Frontiers in Bioscience-Landmark (FBL) is published by IMR Press from Volume 26 Issue 5 (2021). Previous articles were published by another publisher on a subscription basis, and they are hosted by IMR Press on imrpress.com as a courtesy and upon agreement with Frontiers in Bioscience.
1 Biochemistry Laboratory, Department of Zoology, School of Biological Sciences, Dr. Harisingh Gour Central University, Sagar, 470003, Madhya Pradesh, India
2 Insect Immunity Lab, Department of Zoology, School of Biological Sciences, Dr. Harisingh Gour Central University, Sagar-470003 (M.P.), India
3 Biochemistry and Molecular Biology Lab, Department of Zoology, Banaras Hindu University, Varanasi,221005, Uttar Pradesh, India
Abstract
Global metabolism of cancers exhibits a peculiar phenotype that is lactate acidosis (high lactate with acidic pH) in tumor microenvironment. Why tumor microenvironment becomes so responsive towards lactate is still not clear. In this review we have discussed lactate generation and recycling either exogenously, directly or indirectly by cancer cells via some transporters. Tumor cells in hypoxia use glucose rapidly and produce lactate while cells which have profuse oxygen supply take up lactate and use it for energy production which is referred as lactate shuttling between tumor cells. Escaping immune evasion which is also an emerging hallmark of cancer cells has also been discussed in this review with respect of lactate acidosis.
Keywords
- Cancer metabolism
- Lactate
- LDH-A
- MCTs
- Monocarboxylate transporters
- p53
- PFKFB3
- Review
Metabolic impairment is a signature of cancer cells, but there is lack of understanding about the fate of lactate signaling in tumors emerging in their local microenvironment. Lactate also plays significant role in encouraging inflammation in tumor and stimulating angiogenesis in cancer cells (1). In 1924 Otto Warburg found the insightful differences between metabolic pathways of normal cells and cancer cells but misunderstood that phenomenon as mitochondrial dysfunction. For rapid generation of ATP, cancer cells choose aerobic glycolysis whereby consequently glucose is catabolized to lactate instead of going to TCA cycle for mitochondrial oxidative phosphorylation (2). For regulation of redox homeostasis, cancer cells use glutamine catabolism which helps to support anabolic growth, and maintain intermediates of TCA cycles which helps in proliferation of cancer cells (3). The solid tumors and cancer cells maintain extracellular acidity because of high lactate production and to maintain metabolism, tumor cell must drive out lactate from the cell, which they do via transporters called as monocarboxylate transporters (MCTs) meant for H+ coupled lactate efflux. Generally cancer cells are hypoxic and they use glucose for rapid energy production and release lactate which is further utilized by oxygenated cancer cells (4). Glycolysis in hypoxic condition results into lactic acid production (5). Studies in 1980s described that muscle and brain tissues are proficient in oxidizing lactate as a substrate for energy production and scientific community was puzzled whether can lactate serve as bioenergetic tool? (6).
It has been observed that lactate is transferred across biological membrane by specialized mono-carboxylate transporters (MCTs) and the phenomenon is termed as “lactate shuttle” (7). Members of monocarboxylate transporter (MCT) family (MCT1-4) are H+/lactate bidirectional symporters and in recent times they have emerged as attractive target for anticancer drug development (8). While MCT1 is ubiquitously distributed, MCT4 is strongly expressed in glycolytic tissues like cancer cells and is a target of HIF1α. Simultaneous inhibition of MCT1 and MCT4 has been reported to block the export of lactate and inhibit glycolysis independent of AMPK (AMP activated protein kinase) (8). MCT4 has also been implicated in lactate efflux from glycolytic muscle fibers and astrocytes in the brain (7). Further existence of lactate receptor also has been found in brain thereby suggesting intricate link between cerebral metabolisms to different types of tumors. Recent findings suggest that it can act like fuel to tumor cells. Studies by Faubert et al., have demonstrated that human non-small lung carcinoma cells (NSCLC) utilize lactate with high 18fluorodeoxyglucose uptake. Deletion of MCT1 from tumor cells in mice was reported to exclude lactate-dependent metabolite labeling (13C-lactate), thereby confirming that cancer cells take up lactate autonomously (9). LDH (lactate dehydrogenase) has been reported to be present in cytosol and in the outer membrane of mitochondria, where it oxidizes lactate to pyruvate and produce NADH. Aerobic glycolysis regenerates NAD+ upon reduction of pyruvate into lactate by cytosolic LDH (10). In brain malate aspartate shuttle (MAS) is present as an alternative pathway for NAD+ generation for aerobic glycolysis (6). An increased understanding of glycolytic pathway and lactate metabolism has assumed greater significance in recent times with respect to cancer therapeutics.
In rapidly proliferating tumor cells due to elevated rate of tumor growth, their metabolic requirements surpass the vasculature ability to provide oxygen and nutrients. This results in scarcity of intracellular oxygen and nutrition, making the blood vessels hypoxic. Increased diffusion distances of 100 μm or more may result in diffusion-limited or chronic hypoxia (11). Tumor cells have been observed to show a metabolic adaptive response to hypoxic condition and rely on glycolysis for ATP generation to different extents by switching from oxidative to glycolytic pathway and this metabolic strategy is mainly permitted by the over expression of LDH-A which is a direct target of Hypoxia inducing factor (HIF-1a) (12) and glucose transporters, together with a decreased dependence on the oxidative pathway (i.e. pyruvate to lactate to acetyl-CoA). Hypoxia is a necessary feature of most of the solid tumors and it is commonly thought that lactate production and hypoxia are related in cancer, but sometimes it is not true for all the tumors. Aerobic glycolysis (Warburg effect), increased glutaminolysis and low pressure in blood vessels may also result in the accumulation of lactate in non-hypoxic areas (13). So, lactate and hypoxia can be linked in normoxia conditions too (14).
LDH-A coverts pyruvate into lactate, which is the most probable mechanism in tumor cells acidosis. Main source of acid in cells is either via generation of H+ or by carbonic acid (HCO3-) and lactate. To overcome the acidification, cells regulate H+ export and HCO3- import via specific transporters and exchangers (15). Hypoxia up-regulates the expression of LDH-A (pyruvate to lactate conversion) and monocarboxylate transporter 4 (MCT4), but down-regulates MCT1 expression and LDH-B (Lactate to pyruvate conversion). Thus during metabolic transformation from normal to malignant phenotype there is increased consumption of glucose and increase in extracellular acidosis due to high production of lactic acid. It has been suggested that persistent activation of glycolysis will lead to environmental acidosis, which will be toxic to the normal cells but will be harmless to the cancerous cells (16-18).
Glucose is an essential metabolic energy source for all living organisms and a structural precursor for cellular biosynthesis of proteins, lipids, and nucleic acids with ATP generation being the essential metabolic process for energy supply to the cells. Mammalian cells generate their ATP through glycolysis in the cytoplasm and oxidative phosphorylation (or respiration) in the mitochondria. Although normal cells depends on oxidative phosphorylation for ATP production from glucose, the same is not true for hypoxic cancer cells as they obtain their ATP by glycolysis and the final conversion of glucose to lactate. It is still perplexing as to why cancer cells choose aerobic glycolysis despite being an inefficient mechanism for ATP production rather than more efficient mitochondrial respiration for ATP production. Glycolytic pathway is relatively inefficient, requiring one glucose molecule for the net production of two ATP molecules whereas via oxidative phosphorylation one glucose molecule will yield thirty-six ATP molecules (16). It is now well established that although oxidative phosphorylation can produce more energy per molecule of glucose than glycolysis but glycolysis is capable of producing ATP at a faster rate as long as glucose supplies are unlimited. It is now well established fact that cancer cells up regulate the glycolytic pathway and over express glycolytic enzymes (19).
Dependence of cancer cells on glycolysis increases as malignant transformation increases and this gets support from the fact that transition from pre-malignant lesion to invasive cancer involves increased glucose uptake (20, 21). Thus majority of tumors even in aerobic conditions has been observed to invest heavily in glycolysis as their main source of energy, showing an alteration in their glycolytic and mitochondrial machinery with a preference to produce lactic acid to obtain immediate energy (22). This metabolic switch from oxidative phosphorylation ensures sufficient energy from glucose (23, 24). This observation that cancer cells exhibit glycolysis with lactate secretion and mitochondrial respiration even in the presence of oxygen is known as Warburg effect (25, 26) and has been considered a key driver for cancer cell proliferation, malignancy, metastasis and therapeutic resistance (27). Warburg's hypothesis was postulated by the Nobel laureate Otto Heinrich Warburg in 1924 (2). Overall, this is achieved by transcriptional activation of LDH-A which encodes lactate dehydrogenase A and converts pyruvate to lactate (28).
Lactate dehydrogenase is a tetrameric protein, which consist of two types of subunits, the M type (pre-dominantly expressed in skeletal muscle) and the H type (pre-dominantly expressed in heart) contributed by the two genes (LDH-A & LDH-B) (29, 30). These two subunits combine in different ratio to give rise to five isozymic forms (M4, M3H, M2H2, MH3 & H4), a third gene, LDH-C (also known as LDH-X), is reportedly expressed in testes and sperm (31). The numbers were assigned according to the different electrophoretic mobility of the isozymes, LDH-5 shows the lowest mobility, whereas LDH-1 is the fastest moving enzyme in this family (Figure 1). In most of the mammalian tissues, LDH isozymes are expressed in a tissue specific manner. With the exception of LDH-C, all the other five isozymes are mailnly localized in the cytosol of somatic cells, but they have also been observed in mitochondria. In different types of tumors, differential expression of these isozymes has been reported (32). In humans, each subunit have a molecular mass of about 35 kDa, differing in amino acid compositions, but possess a high level of homology among different types of human subunits and also among subunits of the same type in different species (33).
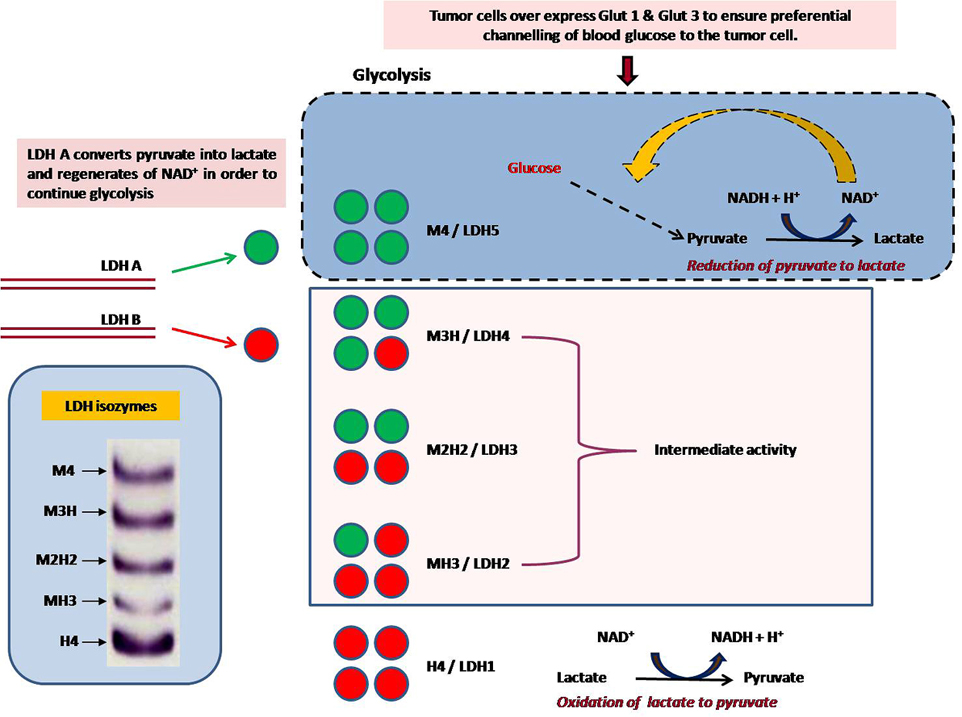 Figure 1
Figure 1Overproduction of lactate is a hallmark of cancer cells. In tumors it can act as an alternate metabolic fuel and also as signalling molecule. The secreted lactate lowers pH of the cellular matrix and helps stimulate angiogenesis and metastasis. Lactate is produced exclusively by LDH. LDHs catalyze interconversion of pyruvate lactate, with concomitant interconversion of NADH and NAD+. M4-LDH (LDH-5, LDH-A) and M3H LDH preferentially convert pyruvate to lactate, and thereby prevents entry of pyruvate to the TCA cycle. This reaction regenerates the cofactor NAD+ and, therefore, permits glycolysis as a self sustained pathway. Thus, up-regulation of LDH-A alone alone can ensure efficient glycolysis in the tumour cells.
Due to the lack of oxygen that is the final electron acceptor in the electron transport chain, M4-LDH (LDH-5, LDH-A) and to a lower extent M3H LDH converts pyruvate to lactate as the final product of glycolysis, instead of channeling it in the TCA cycle in the mitochondria (32). During this reaction cofactor NAD+ is regenerated which permits the continuation of glycolysis (33). In tumor cells LDH-A is up regulated thereby ensuring an efficient aerobic glycolysis in tumour cells, but in normal condition in normal cells, LDH is not so necessary and generally use the aerobic oxidation pathway (32, 34-37). In contrast, H4 LDH and H3M LDH which are heart-specific forms, promote the conversion of lactate to pyruvate, thereby favoring its entrance into the Krebs cycle (33, 38). LDH-M has higher Vmax and the overall turnover rate of LDH-M as compared to LDH-H is two-fold higher. In contrast LDH-H isoform have exhibited about a three-fold increase in the ability to bind pyruvate compared with the -M one due to a lower Km value for pyruvate and thus LDH-H is more sensitive to inhibition by high pyruvate concentrations (33). In different types of tumors, differential expressions of LDH isozymes have been reported (32). Abnormally high LDH activity in cancerous tissues vis-a-vis decreased LDH activity with tumor regression has been demonstrated in animal models (32, 35-37). Recently, LDH-A activity down regulation has been reported to affect mitochondrial bioenergetics and to retard proliferation of tumor cells in culture (39-43). Thus, LDH seems to be a relevant and suitable candidate to study anticancer potential of newly formulated drugs.
At upstream level, the committed step of glycolysis is catalyzed by phosphofructokinase1 (PFK1). 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFK-2/FBPase) has been reported to catalyze the synthesis and degradation of fructose 2,6-bisphosphate (F2,6BP) (44), which is a powerful activator of 6-phosphofructo-1-kinase, the rate-limiting enzyme of glycolysis (Figure 2). PFK2 synthesize fructose-2,6-bisphosphate (Fru-2,6-P2) which is the most critical metabolic activator of PFK1. PFK-2/FBPase is encoded by four genes namely PFKFB1, PFKFB2, PFKFB3, PFKFB4 (45, 46) and each isoform being different from other in its kinase and phosphatase activities, tissue distribution and regulatory response to protein kinases. In cancer cell lines inducible PFK-2/FBPase-2 (iPFK-2), which is a splice product of PFKFB3 gene with proto-oncogenic features, has been detected (47). iPFK-2 is a recently described PFK-2 isoform that is encoded by the PFKFB3 gene on human chromosome 10 (48). PFKFB3 gene product has the highest kinase/phosphatise activity ratio, which means that in tissues where it is expressed high glycolytic rates are sustained due to elevated Fru-2,6-P2 levels (49). iPFK-2/PFKFB3, is a ubiquitous gene constitutively expressed in proliferating tissues (47, 50), in transformed cell lines (47, 51), and in various tumors (52) and is considered to play critical role in switching over to glycolytic phenotype by the cancerous cells.
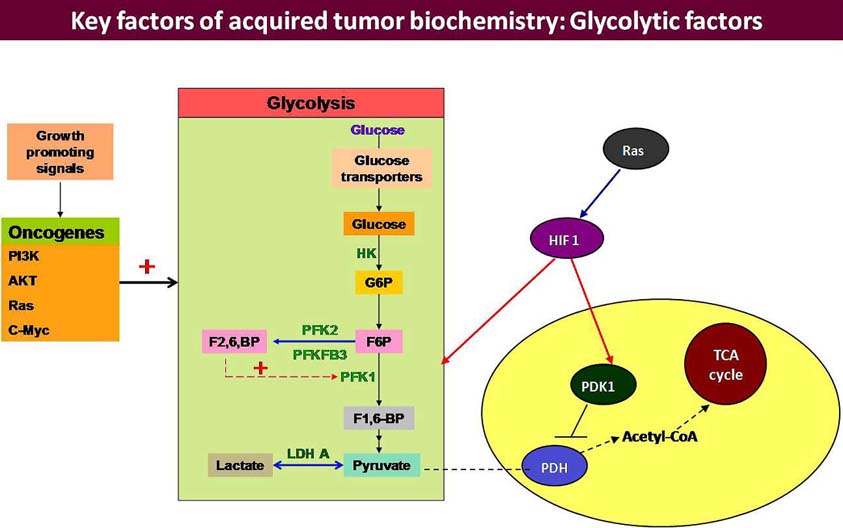 Figure 2
Figure 2Tumor cells over express HIF-1a and initiate a transcription programme to up-regulate glucose metabolizing genes and all the enzymes of glycolytic pathway. Tumor cells over express Glut 1 and 3 to ensure preferential channelling of blood glucose to the tumor cell. There are three rate limiting enzymes of glycolytic pathway: HK II, PFK1 & PK and all of them have been reported to be over activated in the tumor cells. Amongst them, PFK1 occupies a sensitive position because it is regulated by another enzyme PFK2/FBPase 2 which is exclusive for the conditions of additional glycolytic demand, including carcinogenic progression. 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases (PFKFB) are bifunctional enzymes that catalyze either the ATP-dependent phosphorylation of F6P to F2,6BP (PFK2 activity) or the dephosphorylation of F2,6BP to F6P (FBPase activity). The PFKFB family comprises four members among which PFKFB3 is more important because it has high PFK2 but almost no FBPase activity. PFKFB3 sustains high-rate of glycolysis and is highly expressed in several types of human tumors and down regulation of PFK2 has been correlated with regression of certain tumors in vitro. F2,6BP and PFKFB3 are the main allosteric activator of PFK1 and exerts an important contribution to the glycolytic switch.
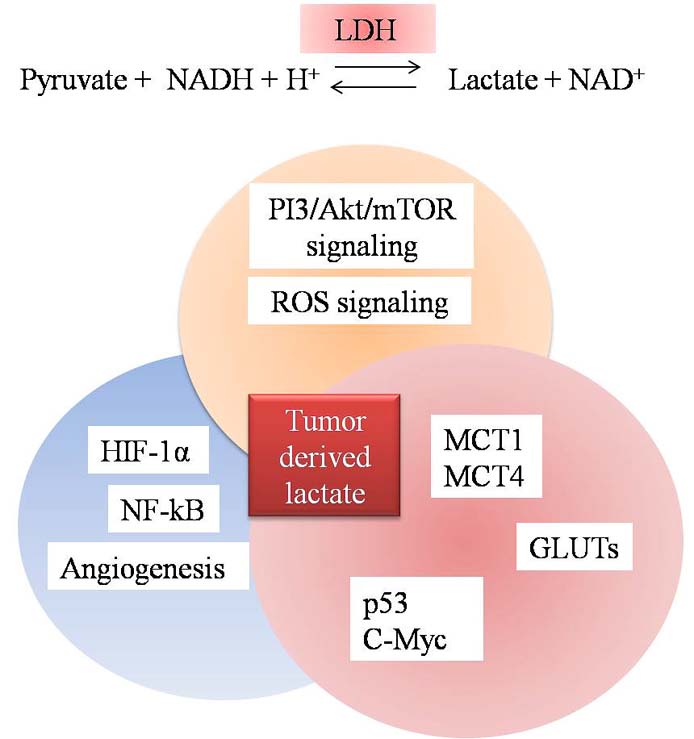 Figure 3
Figure 3Transcription factors and other proteins are modulated during metabolic reprogramming of cancer.
HIF has been reported to induce the gene for PFKB3 (53) in many primary aggressive neoplasms, including colon, ovarian, breast and thyroid carcinomas (52). Once transcribed, the protein is activated by phosphorylation (53), whereas inhibition of inducible PFK-2 protein expression results in decrease of 5-phosphoribosyl-1-pyrophosphate, which is a product of the pentose phosphate pathway (PPP) and important precursor for nucleic acid biosynthesis (47). Down regulation of PFK2 has been correlated with regression of certain tumors both in vitro (54) and in vivo (36, 37).
PDH (Pyruvate dehydrogenase) is another enzyme that catalyzes the conversion of pyruvate to acetyl-coenzyme A, which further enters into the Krebs cycle and provides ATP to the cell. PDH activity is under the control of pyruvate dehydrogenase kinases (PDKs). PDK1 encodes pyruvate dehydrogenase kinase 1, inactivates PDH (the enzyme responsible for conversion of pyruvate to acetyl-CoA), thereby shunting pyruvate away from the mitochondria (22). In cancer cells under hypoxic conditions, conversion of pyruvate to lactate occurs and this reaction is catalyzed by lactate dehydrogenase 5 (LDH5), encoded by lactate dehydrogenase A, regardless of the presence of oxygen (aerobic glycolysis/Warburg effect) (Figure 2).
Some reports suggest that cancer cells appear to use glycolytic metabolism even before exposure to hypoxic conditions. For example, leukemic cells although reside within the blood stream at higher oxygen tensions as compared to cell in most normal tissues, yet leukemic cells has been observed to be highly glycolytic (36, 55, 56). It has been suggested that activation of aerobic glycolysis can impart protection against cell death induced by reactive oxygen species (ROS) such as hydrogen peroxide (57-59) and thus activation of aerobic glycolysis may not be with the sole purpose of proliferation (60).
In cancer cells, splice variant M2 (fetal) isoform of pyruvate kinase has been reported in which several amino acids are added, including a tyrosine which gets phosphorylated in tumors with activated tyrosine kinase signaling and inhibits the feed forward loop. This causes the last step in glycolysis to be slowed in cancer cells, thereby leading to slow entry of pyruvate into the mitochondria or what is known as delayed TCA cycle. End result is accumulation of pyruvate in the cell, which in order to keep glycolysis active gets converted into lactate and secreted. Lactate which is the end product of glycolysis, is thus produced in large excess in tumors, thereby constituting an alternative metabolic fuel for proliferating cancer cells (61, 62).
Metabolic reprogramming in cancer cells also deregulate HIF-1α, c-Myc and p53 expression (Figure 3) (63). Up regulation of HIF-1α and c-Myc stimulates growth factors which further stimulate receptor tyrosine kinase (RTKs) to activate PI3K/Akt axis (64). Highly glycolytic cancer cells also over express GLUT transporter, thereby increasing glucose uptake to fulfill the energy demands of cancer cells. Among these transporters GLUT1 is over expressed in most of the cancer cells and transcription factor HIF-1α increases expression of GLUT1 (65). GLUTs have been proposed as predictive biomarkers for progression of cancer. In a recent reports GLUT3, to a lesser extent has been proposed as a therapeutic target for treatment of glioblastoma (66). GLUT3 has also been reported to be over expressed in prostate cancer (66). Over expression of glucose transporters in various cancers assumes significance from the fact that they are associated with glucose metabolism and lactate production. Recently up regulation of GLUT3 mRNA, protein and glucose uptake by cAMP pathway was characterized in breast cancer cells and was suggested to be a therapeutic target for cancer therapeutics especially in breast cancer (67). GLUTs are also interlinked with pathways like PI3K/Akt, mTOR, HIF, RAS and p53, thus decreased expression of GLUT might help in reducing cancer and also inhibition of aerobic glycolysis in cancer (68). Tumor suppressor pathways have also been suggested to regulate cellular metabolism and coordinate nutrient utilization. In cancer cells mutant p53 has been observed to induce the expression of LDH-A and promotes tumor acidification due to increased production of lactate (68). Loss of p53 has been reported to affect TIGAR pathway (69) as well as oncogenes like c-myc (70) and transcription factors such as hypoxia inducible factors-1α (HIF-1α) (71). Inactivation of p53, which is one of the most commonly mutated genes in cancer, has been suggested to trigger the Warburg effect due to its involvement in the activity of cytochrome c oxidase, a protein complex involved in oxidative phosphorylation (72). Recently regulation of energy metabolism has been suggested to be a novel function of p53. Due to decline in transcription of glucose transporters like GLUT1 & GLUT4, reduced uptake of glucose by cancer cells has been observed. p53 also decreases glycolysis by activating the TIGAR (TP53 inducible glycolysis and apoptosis regulator) gene which declines F2,6,BP and phosphoglycerate mutase levels in the tumor cells. It has also been described to activate NADPH production by channelling glucose into pentose phosphate pathway (69). Thus, according to new evidences, it is now evident that the level of wild type p53 alone can acts as a determinant of tumor cell survival/death (Figure 4).
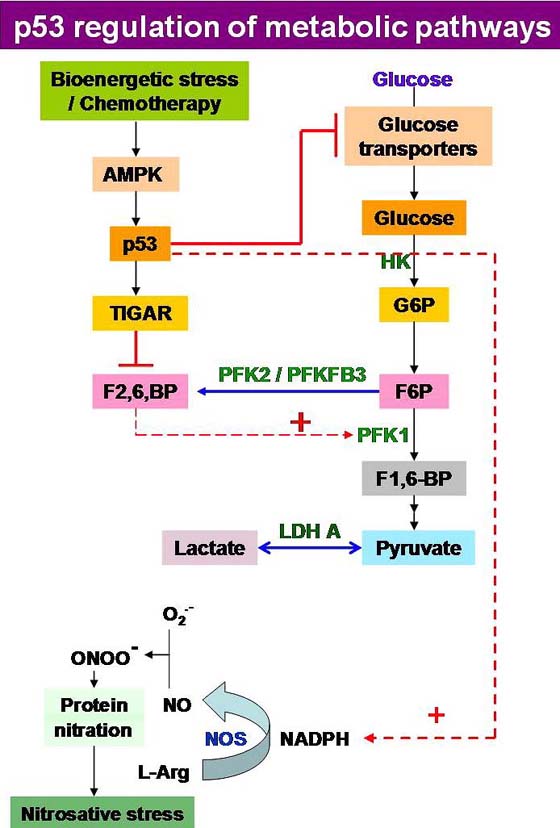 Figure 4
Figure 4Regulation of energy metabolism is a novel function of p53 in tumor suppression. p53 reduces glucose uptake, by repressing transcription of glucose transporters GLUT1 and GLUT4 and decreases glycolysis by activating the TIGAR gene which declines F2,6,BP and phosphoglycerate mutase levels in the tumor cells. p53 has also been described to activate NADPH production by channelling glucose into PPP. Loss of wild type p53 allows apoptosis evasion during tumor progression in many neoplasias, like leukemia and lymphoma. Level of wild type p53 can alone act as determinant of tumor cell survival/death.
Several mechanisms have now been reported through which p53 can slow glycolysis and therefore counteract the increase in glycolysis that is characteristic of cancers. It can reduce glucose uptake, by repressing transcription of the glucose transporters GLUT1 and GLUT4 (73) and declines glycolysis by activating the TIGAR gene which lowers fructose-2,6-bisphosphate and phosphoglycerate mutase levels in cells (69), with a concomitant stimulation of NADPH generation leading to the diversion of glucose through the pentose phosphate pathway (74), thus impeding flux through various steps of the glycolytic machinery (Figure 4).
Tumor suppressor p53, which is considered guardian of the genome and most studied in cancer, interestingly has been observed to be repressed in majority of tumors and has been proposed to be responsible for the metabolic shift of tumor cells to Warburg phenotype (75). p53 can increase the use of the TCA cycle and promote oxidative phosphorylation in the mitochondria by up regulating transcription of glutaminase-2 and cytochrome C oxidase. It can also indirectly regulate glycolysis by modulating the nuclear factor-κb pathway (76). Expression of p53 has been reported to limit the activity of Iκb kinase-α (IKKα) and IKKβ, thereby restricting the activation of NF-κb and dampening the expression of glycolysis-promoting genes such as GLUT3. Reduced expression of p53 has been reported to be accompanied by a switch from oxidative phosphorylation to glycolysis (77). Due to the loss of tumor suppressors TP53 and PTEN, along with concurrent activation of AKT and mTOR pathways, there is activation of hypoxia-induced factor (HIF) activity in cell’s response to hypoxia. Absence of oxygen allows HIF-1α and HIF-2α proteins to form dimers with their beta-counterparts (HIF-1β and HIF-2β), thereby leading to the stabilization of the HIF transcription factor complex. Activation of HIF can increase the transcription of hundreds of genes, which in turn will promote angiogenesis, cell migration and survival. p53 thus can regulate many genes involved in energy metabolism including lactate dehydrogenase A and pyruvate dehydrogenase kinase, both of which keep pyruvate away from the mitochondria, and 9 of the 10 enzymes required for glycolysis via AKT-mTOR mediated HIF stabilisation. Infact, class I PI3K, as well as its downstream targets AKT, extracellular regulated kinase (ERK), and ribosomal protein S6 kinase-1 (RSK1), which activate mTOR, are all well-documented oncoproteins (78).
In rapidly proliferating cancer cells, hypoxia like condition is created, thereby leading to the increased expression of transcription factor like HIF in concert with oncogenes like c-Myc and Ras; thereby leading to the promotion of glycolysis and preferential conversion of pyruvate to lactate due to inhibition of pyruvate dehydrogenase by PDK, thereby leading to the continuous regeneration of NAD+ which in turn supports proliferation of tumor cells (Figure 2) (79). Earlier lactate was considered as the byproduct of glycolysis and major cause of muscle fatigue due to acidosis induced tissue damage (80). In tumors dual role for lactate has been suggested; it can act both as a metabolic fuel and signaling molecule during tumor metabolism and angiogenesis. Exquisite metabolic symbiosis has been reported to occur not only between oxidative and glycolytic tumor cells but also between tumor cells and stromal cells, including endothelial cells and fibroblasts (81). Both oxidative cancer cells and endothelial cells lining tumor blood vessels can take up lactate released by glycolytic tumor cells (61). Lactate derived from glycolytic tumor cells could be used as a main source of metabolic fuel (61). Oxidative tumor cells has special feature like they can also use lactate instead of (or in addition to) glucose, thereby sparing available glucose, thereby facilitating their diffusion deeper into the tumor tissue to fuel the hypoxic cells located at distances farther away from tumor blood vessels (61). Lactate as an energy source requires the conversion of lactate into pyruvate (and back) as well as activation of mechanism to transport lactate into and out of tumor cells by specific transporters like MCT1 and MCT4, which have emerged as key players in this process. MCT1 is a high affinity lactate transporter which facilitates exogenous lactate uptake by oxidative tumor cells whereas MCT4 facilitates the release of glycolysis-derived lactate (61). Preferential expression of MCT1 transporter and LDH-1 with a concomitant elevation in the activity of PDH in tumor fibroblasts has been reported, suggesting the metabolic use of lactate produced by tumor cells, thereby preventing the development of a hostile acidic microenvironment (32). Other workers have reported that tumor-associated fibroblasts can instead undergo aerobic glycolysis and utilize the released lactate to feed the adjacent oxidative cancer cell (62).
In recent times existence of a metabolic symbiosis between hypoxic and aerobic cancer cells has been demonstrated, during which aerobic cells take up the lactate produced by the hypoxic and utilize it as their principal substrate for oxidative phosphorylation (61). By downregulating oxidative phosphorylation hypoxic tumor cells has been observed to consume glucose in a very efficient manner, thereby maintaining redox homeostasis (22). This is achieved by repression of MCT1 by hypoxia which transports lactate into rather than out of cancer cells. This facilitates the efficient uptake of lactate by MCT1 in concert with the O2- dependent expression of LDH-B, to utilize lactate as an energy substrate by aerobic cancer cells, thereby freeing these cells from the need to take up large quantities of glucose (61). In breast cancer cell line MCF7 and in cancer associated with fibroblasts, expression of MCT4 has been observed to be induced, which in turn has been reported to impart protection against apoptosis, when the cell lines are co cultured. These findings have suggested the presence of stromal-epithelial lactate shuttle in human tumors analogous to lactate shuttle present in brain as well as in muscles. MCT1 which is a transporter of lactate uptake was also found to be up regulated in MCF7 when co cultured with fibroblasts (62). Transformed prostate epithelial (TPE) and prostate cancer cells both express MCT1 and MCT4 depending upon rate of proliferation and aerobic glycolysis. Positive correlation has been observed between MCT4 and MCT1 in human prostate cancer tissue based on L-lactate shuttle (82). NMR studies have also documented that hepatoma and glioma utilize lactate in vivo (83). Recent findings describe that HeLa cells and SiHa cells show intracellular lactate signaling, which is dependent on extracellular lactate uptake by MCT-1, thereby encouraging glutamine uptake and metabolism in cancer cells. Lactate induces c-Myc activation by stabilizing HIF-1α. Glutaminase 1 (GLS1) and glutamine transporter is also activated in response to hypoxia (84).
Moreover multiple potential binding sites has been suggested on the MCT1 gene promoter for known ROS-responsive transcription factors such as cAMP response element-binding protein (CREB), activated protein-a (AP-1), stimulating protein-1 (SP-1), nuclear factor-κB (NF-κB), and nuclear factor erythroid 2 (NF-E2, or Neff) (85). Lowering of the pH of the cellular matrix by the secreted lactate in tumors may influence remodeling of the matrix and promote angiogenesis. Up regulation of glycolytic machinery in tumors can make them acidotic and encourage the selection of motile cells which break free of the basement membrane and facilitate metastasis (86, 87). Further it can also regulate cellular redox state through actions of lactate dehydrogenase (LDH) by exchanging and converting it to its more oxidized analogue pyruvate. Apart from this due to its autocrine, paracrine and endocrine like action, it has been suggested to act as an important signaling molecule and a new term “lactormone” has been coined for it because it is taken up by distal tissues and organs when it is released into systemic circulation and affects the redox state in the cells, tissues and organs. Lactate also behaves as a signaling molecule that promotes angiogenesis and act as immunosuppressive agent during cancer (88). Metabolic deregulation can cause alteration in the expression of transcription factors like HIF-1α and c-Myc, which is activated by growth factors that further stimulate receptor tyrosine kinase (RTKs) dependent up regulation of PI3K/Akt pathway and Ras (Figure 3). Thus, pharmacological reduction of lactate production and excretion is expected to reduce the invasive potential of tumor cells making them more susceptible to apoptosis.
Immune system has an important role in repressing cancer and escaping immune surveillance has emerged as new hallmark of cancer cells (3). Cancer and immune cells exhibit similarities on the basis of metabolism to maintain proliferation and survival. Metabolism is also considered as an essential regulator of immune cell function (89). Like cancer cells, T cells also require glucose for their function and survival. It is well established that cancer cells express glucose transporters (GLUT) and both T cells and cancer cells has been observed to compete for glucose and thereby its depletion. It has been shown that phosphoenol pyruvate (PEP), which is an intermediate during glycolysis, is essential for T-cell receptor-mediated Ca2+ NFAT (nuclear factor of activated T cells) signaling (90).
Recently, it has been demonstrated that LDH-A derived lactic acid selectively restrain T and NK cell activation and tumor immune surveillance (Figure 5) (91). LDH-A expression in human melanoma patients has been linked to T cell survival and activity (92) and some researchers have suggested some non metabolic functions of glycolytic enzymes such as GAPDH (glyceraldehyde 3-phosphate dehydrogenase) and lactate dehydrogenase especially LDH-A. GAPDH has also been reported to act as energy sensor and regulate cytokine level in response to glucose concentration in the cell. In low glucose concentration, translation of mRNA encoding IFN-γ and IL-2, including other cytokines, was observed to occur due to the binding of GAPDH to AU-rich elements in 3’-untranslated region (UTR) (93). It was expected that LDH-A may also engage in RNA binding. Both GAPDH and LDH-A use NAD+ as cofactor thus it is likely to participate in mRNA binding function in glycolytic pathway. During glucose scarcity, increased intracellular level of NAD+ has been anticipated to compete with GAPDH for RNA binding (94). Some studies have shown that T Cells exclusively expresses “A subunit” of LDH, which is perilous for proper production of IFN-γ (95). Lactate which is secreted from tumor cells is able to induce and stimulate inflammation which may increase IL-17A secretion by macrophages and T cells in response to chronic inflammation. Lactate secretion can also inhibit dendritic cells activation and also prevent monocyte migration and cytokine release. Tumor derived lactate also helps in angiogenesis via induction of IL-8 and NF-kB (Figure 5) (96).
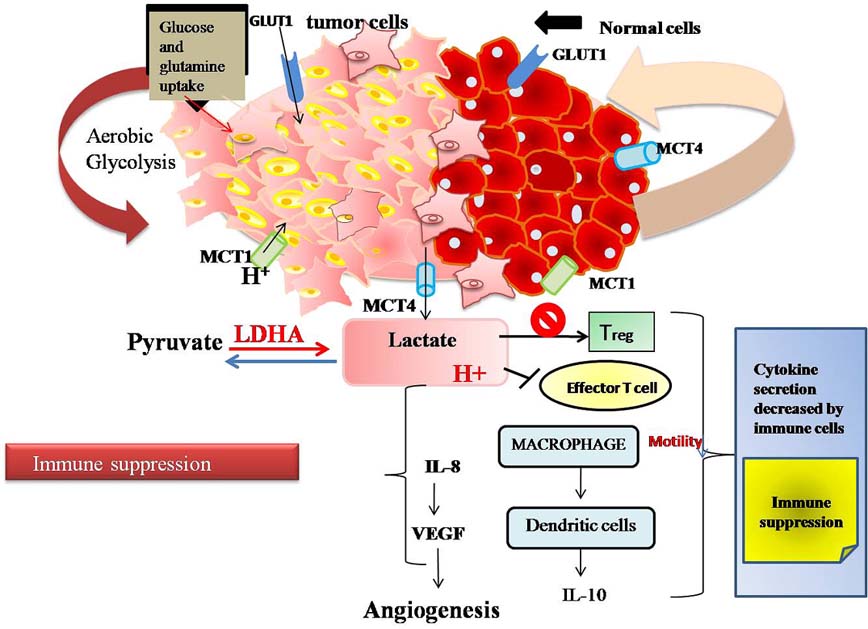 Figure 5
Figure 5Aerobic glycolyis produces lactate that impairs function of regulatory T cells and effecter T cells, thereby leading to a decrease in cytokine production and immune suppression.
Immunometabolic studies on murine lung carcinoma have suggested that myeloid specific deletion of LDH-A, helps to suppress tumor growth and thus macrophage may have T cell immunosuppressive activity by expression of LDH-A (97). Cancer cells bypass immune surveillance by lactate in extracellular environment, which tend to inhibit effector T-cell response and by T-regulatory (Treg) cells, its up regulation (98). According to recent reports, immunoncology and immunometabolic studies have the potential to target lactate signaling for targeted anticancer therapies. Treg cells function in lactate rich microenvironment and Treg dependent Foxp3 transcription factor has been reported to rewire T cell metabolism by quashing c-Myc and glycolysis and promoting oxidative phosphorylation, thereby making Treg cells resistant to acidic environment created by lactate production (99). Cancer originated lactate is now considered as immunosuppressive metabolite that can have key role in developing anticancer therapies. Studies by Kreutz el al., have reported novel mechanism responsible for immune escape during tumor which is by failing to activate cytotoxic CD8+ and NK cells (natural killer cells) (92) . Studies have revealed that lactate stimulates an autocrine pathways, that stimulates NF-kB and IL-8 and helps in endothelial cell migration and cancer progression (100).
Lactate can be thought of as an interactive oncometabolite in metabolic reprogramming of cancer as well as in avoiding immune destruction; both being emerging hallmarks of cancer cells. Further lactate contributes in T-cell anergy, signifying that it can also acts as immunosuppressant outside hypoxic tumor core. Tumor cells increase excessive uptake of glucose via glucose transporters and generate lactate in the tumor microenvironment where they recycle NAD+. Based on the information about tumor generated lactate; GLUT and MCTs have been proposed as target for cancer therapeutics and drug discovery. With an increase in understanding of lactate signaling, there is a need to develop non overlapping strategy to target cancer that selectively target cancer cells but does not interfere with immune cell metabolism. Still the consequences of tumor-induced changes on immune cell metabolism are still in infancy and beginning to be elucidated both from basic understanding of cancer development, progression and therapeutics point of view. An in-depth understanding of transporters and lactate metabolism vis-à-vis alterations in the metabolism of cells of innate and adaptive system during tumor development and its therapeutics may lead to identification of key parameters/control points that can be targeted for the treatment of cancer. Increased understanding of lactate as a signaling molecule, its journey from dead end product of glycolysis to survival of immune and tumor cells and how selectively metabolism of tumor cells can be targeted without adversely affecting the immune cells has got a pivotal role to play in future cancer therapeutics.
DR, RAN and SKC are grateful to Dr. Harisingh Gour Central University, Sagar for fellowship. This work was financially supported by a project from UGC-Faculty Research Promotion Scheme (F.30-12/2014/BSR) and SERB (SERB/LS-816/2013), Govt. of India, sanctioned to RKK. The authors are thankful to Department of Zoology, Dr. Harisingh Gour Central University, Sagar, for providing infrastructural facilities and financial support.





