Frontiers in Bioscience-Landmark (FBL) is published by IMR Press from Volume 26 Issue 5 (2021). Previous articles were published by another publisher on a subscription basis, and they are hosted by IMR Press on imrpress.com as a courtesy and upon agreement with Frontiers in Bioscience.
Retraction of Frontiers in Bioscience-Landmark 2024, 29(12)
1 Department of oncology, diamond Bay Hospital, Second Affiliated Hospital of Dalian Medical University, Dalian, 116036, China
2 Department of Cardiology, Second Hospital of Dalian Medical University, Dalian, 116036, China
3 Department of Urology, Second Hospital of Dalian Medical University, Dalian, 116036, China
4 General Department, Second Hospital of Dalian Medical University, Dalian, 116036, China
Abstract
Colorectal cancer (CRC) is one of the most common malignancies worldwide. However, therapies against CRC have not been completely effective. Astragaloside IV (AS-IV) has shown anti-tumorigenic properties in certain cancers, but its role in CRC remains unclear. In this study, we investigated the therapeutic effect of AS-IV in CRC and explored its underlying mechanism. The results showed that AS-IV dose-dependently inhibited the proliferation of CRC cells and suppressed tumor growth in CRC xenograft mouse models. In addition, AS-IV promoted cell cycle arrest in the G0 phase, associated with increased expression of p21. Furthermore, flow cytometry demonstrated that AS-IV promoted apoptosis of CRC cells in a dose-dependent manner. AS-IV induced caspase-dependent apoptosis, which involved the increased release of cytochrome c and Omi from the mitochondria into the cytoplasm and the up-regulation of Bax/Bcl-2 ratio, as well as the activation of PARP and caspase cascade (caspase-3 and -9). Taken together, our study has identified a novel function of AS-IV and provided a molecular basis for AS-IV’s potential applications in the treatment of CRC and other cancers.
Keywords
- Astragaloside IV
- Colorectal cancer
- Cell cycle
- Apoptosis
- Caspase
Colorectal cancer (CRC) is one of the most common malignancies worldwide. Although surgery, new chemotherapeutic agents and targeted therapies have significantly reduced the mortality of CRC in the past 20 years, the treatment benefits are still limited and the 5-year survival rate is relatively low compared with other types of cancer (1, 2). Thus, it is urgently needed to develop novel and effective therapeutic agents.
Astragaloside IV (AS-IV, 3-O-β-D-xylopyr-anosyl-6-O-β-D-glucopyranosylcyl-cloastragenol) is one of the major active compounds of Chinese traditional herb Astragalus membranaceus. According to previous studies, AS-IV exerts a number of pharmacological effects, including immunoregulatory, neuroprotective, anti-oxidative, anti-inflammatory and anti-tumor function (3-8). It was reported that AS-IV inhibits the migration and invasion of human lung cancer and breast cancer cells (8, 9). AS-IV also promotes the sensibility of anti-tumor drugs, such as cisplatin, fluorouracil and gefitinib (10-12). However, the pharmacological effect of AS-IV on CRC is not well-explored.
It is well-known that the imbalance between cell proliferation and cell death contributes to cancer initiation and development (13). Cell proliferation is regulated by DNA replication- and cell cycle-related proteins, such as Proliferating cell nuclear antigen (PCNA), Ki67, cyclin-dependent kinases (CDKs) and p21/WAF1/Cip1 (p21) (14). Cell death is governed by cell apoptosis, which is characterized by cell shrinkage, chromatin condensation, DNA fragmentation and formation of apoptotic bodies (15). Apoptosis can be initiated through two pathways. Both pathways induce cell death by caspase activation (16). In the extrinsic pathway, the death receptors recognize their ligands, resulting in the activation of caspase-8 and -10 (17). In the intrinsic pathway, cells apoptosis is triggered by cell stress, including production of nitric oxide, disruption of the mitochondrial transmembrane potential, permeabilization of the outer membrane, release of apoptogenic proteins such as cytochrome c (Cyt c). Cyt c binds with apoptotic protease activating factor-1 (Apaf-1) and ATP, which in turn activates caspase-9 and caspase-3 (18).
In this study, we investigated the therapeutic effect of AS-IV on CRC and explored its underlying mechanism. The effects of AS-IV on cell cycle and apoptosis in CRC cells were specifically examined.
AS-IV (purity > 99%) was obtained from J&K Scientific Ltd., (Beijing, China). Antibodies to p21, Cyt c and Omi were purchased from Abcam (Cambridge, MA); Antibodies to Bcl-2, Bax, PARP and PCNA were purchased from Santa Cruz Biotechnology (CA, USA); Antibodies to cleaved caspase-3 and cleaved caspase-9 were purchased from Cell Signaling Technology (Danvers, MA). Z-VAD-FMK was purchased from R&D Systems (Minneapolis, MN).
HT29 and SW480 cells were cultured on a 96-well plate and treated with various doses of AS-IV for 24 h. Cell viability was then measured by the CCK-8 kit (Beyotime Biotechnology, Shanghai, China) according to the manufacturer’s instructions.
HT29 and SW480 cells at the logarithmic growth phase were incubated with serum-free medium for 24 h. After treated with various doses of AS-IV for 24 h, cells were harvested and resuspended with cold 70% ethanol at -20˚C overnight. Then the suspension was incubated with propidium iodide (PI) for 30 min at room temperature. Cell cycle distribution was measured by a flow cytometry (ThermoFisher Scientific, USA).
The lysates of the mitochondria and cytoplasm were separated using the Mitochondria/Cytosol Fractionation kit (Abcam, Cambridge, UK). Proteins were extracted from cells or tissues using RIPA lysis buffer (SunShine Biotechnology, Nanjing, China) according to the manufacturer’s protocol. Equal amount of protein samples were separated by 10% SDS-PAGE gel and then transferred into PVDF membranes (Millipore). After blocking with 5% non-fat milk, the membranes were incubated with respective primary antibodies and secondary antibodies. The antibody-bound proteins were visualized using enhanced chemical luminescence (ECL, Bio-Rad Laboratories) and quantificated by ImageJ software.
HT29 and SW480 cells were treated with various doses of AS-IV for 24 h. After wash with ice-cold PBS, cells were resuspended in Annexin V binding buffer and incubated with FITC-conjugated Annexin V/PI (ThermoFisher, USA) for 15 min at room temperature. The cells were then analyzed with Attune NxT Flow Cytometer (ThermoFisher Scientific, USA).
Ten athymic BALB/c mice (nu/nu, 20-25 g body weight) were obtained from the Animal Center of Dalian Medical University (Dalian, China). Mice were housed under controlled conditions (25±2°C, 70% humidity and 12-hlight-dark periods) and fed on regular sterile chow diet and water ad libitum. The experimental protocol was in accordance with the guidelines of Institutional Animal Care and Use Committee (IACUC) of Dalian Medical University. All mice were subcutaneously inoculated with 5×106 HT29 cells and then randomly divided into two groups: One group was received daily gavage of AS-IV (20 mg/kg) for 30 days and another group was administrated with equivalent water. Animals were monitored for signs of tumor growth. Tumor volumes were calculated per 5 days. Upon termination, tumor tissues were collected for immunohistochemical analysis.
Tumor sections were prepared essentially by standard protocol. After the sequential processes of rehydration and antigen retrieval, the sections were incubated with antibody against PCNA/p21/cleaved caspase-3 overnight, and subsequently incubated with secondary antibody for 30 minutes. Diaminobenzidine (DAB) was used for performing color reactions. Sections were visualized with a fluorescent microscope.
Apoptotic cells were identified using the TUNEL-based ApopTag Peroxidase In situ Apoptosis Detection Kit (Millipore) according to the manufacturer’s instructions.
All results were presented as mean ± SD. The statistical significance of the studies was analyzed using One-way ANOVA. The difference was considered statistically significant at P < 0.0.5.
To gain insight into the anti-tumor effect of AS-IV, CRC cell HT29 and SW480 were treated with series doses of AS-IV for 24 h. The results showed that AS-IV inhibited the proliferation of HT29 and SW480 cells in a dose-dependent manner (Fig.1A and 1B). In addition, AS-IV dose-dependently induced an accumulation of cells in G0 phase, accompanied by a decrease of cells in the S phase (Fig.1C and 1D). We further investigated the effect of AS-IV on the cell cycle regulatory protein, p21. The results showed that AS-IV dose-dependently increased the expression of p21 in CRC cells (Fig.1E-1F). These results demonstrate that AS-IV inhibits cell proliferation and induces cell cycle arrest in CRC cells.
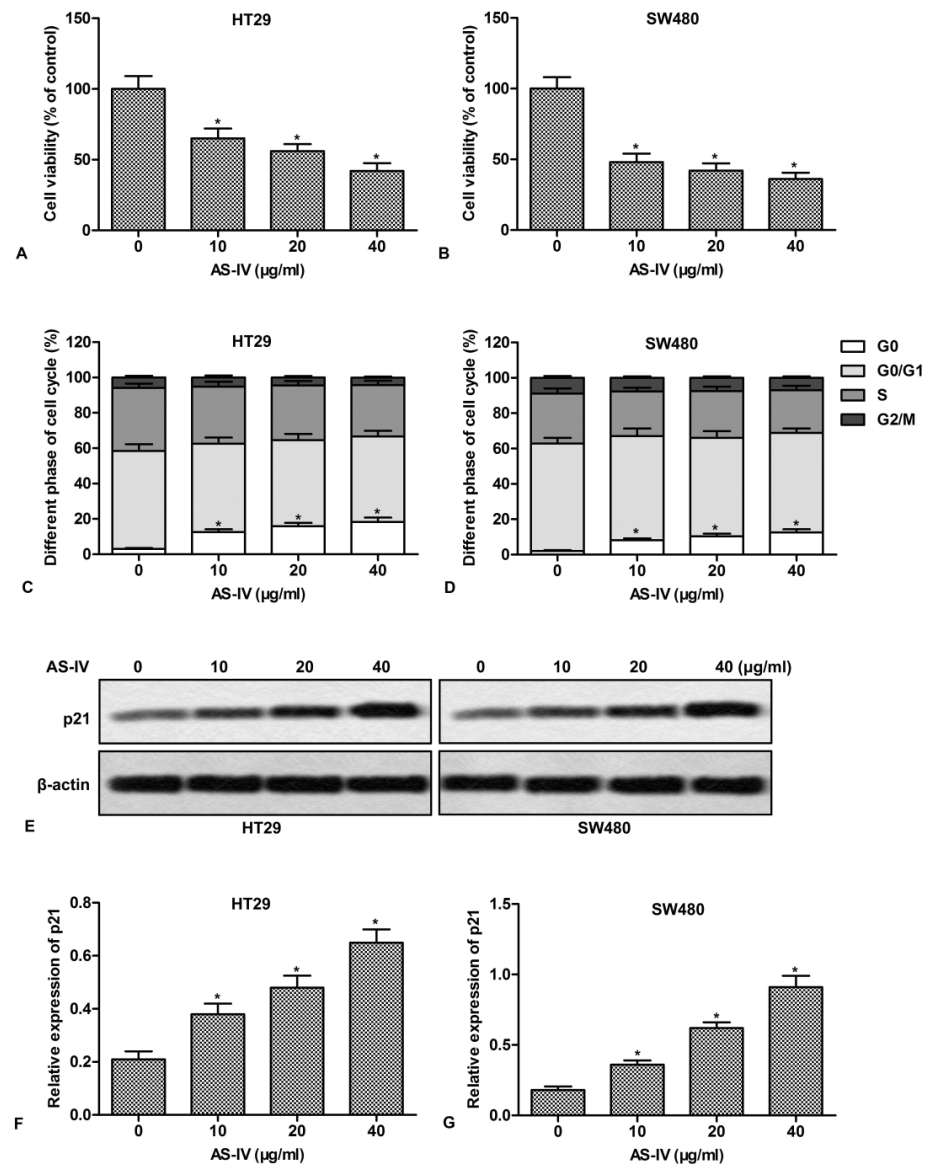 Figure 1.
Figure 1.AS-IV inhibits cell proliferation and induces cell cycle arrest in CRC cells. HT29 and SW480 cells were treated with various doses of AS-IV (0-40 μg/ml) for 24 h. (A-B) Cell proliferation was assayed by CCK-8 Kit. The relative activity was presented as percentages of control. (C-D) Cells were stained with PI (20 μg/ml) to determine the percentages of cells in the G0, G0/G1, S and G2/M phases. (E) The expression of p21 was measured by Western blot. (F-G) Quantitation of Fig.1E. *P < 0.0.5 versus untreated cells.
We next examined the effect of AS-IV on cell apoptosis. As shown in Fig.2A and 2B, AS-IV promoted apoptosis of CRC cells in a concentration-dependent manner. In addition, AS-IV also increased the ratio of Bax/Bcl-2 in CRC cells (Fig.2C-2E). Since mitochondria play a crucial role in the transduction of apoptotic signaling, the expression of mitochondria-related apoptogenic proteins was further examined. The results showed that AS-IV dose-dependently increased the release of Cyt C and Omi (Fig.2F-2J).
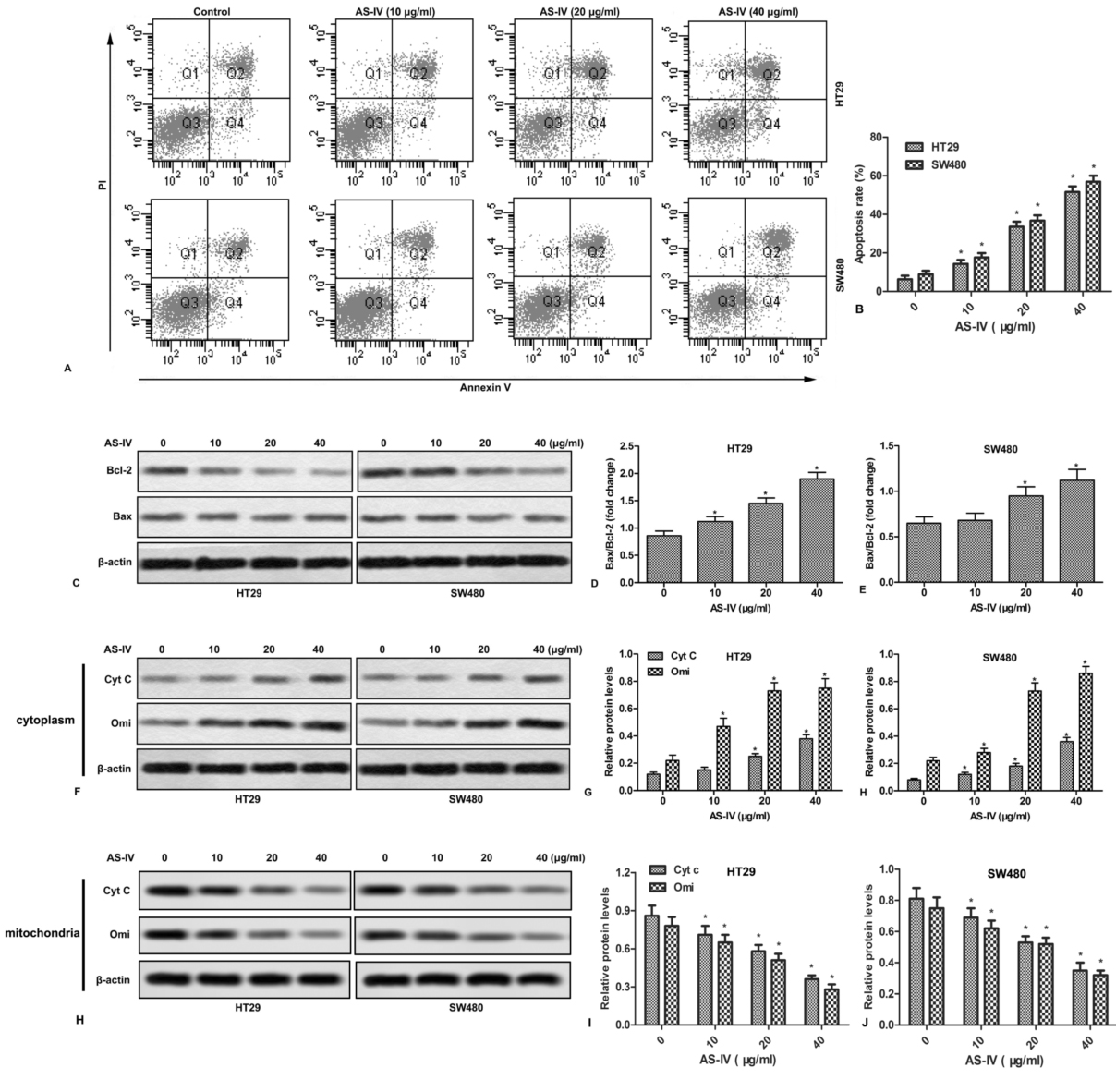 Figure 2.
Figure 2.AS-IV promotes apoptosis and mitochondrial events in CRC cells. HT29 and SW480 cells were treated with various doses of AS-IV (0-40 μg/ml) for 24 h. (A) Cell apoptosis was measured by flow cytometry. (B) Quantitation of apoptotic cells. (C) The expressions of Bax and Bcl-2 were detected by Western blot. (D-E) Quantitation of Figure 2C. (F) The expressions of Cyt c and Omi in cytoplasm were measured by Western blot. (G-H) Quantitation of Figure 2F. (H) The expressions of Cyt c and Omi in mitochondria were measured by Western blot. (I-J) Quantitation of Figure 2H. *P < 0.0.5 versus untreated cells.
Based on the above findings, we then investigated whether the activation of caspase cascades is involved in AS-IV-induced apoptosis. Western blot showed that AS-IV increased the expression of PARP, cleaved caspase-3 and cleaved caspase-9 in a concentration-dependent manner (Fig.3A-3C). To explore the contribution of caspase to AS-IV-induced apoptosis, cells were pretreated with Z-VAD-FMK, a general caspase inhibitor. As shown in Fig.3D and 3E, Z-VAD-FMK efficiently suppressed AS-IV-induced expression of PPRP. More importantly, Z-VAD-FMK pretreatment dramatically blocked AS-IV-induced apoptosis (Fig.3F). These results indicate that caspase is essential for AS-IV-induced apoptosis.
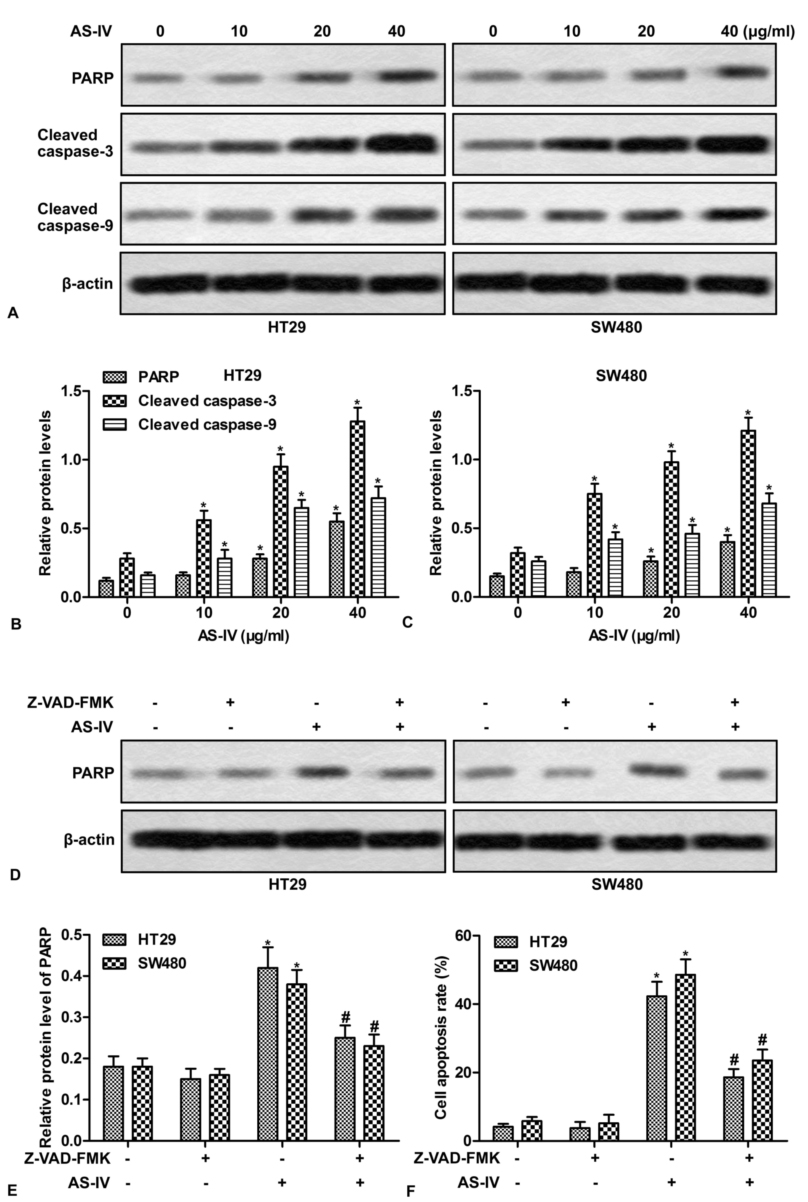 Figure 3.
Figure 3.The activation of caspases is required for AS-IV-induced apoptosis. (A) HT29 and SW480 cells were treated with various doses of AS-IV (0-40 μg/ml) for 24 h, the expressions of PAPR, cleaved caspase-3 and cleaved caspase-9 were detected by Western blot. (B-C) Quantitation of Figure 3A. (D) HT29 and SW480 cells were pretreated with Z-VAD-FMK (40 μg/ml) for 1 h, followed by exposure to 40 μg/ml of AS-IV for 24 h. The expressions of PARP and cleaved caspase-3 were detected by Western blot. (E) Quantitation of Figure 3D. (F) Quantitation of apoptotic cells. *P < 0.0.5 versus untreated cells. #P < 0.0.5 versus AS-IV treated alone.
To investigate the in vivo anti-proliferation effect of AS-IV, CRC xenograft mice were administrated with AS-IV. The results showed that AS-IV significantly increased survival rate and inhibited tumor growth in vivo (Fig.4A-4C). In addition, Immunohistochemistry assay demonstrated that AS-IV decreased the expression of PCNA, a proliferation-related protein in tumor tissues (Fig.4D-4E). AS-IV also promoted cell apoptosis and increased the expression of p21 and cleaved caspase3 in tumor tissues (Fig.4F-4K). These results indicate that AS-IV inhibits tumor growth and induces apoptosis in vivo.
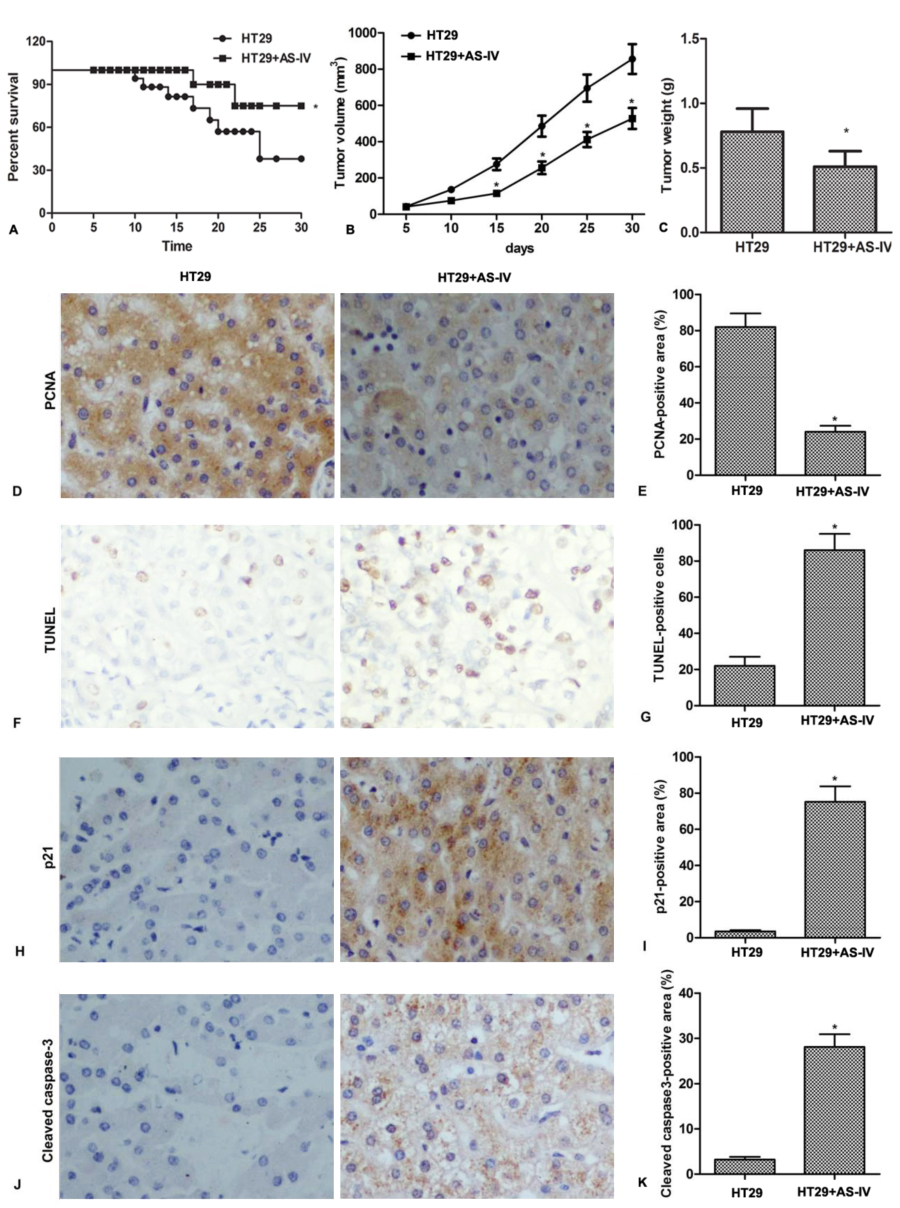 Figure 4.
Figure 4.AS-IV inhibits tumor growth and induces apoptosis in vivo. Mice were inoculated with HT29 cells, followed by intragastrical adminstrated with AS-IV (20 mg/kg/day) for consecutive 30 days. (A) The survival rates were calculated. (B) Tumor volumes were calculated per 5 days. (C) Tumor weights were measured at day 30. (D-E) The expression of PCNA in tumor tissue was detected by immunohistochemistry. (F-G) Apoptotic cells in tumor tissues were identified using the TUNEL-based Apoptosis Detection Kit. (H-K) The expressions of p21 and cleaved caspase-3 in tumor tissue were detected by immunohistochemistry. n = 5. *P < 0.0.5 versus control.
Numerous studies have demonstrated that some anti-inflammatory compounds, such as sinomenine and berberine act as cancer preventive and therapeutic agents (19, 20). AS-IV is widely used to treat immunodeficiency and to ameliorate the side effects of antineoplastic drugs (21). AS-IV has shown in vitro anti-proliferation and anti-migration activities in a variety of tumor cell lines (8, 9). However, its pharmacological effect on CRC remains unknown. In this study, we first demonstrated that AS-IV inhibited the proliferation of CRC cells in vitro and suppressed the growth of CRC xenograft tumor in vivo. Further studies indicated that cell apoptosis induction and cell cycle arrest were involved in the anti-CRC function of AS-IV.
p21, also known as cyclin-dependent kinase inhibitor 1 or CDK-interacting protein 1, is capable to inhibit all cyclin/CDK complexes (22, 23). p21 represents a major target of p53 activity and is associated with cell cycle arrest (24, 25). Previous studies have shown that bone marrow mesenchymal stromal cells affect the cell cycle arrest in acute lymphocytic leukemia cells via p21 down-regulation (26). HNRNPK, an effective prognostic marker of gastric cancer, inhibits gastric cancer cell proliferation through p53/p21/CCND1 pathway (27). In this study, we found that AS-IV dose-dependently inhibited the expression of p21 in CRC cells, resulting in an accumulation of cells in G0 phase. However, AS-IV treatment did not affect the expression of p53 (data not shown), indicating that AS-IV-induced p21 up-regulation was p53-independent. Throughout the cell cycle, PCNA has a high expression level (28). Down-regulation of PCNA resulted in the inhibition of cell cycle transformation, depression of DNA replication and increase of cell death in cancer cells (29). Our results also showed that AS-IV treatment inhibited the expression of PCNA in tumor tissues.
It is well known that cancer progression is attributed to the imbalance between cell proliferation and cell apoptosis. As a result, numerous natural products exert anticancer function by promoting apoptosis. For example, Hopea odorata extract inhibits hepatocellular carcinoma via induction of caspase-dependent apoptosis (30). Curcumin induces DNA damage and apoptosis through generation of reactive oxygen species and regulating mitochondrial membrane potential in melanoma cancer cells (31). In the present study, AS-IV promoted apoptosis of CRC cells in vitro and in vivo, as demonstrated by the increase of Annexin V-labeled cells and the change of apoptosis-related proteins.
The transduction of apoptotic signaling depends on the change of mitochondrial membrane potential and permeability. The sustained elevation in calcium levels leads to the translocation of Cyt c and Omi/HtrA2 from the intra-membrane space into the cytoplasm (32). The release of Cyt c activates caspase-9, which in turn leads to the activation of caspase-3 and caspase-7 (33). Omi/HtrA2 is an antagonist of inhibitors of apoptosis (IAPs). It complexes with different IAPs and prevents their ability to bind caspases (34). Overexpression of Omi/HtrA2 induces apoptosis in human cells, and its down-regulation attenuates cell death (35). In the present study, we found that AS-IV increased the release of Cyt c and Omi in a dose-dependent manner, which may be contribute to the apoptosis-induction of AS-IV. Furthermore, mitochondrial apoptosis induced by Bax can be prevented by Bcl-2 via inhibiting the release of Cyt c (36). We also found that AS-IV treatment increased the ratio of Bax/Bcl-2, which further demonstrated the role of the mitochondria in AS-IV-induced apoptosis.
Caspase-3 is an executioner of apoptosis. Activated caspase-3 leads to the cleavage of PARP, a family of proteins involved in programmed cell death (37, 38). Our study also showed that AS-IV promoted the cleavage of PARP, caspase-3 and caspase-9. Z-VAD-FMK, a general caspase inhibitor, blocked the activation of caspase-3 and PARP, and abolished AS-IV-induced apoptosis, indicating that AS-IV-induced apoptosis depends on caspase activation.
In conclusion, our study demonstrated that AS-IV suppressed the growth of human CRC cells in vitro and in vivo, which may be possibly attributed to cell cycle arrest and apoptosis induction. The results suggest that AS-IV may be a promising therapeutic agent for CRC.
We sincerely appreciate the technical support from Second Hospital of Dalian Medical University.
Abbreviations: CRC, Colorectal cancer; AS-IV, Astragaloside IV; PCNA, Proliferating cell nuclear antigen; CDKs, cyclin-dependent kinases; Cyt c, cytochrome c; Apaf-1, apoptotic protease activating factor-1




