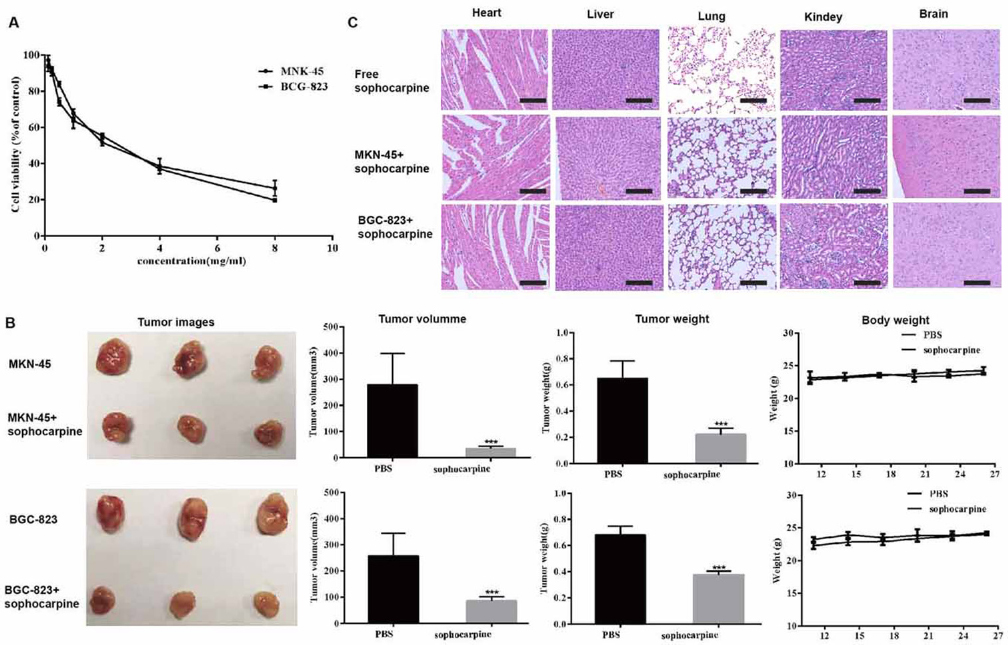
To determine whether sophocarpine was toxic to gastric cancer cells, a cytotoxicity assay was performed using the CCK-8 assay. Cell viability of MKN45 and BGC-823 cell lines treatment with sophocarpine was decreased significantly in a dosage-dependent manner. The estimated CC50 of sophocarpine was 2.45 mg/mL on MKN45 and 2.07 mg/ml on BGC-823 cell lines, respectively (Figure 1A).
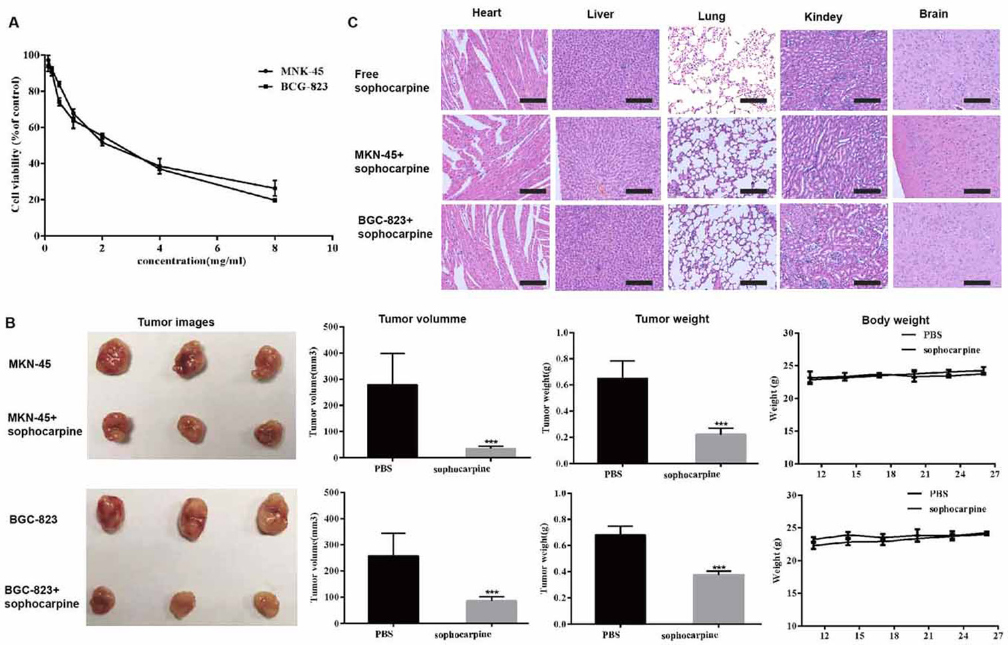 Figure 1
Figure 1The cytotoxicities of sophocarpine on MNK45 and BGC-823 cell lines. (A) Cell viabilities of MNK45 and BGC-823 cells measured by CCK-8 assay, after the treatment of different concentrations of sophocarpine for 48 h. (B). The tumor images, volume, weight and the body weight of the nude mice after treatment for 26 days. (C). H&E staining analysis of the main organs (heart, liver, lungs, kidneys, and brain) in three groups. All data are shown as the mean ± SD, n=3. *p < 0.05, **p < 0.01, or ***p < 0.001, versus the control group. Scale bar was 50 μm.
Sophocarpine was not merely effective in vitro but also acted as an effective anti-cancer regimen in vivo. We evaluated the effect of sophocarpine in vivo using an established the gastric cell lines xenograft model. As shown in Figure 1B, sophocarpine exerted a significant synergistic inhibitory effect on MKN45 and BGC-823 xenografts, while the tumor volume and weight of sophocarpine treated groups were significantly decreased compared with the PBS group (p <0.05, respectively).
Meanwhile, the weight of the nude mice had no significant change compared to the control group (p>0.05, respectively). Moreover, the HE results showed no histopathologic changes between the mice treated with sophocarpine, indicating that that sophocarpine did not induce any systemic side toxicity (Figure 1C).
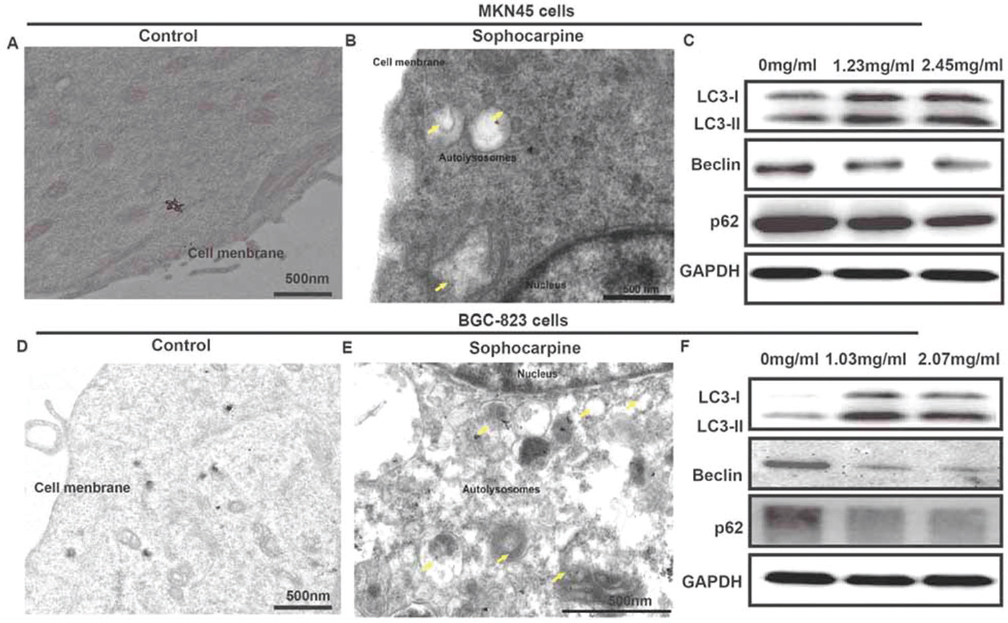
We used TEM to observe the ultrastructure of the two gastric cancer cells after treated with sophocarpine. As presented in Figure 2A&B, autolysosomes could be clearly observed around the nucleus in MKN45 cells treated with sophocarpine, compared to the control group. Furthermore, some autophagososomes even contained organelles, such as an endoplasmic reticulum. In addition, the proteins of the autophagy LC3-I, LC3-II, Beclin and p62 were detected. Compared with 0 mg/ml group, the expression of the LC3-I, and LC3-II proteins in groups treated with sophocarpine were significantly increased, while Beclin and p62 proteins were conversely depressed in a dose-dependent way (Figure 2C). Consistently, the BGC-823 cells showed similar tendency after treatment of sophocarpine. These results provided direct evidence that the two gastric cancer cells underwent autophagy when treated with sophocarpine.
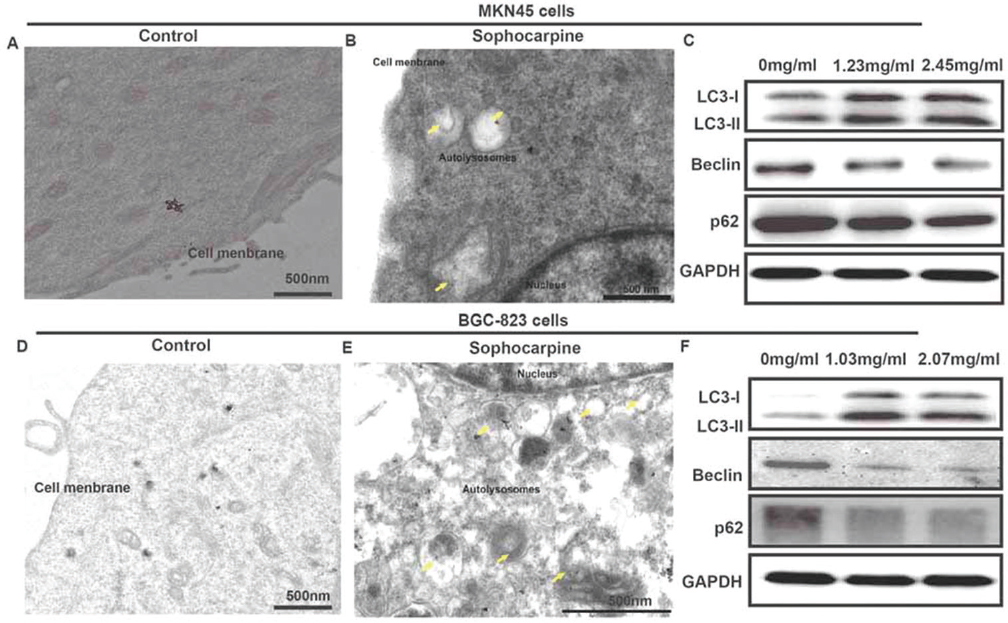 Figure 2
Figure 2Sophocarpine regulates cell autophagy of MNK45 and BGC-823 cell lines. (A&B) TEM images of MNK45 cells after incubation with or without sophocarpine for 48 h. (C) The expression of the LC3-I, LC3-II, Beclin and p62 proteins of the groups were detected by western blot in MNK45 cell lines, after the treatment of different concentrations of sophocarpine for 48 h. (D&E) TEM images of BGC-823 cells after incubation with or without sophocarpine for 48 h. (F) The expression of the LC3-I, LC3-II, Beclin and p62 proteins of the groups were detected by western blot in BGC-823 cell lines, after the treatment of different concentrations of sophocarpine for 48 h. Scale bar was 500 nm. Yellow arrows represented autolysosomes.
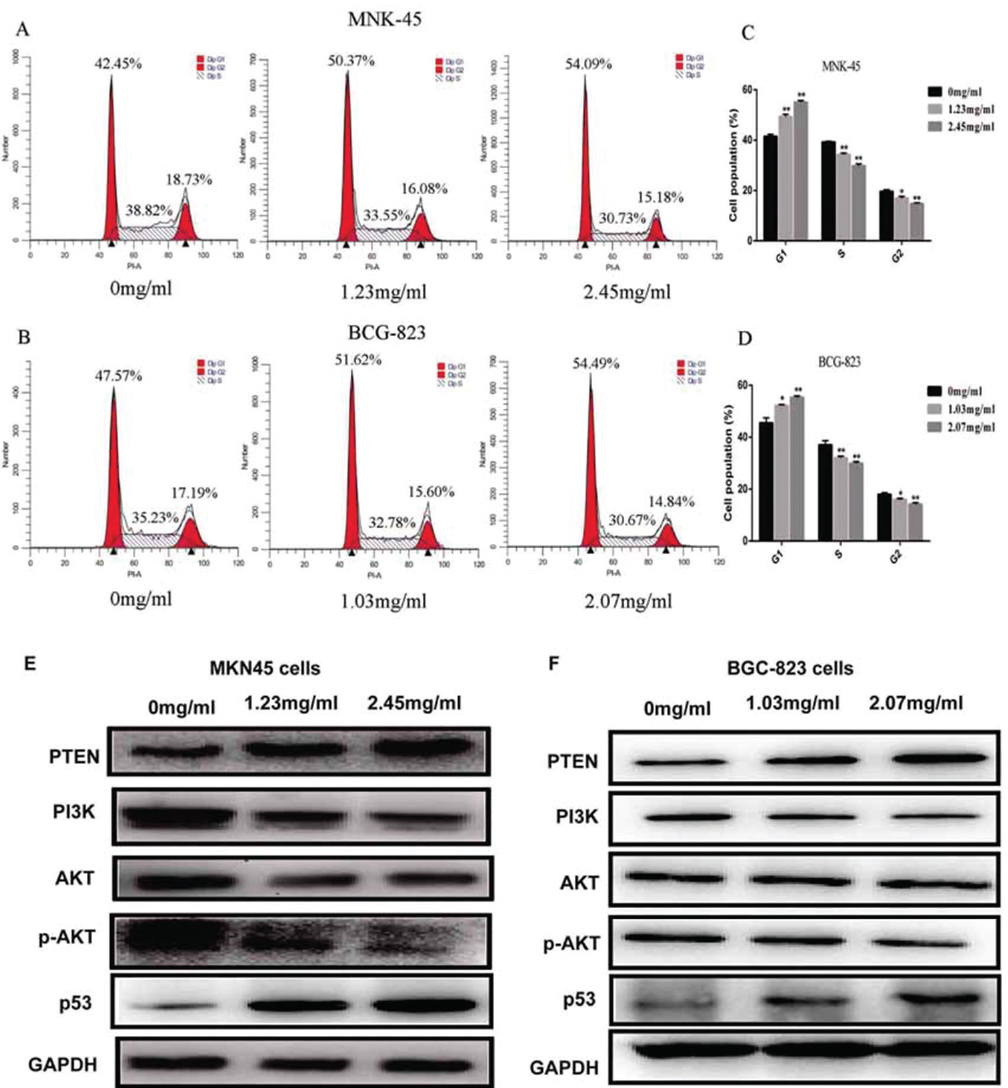
To explore whether sophocarpine inhibits cell growth by interrupting the cell cycle progress, cellular DNA was analyzed and stained with propidium iodide (PI). The cells were analyzed using flow cytometry. The profiles were shown in Figure 3. Obviously, an increase in the G1 population was observed in MKN45 cells after the treatment with sophocarpine at the dose of 1.23 mg/ml and 2.45 mg/ml (p<0.05) and at the dose of 1.03 mg/ml and 2.07 mg/ml in BGC-823 cells (p<0.05); and the significant decrease in the G2/M and S population was observed when treated with the sophocarpine at the two doses in comparison with the untreated cells (p<0.05) (Figure 3A-D).
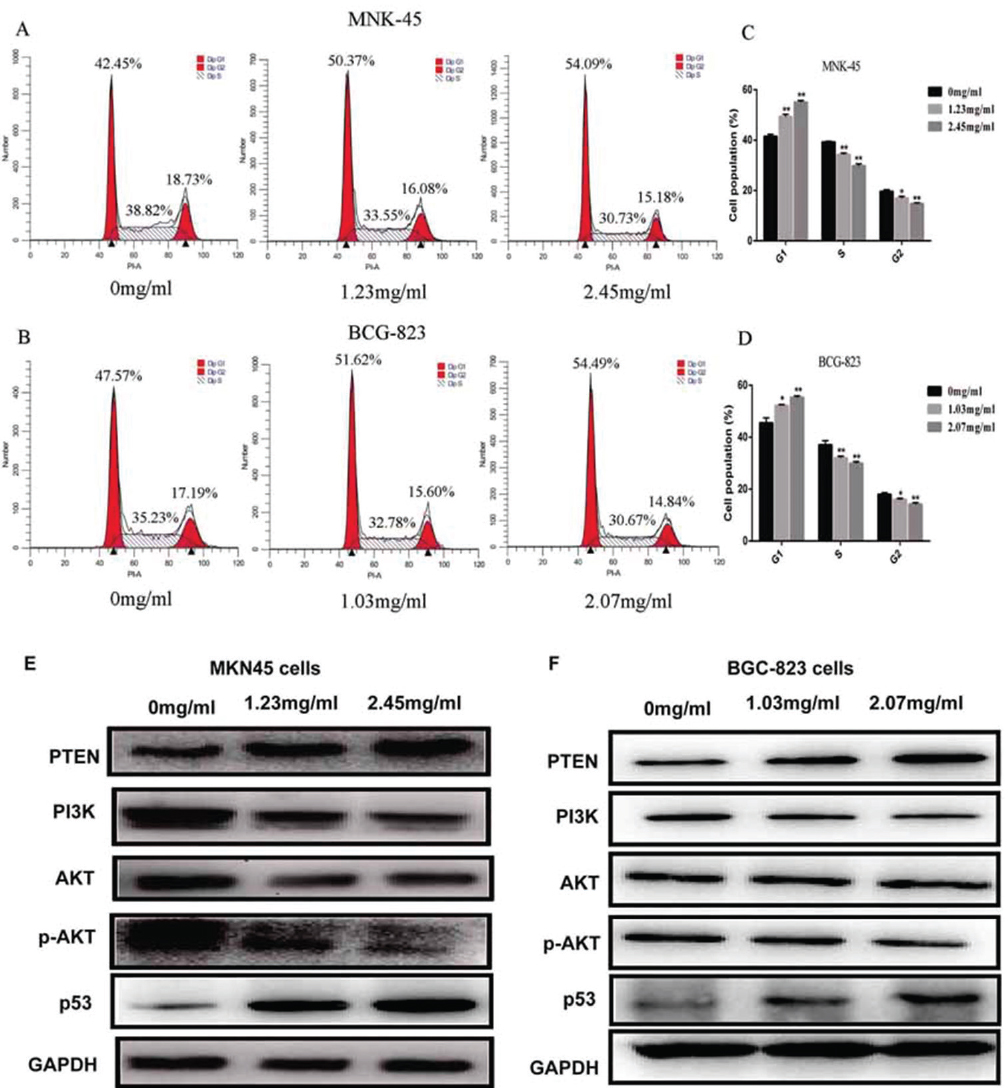 Figure 3
Figure 3Sophocarpine arrests GC cell cycle in G1 phase through PTEN/PI3K/AKT pathway. (A&B) The cell cycle images of MKN45 and BGC-823 cells treated with sophocarpine for 48 h. (C&D) The percentage of MKN45 and BGC-823 cells in G1, S and G2/M phases treated with sophocarpine for 48 h. (E&F) The expression of the PTEN, PI3K, AKT, p-AKT and p53 proteins of the groups were detected by western blot in MKN45 and BGC-823 cell lines treated with sophocarpine for 48 h. All data are shown as the mean ± SD of three independent experiments. *p < 0.05, **p< 0.0.1, or ***p< 0.001, versus the control group.
Furthermore, the expression of the PTEN protein in groups treated with sophocarpine was significantly increased while PI3K and p-AKT proteins were conversely depressed in both MKN45 and BGC-823 cell lines, compared with 0 mg/ml group (Figure 3E-F). Meanwhile, the expression of the PI3K was significantly decreased when treated with the sophocarpine in the two cells. Results showed that sophocarpine treatment could regulate the PTEN/PI3K/AKT pathway in MKN45 and BGC-823 cell lines dose-dependently.
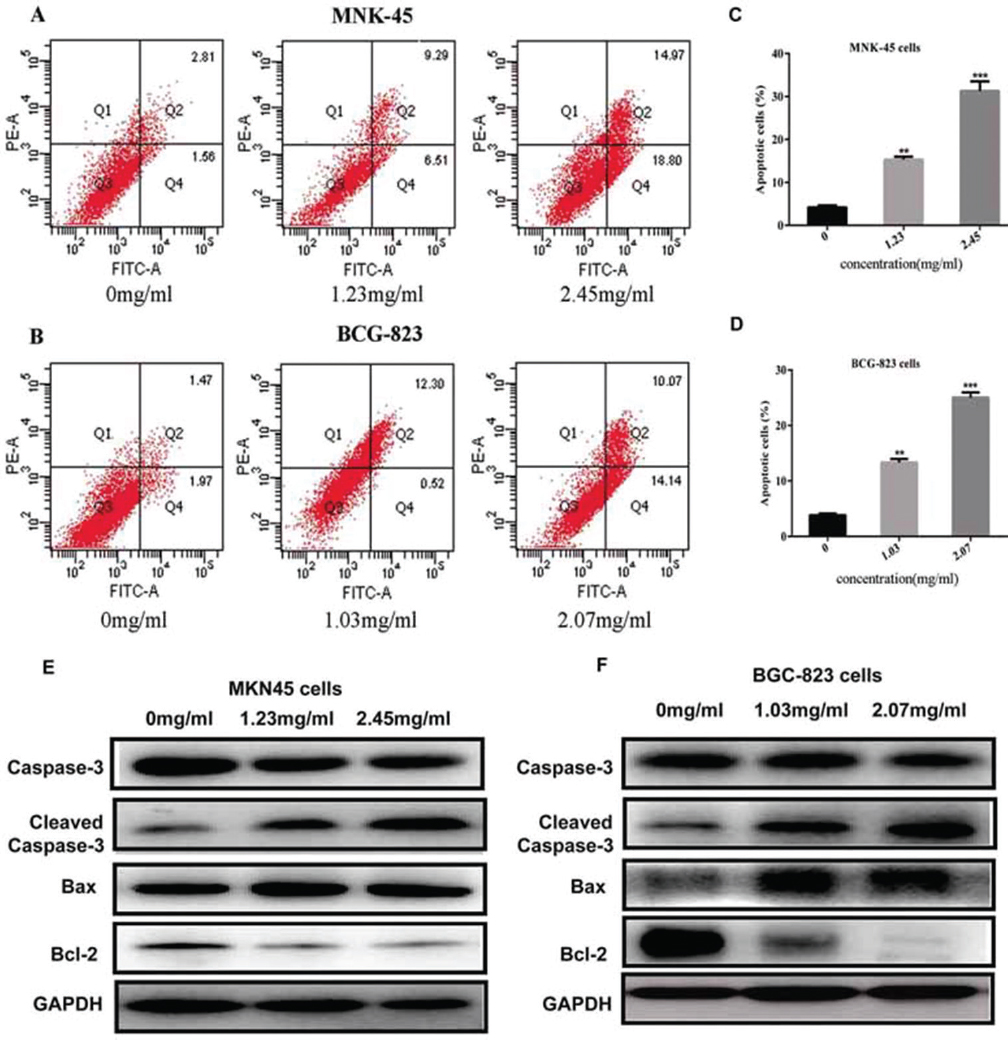
In the experimental group, the numbers of apoptotic cells significantly increased with drug concentrations. Nuclear fragmentation and petal-like arrangement were observed at various concentrations, which were considered as one of apoptotic body formation process. After 48 h sophocarpine treatment on the two cell lines, the incidence of apoptotic cells was 4.37 ± 1.25%, 15.80 ± 0.78%, 33.77 ± 1.2.1%, at the concentrations of 0, 1.25, and 2.45 mg/ml on MKN45 cell lines, respectively. And 3.44 ± 0.86%, 12.82 ± 1.78%, 24.2.1 ± 1.58%, at the concentrations of 0, 1.25, and 2.45 mg/ml on BGC-823 cell lines, respectively. Compared to apoptotic rate of control groups, sophocarpine treatment significantly promoted apoptosis of the two cells (p<0.05). Furthermore, the incidence of apoptosis was further increased with the concentration of sophocarpine (Figure 4A-D).
 Figure 4
Figure 4Sophocarpine induces cell apoptosis through Caspase pathway. (A&B) The apoptosis images assessed by Annexin-V/PI staining in both the MKN45 and BGC-823 cell lines treated with sophocarpine for 48 h. (C&D). The apoptosis rates of MKN45 and BGC-823 cells treated with sophocarpine for 48 h. (E&F) The expression of the Caspase-3, Cleaved Caspase-3, Bax and Bcl-2 proteins in MNK45 and BGC-823 cell treated with sophocarpine for 48 h. All data are shown as the mean ± SD of three independent experiments. *p < 0.05, **p< 0.01, or ***p< 0.001, versus the control group.
Consist with the flow cytometry, the expression of cleaved capase-3 and Bax proteins in groups treated with sophocarpine was significantly increased while Bcl-2 protein was conversely depressed in both MKN45 and BGC-823 cell lines (Figure 4C&-D). Taken together, these results showed that sophocarpine treatment could regulate the Caspase pathway in MKN45 and BGC-823 cell lines in dose-dependent.
Gastric cancer is one of the most common malignant tumors that threaten human health worldwide. The recurrence rate after gastrectomy is terrible in patients with advanced gastric cancer (17-20). Some reports have indicated that many traditional Chinese medicine extracts have a significant effect on cancer treatment; it has great potential in the treatment of gastric cancer (21-23). Sophocarpine is one of the monomeric alkaloids, which are extracted from Sophora flavescens with a strong anti-tumor effect extractive (2, 3). In this study, we found that the sophocarpine had anti-tumor effects both in vitro and in vivo. Furthermore, sophocarpine could induce apoptosis and arrest G2/M phase in the gastric cancer cells, which were highly associated with autophagy and PTEN/PI3K/AKT signaling pathway.
In vitro, a CCK-8 assay revealed that sophocarpine treatment significantly inhibited cell proliferation in MKN45 and BCG-823 gastric cancer cell lines in a dose-dependent manner. Xenograft tumorigenesis analysis in vivo, demonstrated that following sophocarpine treatment, the weight and size of tumors in the two gastric cancer cell lines subcutaneous xenografts were markedly reduced. The results indicated that sophocarpine treatment inhibited the proliferation of gastric cancer cells, in vitro and in vivo. In addition, no weight loss was observed in the treated mice. The safety of the sophocarpine treatment was further confirmed by the histological analysis of the main organs (heart, liver, lungs, kidneys, and brain) using H&E staining, as there were no pathological changes. These results implied that sophocarpine displayed an advantage in drug safety and druggable potential.
Dysregulation of apoptosis is one of the fundamental mechanisms underlying cancer. Activation of apoptotic pathways in cancer cells is essential for cancer therapy, which indicating that an herbal product induces apoptosis has become the subject of great interest. In this study, we revealed that sophocarpine exhibited potent cytotoxicity against MKN-45 and BGC-823 cells.
To gain insight into the mechanisms underlying the anti-cancer effects of sophocarpine, we investigated the possible changes in the cell cycle and apoptosis. We found that the sophocarpine was able to restrain the MKN-45 and BGC-823 cells at G0/G1 phase which caused the decrease in the G2/M and S phase in a dose-dependent way. Consistent with these results, the apoptotic rates of MKN-45 and BGC-823 cells were also increased by sophocarpine in a dose-dependent way. These data broadened our framework for the understanding of the medicinal function of sophocarpine, and provided evidence for the first time that sophocarpine exerted a potent inhibitory effect on gastric cancer cells growth.
Autophagy is a highly conserved lysosome-dependent catabolic pathway in eukaryotes. It is reported that autophagy is closely related to a variety of physiological and pathological process, and might be a potential target during the treatment of tumor cells. Meanwhile, autophagy can block tumor cells in the G2/M phase, and induce more cell apoptosis (24, 25). However, the autologous function of the tumor cell itself is still controversial in its role in tumor development. Sophocarpine was previously reported inducing autophagy in hepatoma cells, thereby inhibiting tumor proliferation. In our study, we observed dozens of autophagosomes which contained organelles in the ultrastructure of gastric cancer cells treated by sophocarpine. Meanwhile, the autophagy-related markers including LC3II were increased, while beclin and p62 were suppressed. Such as an endoplasmic reticulum was clearly observed which provided evidence that sophocarpine could increase autophagy in gastric cancer cells, then inhibit the tumor.
The PTEN/ PI3K/AKT signaling pathway plays a critical regulatory role in regulating the signal transduction activity associated with cell proliferation, apoptosis, differentiation and survival (26, 27). PTEN/ PI3K/AKT signaling pathway is implicated in the initiation and progression of multiple human malignancies (28). Recently, an AKT inhibitor has been proved to strongly reduce the amount of brain cancer stem cells. The decline was correlated to a preferential induction of apoptosis and a suppression of neurosphere formation (29). In this study, the results showed that sophocarpine suppressed the PTEN/ PI3K/AKT pathway in the two gastric cancer cells, which was evidenced by overexpression of PTEN, reduction of PI3K and phosphorylation of p-AKT. Therefore, the downregulation of the PTEN/ PI3K/AKT pathway might have contributed to the inhibitory effect of sophocarpine on the two gastric cancer cells. Emerging evidence has demonstrated that the PTEN/PI3K/AKT signaling pathway is highly assosiated with cell cycle and apoptosis in GC. P53 is involved in the regulation of the cell cycle. Bcl-2 is transcriptionally downregulated by p53, whereas Bcl-2 is transcriptionally downregulated by p53, and Bax is activated by p53. Here, we observed that pro-apoptotic p53 and Bax were overexpressed in GC cells following addition of sophocarpine. We suggest that sophocarpine might downregulate the expression of p53 and, consequently, Bax, while upregulating Bcl-2. This would result in a decrease of the Bax/Bcl-2 ratio, promoting proliferation and inhibiting apoptosis.
These results suggest that sophocarpine suppresses the PTEN/ PI3K/AKT pathway to ultimately inhibit the gastric cancer cells self-renewal, sophocarpine might dysregulate expression of p53, Bax, and Bcl-2. Further studies are awaited to clarify the exact role of such downregulation in the inhibition of the gastric cancer cells by sophocarpine.
The results of the present study suggested that sophocarpine exhibits antitumor properties in gastric cancer cells, in vitro and in vivo. Furthermore, the results indicated that the antitumor properties of sophocarpine may be attributed to the inhibition of proliferation and the induction of apoptosis, which is associated with regulation of PTEN/PI3K/AKT pathway and inducing autophagy in human gastric cancer cells. Therefore, these findings may provide a novel approach for the development of gastric cancer therapy, using sophocarpine, which is derived from the traditional Chinese herb, Sophora flavescens.
Yijie Huang and Xinhua Chen equally contributed to this paper. This work was supported by special Funds for the Cultivation of Guangdong College Students’ Scientific and Technological Innovatio (pdjha0094). We thank LetPub for its linguistic assistance during the preparation of this manuscript.
Abbreviations: GC: gastric cancer; CCK-8: 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfonic acid benzene)-2H-tetrazole monosodium; TCM: traditional herb medicine; TEM: electron microscopic; PI: propidium iodide; HE: haematoxylin and eosin; CC50: the 50% cytotoxic concentrations