Frontiers in Bioscience-Landmark (FBL) is published by IMR Press from Volume 26 Issue 5 (2021). Previous articles were published by another publisher on a subscription basis, and they are hosted by IMR Press on imrpress.com as a courtesy and upon agreement with Frontiers in Bioscience.
1 Department of Environmental Biology, University of Rome, Sapienza, 00185 Rome, Italy
2 Consiglioper la Ricerca in Agricoltura e l,Analisi dell,Economia Agraria, Centro di Ricerca Difesa e Certificazione,Via C.G, Bertero, 22, 00156 Rome, Italy
Abstract
Lipids occur in fungi as major constituents of the membrane systems and minor component in the cell wall; they can store energy in the lipid bodies and, in some cases, they can act as intra-extracellular signals. Fungi contain a various set of lipids, including fatty acids, oxylipins, sphingolipids, phospholipids, glycolipids, and sterols. Current studies in lipids suggest their additional role in cell signalling; for instance, host-pathogen exchange lipid signals at the interface during their interaction. This review aims examining those fungal lipid classes involved in the pathogenic interaction with the host plants. The lipid signals may trigger host immune response as well as functioning as virulence factors altering the lipid homeostasis of the host cells.
Keywords
- Fungal Lipids
- Host-Pathogen Interaction
- Fatty Acids
- Oxylipins
- Sphingolipids
- Review
Lipids are classified as being sparingly soluble in water but readily soluble in organic solvents. Lipids can be divided in two broad categories: lipids with long-chain fatty acid and lipids derived from an isoprene unit (1).
Lipids are the major constituents of plasma membrane and intracellular membranes; they also have other biological functions such as energy storage, signal transduction, and stress response. The role of lipids in host-fungus interactions is gaining momentum since the availability of highly sensitive analytical technologies, including gas chromatography and high-pressure liquid chromatography coupled to mass spectrometry (2).
In this review, we will consider the sole lipids with long-chain fatty acid and we will highlight some biosynthetic and signalling pathway of fatty acids, oxylipins and sphingolipids. Fatty acids (FAs) occur either as free form (FFA) or in conjugation to other molecules, such as glycerol, alcohols or amines. Additionally, FAs can be fully saturated, unsaturated, and linear, branched, or can contain alicyclic rings. Unsaturated FAs occur as frequently as the saturated ones; they may contain several double bond, though one or two are the most usual. FAs can be oxidized thus generating the oxylipins. Usually, FFAs do not accumulate intracellularly for their toxicity; they are very reactive by binding and inactivating several enzymes and proteins.
One distinctive group of lipids, the sphingolipids, contain a backbone of sphingoid long chain base (LCB), a set of aliphatic amino alcohols that includes sphingosine. N-Acylation of the LCB by a fatty acid produces compounds collectively known as ceramides (the basic unit of the sphingolipids). The ceramides can be converted to more complex sphingolipid through the presence of substituent groups linked to the LCB. The main and most common substituents are the phosphate, glycosyl-inositol phosphate and glucose groups.
This review aims providing an overview of the state-of-the-art on the involvement of lipids in host-fungal pathogen interaction, suggesting that lipids may play a key role in the infection processes.
FAs are important metabolic energy sources and the building blocks of membrane lipids as for the FAs incorporated into phospholipids; FA may also serve as an energy reservoir in the form of triacylglycerols and steryl esters stored in lipid droplets.
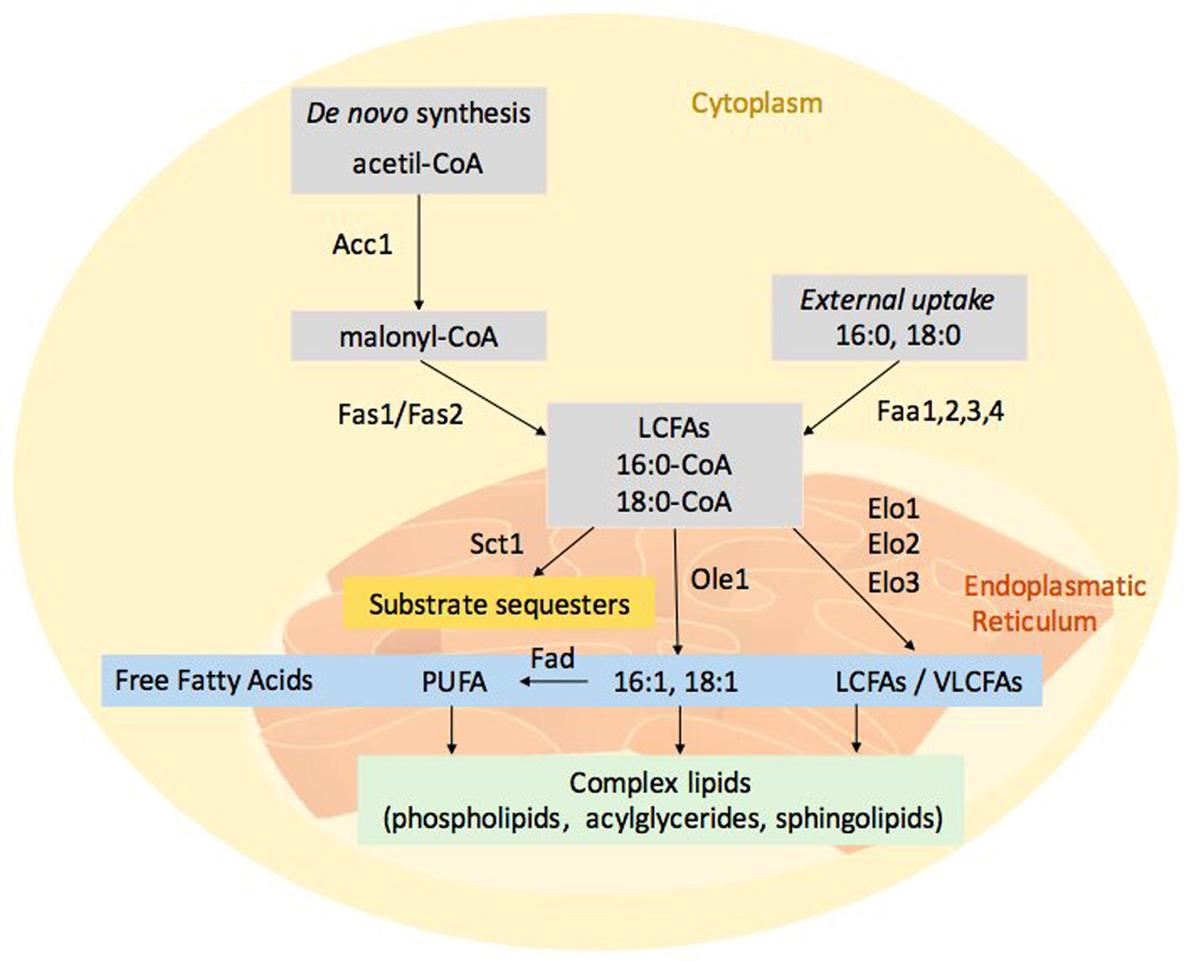
The model organism Saccharomyces cerevisiae has provided valuable insight into the fatty acid synthesis machinery of fungi. In S. cerevisiae, FA synthesis (Figure 1) initiates with the the acetyl-CoA carboxylase (Aac1) and continues with the cytosolic multi-enzyme fatty acid synthase complex (consisting of Fas1 and Fas2); this reaction yields an acyl-CoA with an acyl chain length of 16 or 18 carbon atoms (3). FA synthesis occurs in the cytosol whereas the elongation of the carbon chain takes place in the endoplasmic reticulum (ER) due to the hydrophobic nature of the long and very long chain fatty acids (LCFAs, i.e. C13-21, and VLCFAs, i.e. C22-26). The major VLCFA species in yeast is C26 that is predominantly present in an amide linkage of the ceramide backbone of sphingolipids (4). De novo synthesis uses acetyl-CoA as primers, while the elongation requires malonyl-CoA, which is provided by Aac1. S. cerevisiae encodes for different fatty acid elongation enzymes: Elo1 has specificity for the elongation of C12–16 to C16–18 FAs; Elo2 elongates C16–18 up to C22; Elo3 is involved in the formation of VLCFA, up to 26 carbon atoms (5).
 Figure 1
Figure 1Fungal fatty acid metabolism. Fatty acids derive from both de novo synthesis and external uptake. Acetyl-CoA carboxylase (Aac1) catalyses the carboxylation of acetyl-CoA to malonyl-CoA and cytosolic multi-enzyme fatty acid synthase complex (Fas1p/Fas2) generates the acyl-CoA with an acyl chain length of 16 or 18 carbon atoms. Long chain (LCFAs) and very long chain fatty acids (VLCFAs) are localized in the endoplasmatic reticulum. The elongation machinery presents different fatty acid elongation enzymes, Elo1 has specificity for the elongation of C12–16 to C16–18 FAs, whereas Elo2 elongates C16–18 up to C22 and Elo3 elongates C18 up to C26. Ole1 and other desaturases (Fad) respectively can convert the pivotal fatty acids, 16:0-CoA and 18:0-CoA, in mono and poly-unsaturated fatty acids (PUFAs). FFA can be incorporated into complex membrane lipids (phospholipids, acylglycerides and sphingolipids). Sct1 enzyme is an Ole1 competitor; its action reduces the PUFAs formation.
There are two groups of FAs, saturated and unsaturated. The term unsaturated refers to the presence of one or more double bonds between carbons. The ratio of saturated versus unsaturated FAs contributes to membrane fluidity. The viscosity of the membrane can affect the diffusion and the movement of proteins and other bio-molecules, affecting their activity (6). Unsaturated FAs are the most abundant acyl group in the membrane glycerolipids and consist of a wide range of C14-C26 species from one to six double bonds. In S. cerevisiae approximately 70-80% of glycerolipid acyl chains consist of monounsaturated fatty acids.
Delta-9-fatty acid desaturase (Ole1) and glycerol-3-phosphate acyltransferase (Sct1) control the degree of FA unsaturation. These enzymes compete for the same substrate: 16:0-CoA, a pivotal intermediate in the fatty acid synthesis (7). Ole1 is able to synthesize monounsaturated fatty acid, i.e. palmitoleic 16:1 and oleic 18:1 acid (8), while Sct1 catalyses the production of lyso-phosphatidic acid inserted into the glycerophospholipid synthesis (9). Fatty acids can present more than one double bonds along the carbon chain, in that case, they are defined polyunsaturated fatty acids (PUFAs). PUFAs are biosynthesized via an extension of the saturated-fatty acid pathway. The most common and abundant saturated FA in fungi is the stearic acid 18:0; this is converted to oleic acid, 18:1, by Ole1 and then to linoleic acid, 18:2, and alpha-linolenic acid, 18:3, by other desaturases.
In addition to de novo synthesis, an external uptake of FA actually occurs (Figure 1). FAs can be imported from the environment through a simple diffusion or an active transport, and they can be catabolized after the activation, or rather incorporated after the conversion to a coenzyme A (coA) derivate. Extracellular FA transport across the plasma membranes occurs through transporters and receptors (10). Several enzymes can mediate the transport and the activation of FAs. These enzymes are localized in the plasma membrane as well as in the ER, lipid droplets and peroxisomes (11). Fatty acid transporter (Fat1) mediates FA transport and uptake of LCFAs. Fat1 is also involved in the maintenance of VLCFAs homeostasis through their turnover (12-13).
Cells require long-chain fatty acyl-CoA synthetases, named Faa, for using exogenous free fatty acids (FFAs). At least four Faa have been found in S. cerevisiae (14). Faa1 and faa4 genes encode acyl-CoA synthetases that are required for activating imported exogenous FAs. Faa1 and its paralog Faa4 exhibit a preference for C12-C16 FAs and they are able to import sphingoid chain bases (15). Faa2 and Faa3 can activate only the FAs synthesized within the cell (16). Faa2 has been localized into the matrix side of peroxisome membranes and it is mainly active toward the fatty acids with 9-13 carbon atoms, while Faa3 prefers fatty acids with 16-18 carbons (17).
Lipids derived from both plant and pathogens play important roles during the infection process. When the fungus adheres to the host, an alteration of fatty acid composition occurs in both organisms. This alteration can be caused firstly by re-modulating the uptake of FAs; secondly, by controlling the activity of enzymes involved in fatty acid synthesis (18).
FAs influence the growth of fungi. In vitro assays indicate that the unsaturated FAs, linoleic 18:2 and linolenic 18:3 acids reduce the mycelial growth in several fungal species (19). Fungi can grow over a range of physiological and nutritional conditions that require continuous adaptation of membrane lipids. The expression of several genes encoding enzymes of lipid metabolism is up-regulated upon infection of plant; this resulting in the synthesis, modification, and re-allocation of lipid-derived molecules. Lipid-modifying enzymes regulate the spatial and temporal production of lipid metabolites involved in signalling and membrane proliferation. This spatial-temporal modulation is needed for the establishment of intracellular compartments or compositional changes of lipid bilayers. For instance, the modulation of ole1 gene expression is crucial; ole1 expression responds to a number of different stimuli, including exogenous FFAs. For instance, the active import of environmental LCFAs cause the down-regulation of ole1 expression. Ole1 mRNA is regulated at transcriptional (20) and post-transcriptional level (21). Cis-unsaturated FA products of Ole1 and polyunsaturated species can repress the transcription of the ole1 gene (22-23). Clearly, only the FAs with the cis conformation maintain the repression of ole1 transcription. At post-translational level, unsaturated FAs or PUFAs repress Ole1 (24).
The intracellular lipids of fungi mostly reside within the membranes; some lipids, however, may accumulate as droplets or globules in the cytoplasm. FAs may be part of more complex lipids, as acylglycerides, phospholipids and sphingolipids.
Acylglycerides are the ester of fatty acids linked to glycerol; this class of lipids includes monoacyl, diacyl- and triacyl-esters. FAs are the major carbon source of energy in living system and triacylglycerol are the major constituent of the lipid droplets. Phospholipids contain two fatty acids esterified to the sn-1 and sn-2 positions of a glycerol backbone, and a polar head group attached to the sn-3 position. The phospholipid class comprises phosphatidic acid (PA), phosphatidylserine (PS), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), and phosphatidylinositol (PI). Each phospholipid class includes several molecular species due to a large number of FAs varying in chain length and degree of desaturation. Phospholipids are synthesized predominantly in the ER.
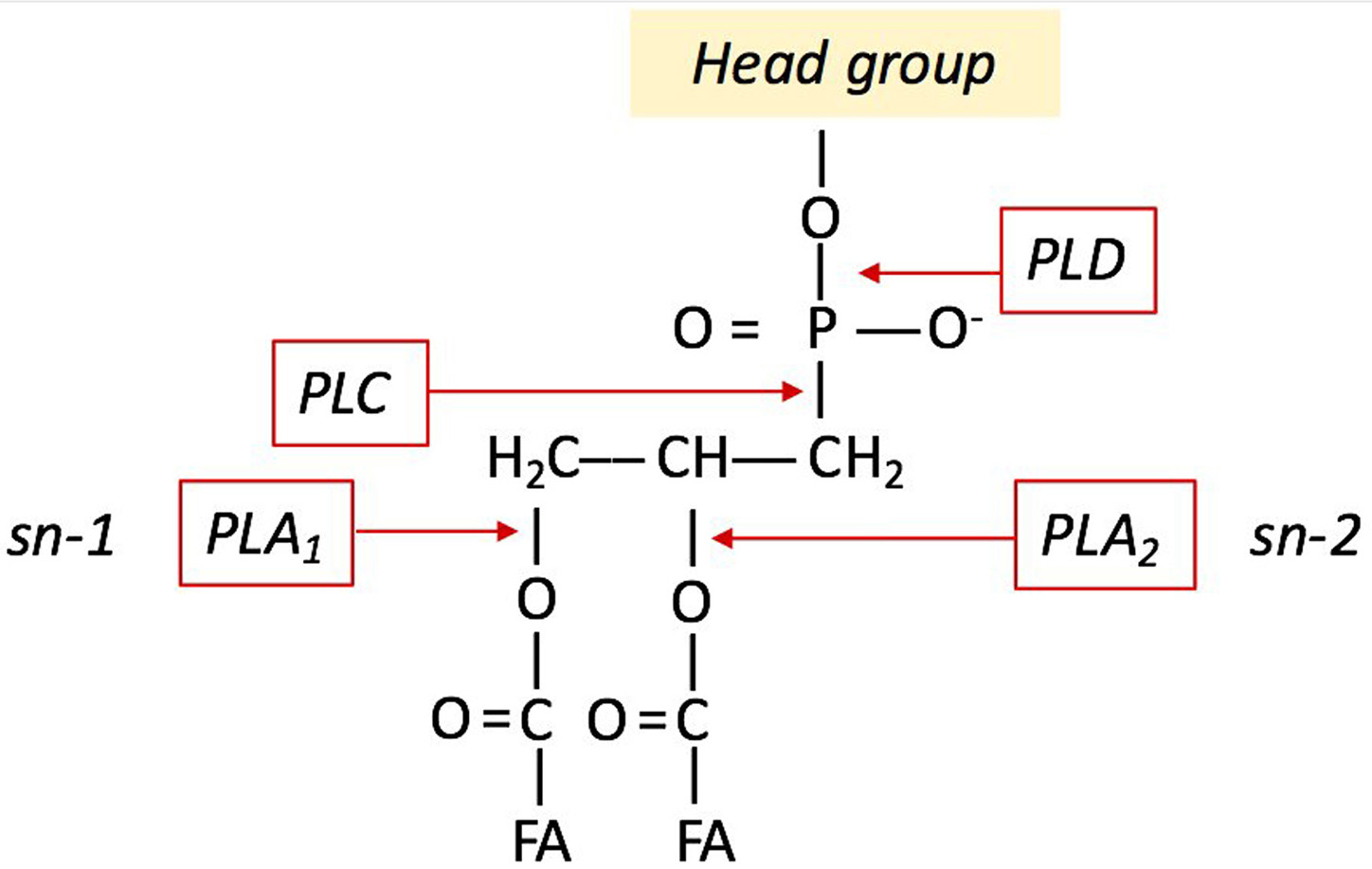
The esterases are a class of hydrolases, which catalyse the hydrolysis of triglycerides to glycerol and FFAs. Several studies indicate that the secreted lipases act as virulence factors in plant pathogenic fungi, also releasing fatty acids from their glycerophosphate backbone (25-26). Phospholipases and phospholipid-derived molecules are crucial signals in plant–pathogen interactions (27-28). Phospholipases can catalyse the conversion of phospholipids into FFAs and they can function as a modulator of many signal transduction pathways. For instance, changes in phospholipid content and phospholipase activities within the host elicit the activation of defence and resistance response to necrotrophic pathogens (29). Figure 2 shows the different sites of phospholipid hydrolysis. The phospholipase D (PLD) catalyses the hydrolysis of PC to generate choline and PA, the phospholipase C (PLC) produces two intracellular messengers, diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3), which mediate the activation of protein kinase C and intracellular calcium release, respectively. The phospholipases A1 (PLA1) and A2 (PLA2) can remove acyl groups from both sn-1 and sn-2 positions yielding to FFAs and lysophospholipids formation. The pathogenic fungus Verticillium dahliae induce PLA activity and reactive oxygen species (ROS) production in soybean cells (30). Plant PLD and PLA1-2 release FAs during the host-pathogen interaction. In Arabidopsis thaliana, oleic acid alters the defence responses (31) whilst the trienoic acids (hexadecatrienoic acid 16:3 and alpha-linolenic acid 18:3) activate the defence responses against avirulent bacterial pathogens (32).
 Figure 2
Figure 2Sites of phospholipid hydrolysis by PLD, PLC, PLA1, PLA1.
FAs represent also the substrates for the formation of the oxylipins, a family of compounds with distinct hormone-like functions, implicated in the host-pathogen interaction (33-34).
Oxylipins constitute a large family of oxidized fatty acids and metabolites derived therefrom. These bioactive lipids are abundant in mammals, plants, bacteria and fungi (35).
Oxylipins can regulate developmental processes and mediate responses to biotic and abiotic stresses in every living organisms (36). Oxylipins have been widely studiend in the plant, given that they have developed a broad range of defence responses to cope with the pathogenic infections. Plant-oxylipins include fatty acid hydroperoxides, hydroxyl-, epoxy-, keto- and oxo-FAs, epoxy alcohols, divinyl-ethers, volatile alcohols or aldehydes, and jasmonic acid (JA) and its corresponding derivatives (37). All these compounds are synthesized by non-enzymatic or enzymatic processes. In the higher plants, linoleic acid 18:2 and alpha-linolenic acid 18:3 represent the most abundant PUFAs. Indeed, PUFAs easily react with O2 generating hydroperoxides into membrane-bound lipids, in turn affecting membrane fluidity (38). The oxidative burst, a rapid and transient production of ROS, is one of the earliest observable aspects of a plant’s defence strategy. The term ROS is used to describe the products of the sequential reduction of molecular oxygen as shown in the scheme:
The superoxide anion (•O2-), hydrogen peroxide (H2O2) and hydroxyl radical (•OH) are the species predominantly detected in plant-pathogen interaction (39). ROS may oxidize PUFAs within the plasma membrane, and biologically active oxylipins are formed (37-40). To limit these oxidation reactions, thus reducing potentially damaging effects, cells have evolved a wide battery of antioxidant defence systems (41). The superoxide radical (•O2-) can behave either as a reducing agent (often reducing Fe3+ to Fe2+) or as a weak oxidizing agent as in its interaction with ascorbic acid. Superoxide radicals (•O2-) are reduced to hydrogen peroxide (H2O2) through the action of superoxide dismutases and then reduced, by a group of heme-containing proteins, the catalases, to water and oxygen. Hydrogen peroxide (H2O2) may be also reduced to H2O by the combined action of both superoxide dismutase and ascorbate peroxidase (42). Hydrogen peroxide (H2O2) is a weak oxidizing agent but in the presence of cellular Fe2+ or other transition metals, may form the hydroxyl radical (•OH), which, lacking charge, may pass through cell membranes, reacting and oxidizing lipids inter alia. The decomposition of hydrogen peroxide by Fe2+ and some Fe3+ complexes is referred to as Fenton reaction (H2O2+ Fe2+ + H+ → H2O + Fe3+ + ˙OH). All these compounds alter the oxidant/antioxidant balance that plays a critical role in lipid peroxidation. Autoxidation is the direct reaction of molecular oxygen with organic compounds; the lipid oxidation involves a free radical mechanism consisting of initiation, propagation and termination steps, frequently overlapping (43). The relative rates of oxidation of C18-series reveal that the higher the number of double bonds C=C the higher the reactivity, that is, alpha-linolenic acid 18:3 is more unstable of linoleic acid 18:2, which in turn is more unstable of oleic acid 18:1 (44). In plants, the formation of non-enzymatic oxylipins occurs in response to pathogen attack; esterified hydroxyl-fatty acids increase significantly in the esterified lipid fraction. Regarding this, esterified hydroxyl-fatty acids achieve levels up to thirty times more abundant than the free oxylipins suggesting that they are generated predominantly in membranes (40).
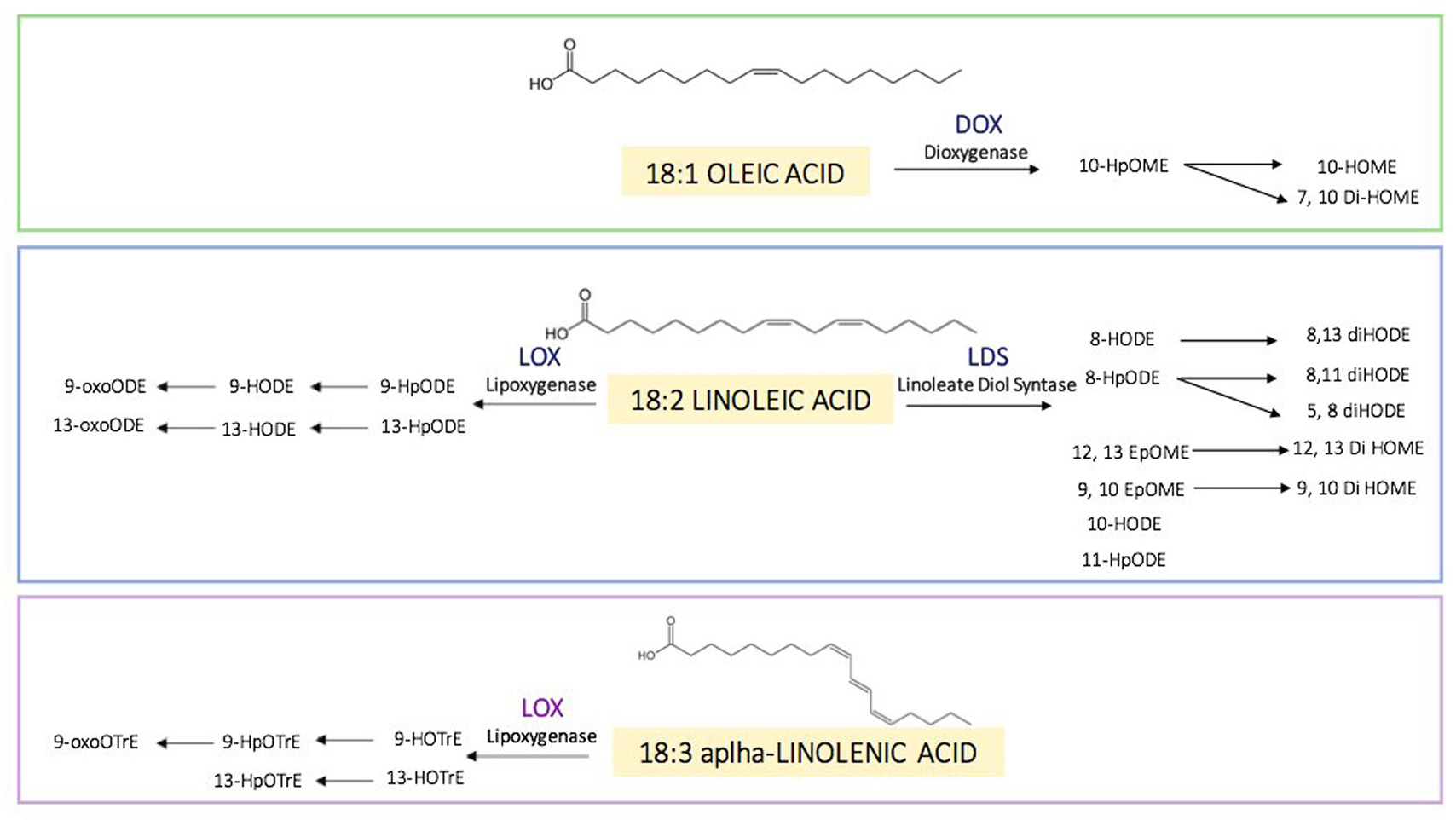
In fungi, the enzymatic routes of oxylipins act primarily on oleic acid 18:1, linoleic acid 18:2 and alpha-linolenic acid 18:3 (Figure 3). The biosynthetic oxylipin pathway usually begins with an initial reaction of peroxidation of these fatty acids that is catalysed by lipoxygenase enzymes (LOXs) (45). The hydroperoxy-fatty acid can be converted to different products by enzymes belonging to the cytochrome P450 enzyme family (46). Some authors suggest that LOXs utilize non-esterified fatty acids but may also act on the esterified acyl chains in triacylglycerol or phospholipid fractions (45). Fungal oxylipin formation privileges the enzymatic route that includes the linoleate diol synthase complex, even if lipoxygenases are active too (47). The Linoleate Diol Synthase (LDS), which catalyses the enzymatic conversion of linoleic acid into dihydroxy-linoleate was the first oxylipin biosynthetic enzyme characterized in the fungus Gaeumannomyces graminis (48). Based on sequence homology studies, LDS of G. graminis aids discovering three oxylipin biosynthetic genes named ppo: psi (precocious sexual inducer)-producing oxygenases in Aspergillus nidulans (49) and in Aspergillus fumigatus (50). PpoA, PpoB and PpoC regulate reproduction, dispersal of spores and mycotoxins production (51-52-53-54). Three different LDS isoforms were identified in Fusarium verticillioides too (55); the deletion of the one of these LDS generates a mutant that produces more mycotoxins, more conidia and resulted more virulent to maize ears (56).
 Figure 3
Figure 3Oxylipins found in fungi and their enzymatic routes. Principal substrates of oxylipins (18:1 oleic, 18:2 linoleic and 18:3 alpha-linolenic acid). FAs can be converted in several oxylipins through the catalytic action of several enzymes: dioxygenases (DOXs), lipoxygenases (LOXs) and linoleate diol synthases (LDSs). Oxylipins formed can be further reduced and oxidised both enzymatic and non-enzymatic action.
The structural similarity of plant and fungal oxylipins prompts the hypothesis that they are important in cross-kingdom communication (57). The oxylipins are molecules capable of signalling; they can drive signals between different organisms, and some study demonstrate that the oxylipins can be sensed by mean of G-protein coupled receptors (58).
In fungi, the oxylipins modulate sexual and asexual sporulation, coordinate the quorum sensing and regulate the density-dependent sporulation (59). The density can determine the colonization of the host and the consequent production of secondary metabolites. In the human pathogenic yeast, Candida albicans, the oxidised farnesol produced from farnesyl pyrophosphate, an intermediate in the ergosterol biosynthesis pathway regulates the quorum sensing and the biofilm formation (60). Even within prokaryotes, lipid molecules (e.g. diffusible signalling factors, DSF) and their oxidized counterpart (e.g. 7,10-diHOME) regulate some crucial event of the life cycle such as virulence and biofilm formation (61).
Since the oxylipins are present and recognized by both the fungus and the plant, they can have multiple effects on the host-pathogen interaction (62). LOX enzymes can produce several oxylipins derived from linoleic acid. Notably, 9-LOX produce the oxylipins 9-HODE and 9-HpODE, which in plant can induce programmed cell death in tomato protoplasts (63), while A. nidulans, responding to these plant 9-oxylipins, promotes cAMP production via G-protein coupled receptors (GPCRs). This signalling pathway has effect on fungal germination, sporulation, and secondary metabolism including mycotoxin synthesis (64). 13-LOXs, acting on linoleic acid, produce the oxylipins 13-HODE and 13-HpODE; in the plant, e.g. during cucumber germination, these oxylipins may be found among the products of lipid bodies mobilization (65), whereas the same oxylipins in Aspergillus suppress mycotoxin production (66-67). A 13-LOX enzyme modifies alpha-linolenic acid 18:3 yielding 13-HpOTrE precursor of jasmonates. The plants produce many derivatives of JA including the methyl jasmonate (MeJA). MeJA is involved during global plant stress, defence and developmental process (68-69; it induces the synthesis of secondary metabolites in the fungus (70).
Thus, oxylipins are central to several host-pathogen interactions. Christensen & Kolomiets in 2011, proposed a hypothetical model of oxylipin-mediated signal communication among plants and fungi. They suggest that the: “fungal lipases could be secreted into plant cells where fatty acid substrates are cleaved and processed by fungal secreted lipoxygenases and/or plant lipoxygenases for oxylipin production. Plant-produced oxylipins are perceived and exploited by fungi to regulate GPCR-, PkaA-, and Ppo-mediated growth, sporogenesis, and mycotoxin production. Host manipulation through oxylipin mimicry (i.e. fungal oxylipins binding to host GPCRs and secretion of JA-Ile-like coronatine into host cells) is also implicated”.
The model is still valid and the missing dots are less and less.
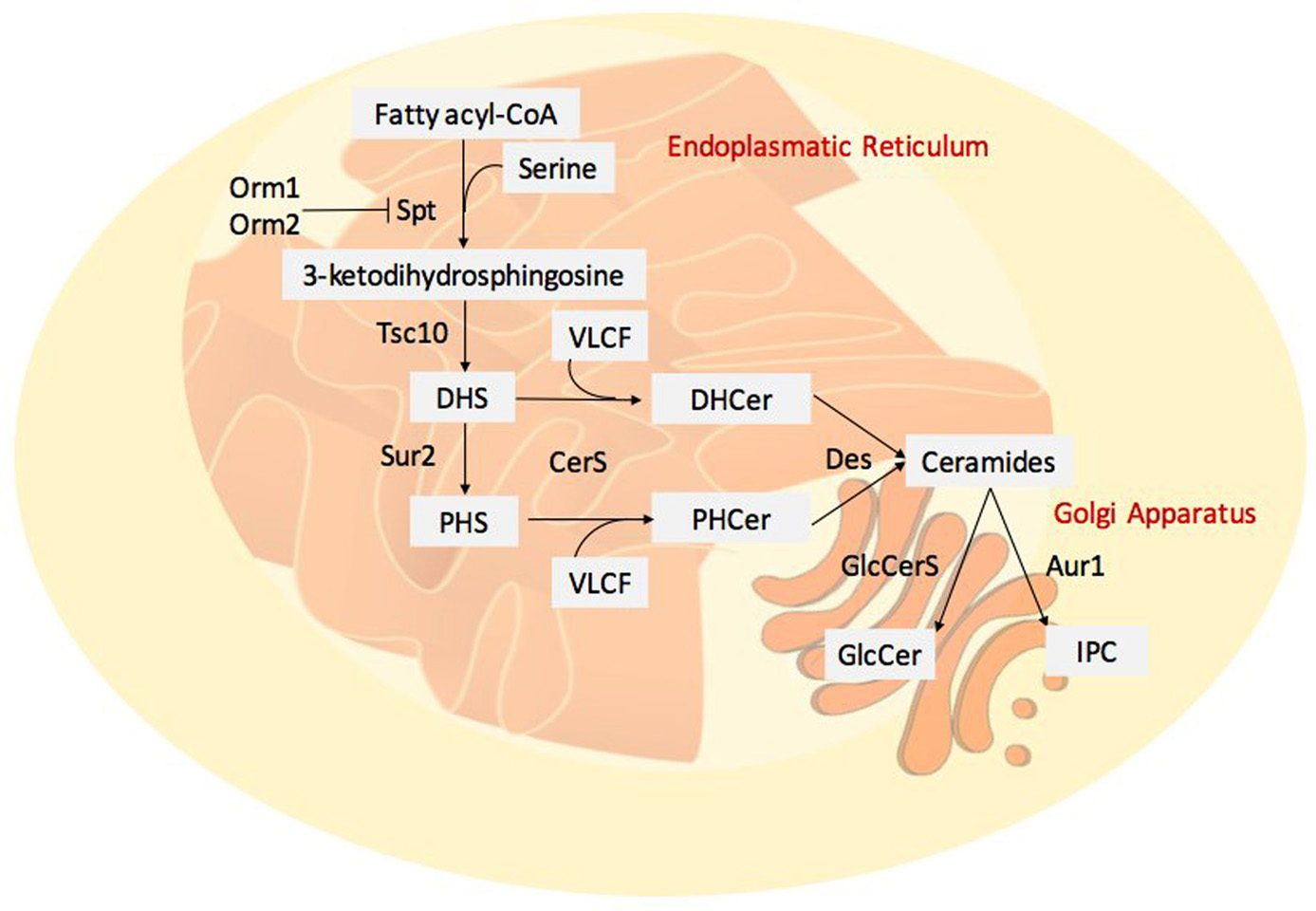
A class of lipids gaining new prominence in both mammalian, plant and fungal research is the sphingolipid family; sphingolipids are components of cell structure and may control cell metabolism (18). In mammals, these class of lipids are involved in several processes such as cell proliferation, stress response and apoptosis (71). Although the role of sphingolipids is better defined in animal systems, they are fundamental in many essential processes in plants including the pollen development, and the response to biotic and abiotic stress (72). In recent years, the role of sphingolipids in pathogenic fungi deserved particular attention. The main themes of interest related to the involvement of sphingolipids in signalling, growth and virulence in this class of pathogens. Sphingolipids play an important role in the regulation of virulence in a variety of fungi (73-74). Sphingolipid synthesis in pathogenic fungi is largely conserved among species, ER is the site of sphingolipid biosynthesis (Figure 4), and then they are transported to other organelles or directed to the plasma membrane. In general, sphingolipid structure consists in a long-chain base (LCB), or sphingoid base, linked to a fatty acid by an amide bond; this structure is defined as ceramide (Cer). The enzyme catalysing the amide bond is the Ceramide Synthase (CerS); in mammalian, six isoforms of CerS exist that can combine different fatty acyl chain length substrate (75). The grade of hydroxylation of FAs and LCBs represents another factor of variability in sphingolipids (76-77). Complex sphingolipids are the glucosylceramides (GlcCer) and phosphoinositol ceramides (IPC), produced by their respective synthase (GlcCerS and IPCS).
 Figure 4
Figure 4Fungal sphingolipid synthesis. De novo synthesis begins with the condensation of a serine with fatty acyl-CoA, it is catalysed by serine palmitoyl-transferase (SPT) complex. Orm1 and Orm2 are able to bind SPT complex and inhibit its activity. 3-ketodihydrosphingosine is formed and the reductase Tsc10 catalyses the formation of dihydrosphingosine (DHS), it can be hydroxylated by a Sphingolipid C4-hydroxylase (Sur2) to generate the phytosphingosine (PHS). The association between the long chain bases (DHS or PHS) and VLCFA is catalysed by the Ceramide Synthase Complex (CerS) leading to dihydroceramide (DHCer) and phytoceramide (PHCer). The desaturases (Des) form the ceramides. Complex ceramides can present several head groups, glucosylceramides (GlcCer) and phosphoinositol ceramides (IPC) are one example.
In the model organism Saccharomyces cerevisiae, de novo synthesis of sphingolipids begins with the condensation of a serine with a fatty acyl-CoA, normally palmitoyl-CoA or stearyl-CoA, catalysed by the serine palmitoyl-transferase (SPT) complex (78-79). Two ER’s proteins Orm1 and Orm2 are able to bind the SPT complex and inhibit its activity; this is an important step in sphingolipid biosynthesis regulation (80). The last reaction generates 3-ketodihydrosphingosine and the reductase Tsc10 catalyses the formation of dihydrosphingosine (DHS) (81). To generate the phytosphingosine (PHS), the Sphingolipid C4-hydroxylase (Sur2) can hydroxylate DHS (82). Ceramide Synthase Complex catalyses the association between the LCB (DHS or PHS) and VLCFA formed of Lag1, Lac1 and Lip1 (83-84). This complex forms dihydroceramides (DHCer) and phytoceramides (PHCer); these compounds can be further desaturated (by Des) to form the ceramides. Complex yeast ceramides present the addition of head groups; for example, the IPC synthase (Aur1) catalyses the transfer of the phosphoinositol to ceramides (85).
The catabolism of sphingolipids can be regulated by the phospholipase C (Isc1) that hydrolyses complex sphingolipids to produce ceramide (DHCer and PHCer); the alkaline dihydroceramidase Ydc1 and its paralog Ypc1 hydrolyse dihydroceramide to FFA and DHS, or PHS (86). Then, sphingoid long-chain base kinases (Lcb4 and Lcb5) can phosphorylate DHS and PHS to produce respectively DHS-P and PHS-P, with function as signalling molecules (87).
Pathogenic fungi cause important diseases in plants and humans. Sphingolipids and their metabolites, apart from their role as essential membrane components, are involved in a variety of cellular processes: growth, cell cycle arrest and apoptosis. Sphingolipids play an important role in controlling the host-pathogen interaction. Pathogens can produce sphingolipid species that are not present in the host (88) as it is for some fungal GlcCers that present a C-9 methyl group; they are a marker of pathogen infection. In fact, the plant defensins, antimicrobial proteins triggered in the innate immunity by these peculiar sphingolipids, kill the pathogen, although the mechanism of fungal cell death is not clear (73). Another study shows that spraying rice leaves with GclCers might function as elicitor and inducer of the pathogenesis-related (PR) protein synthesis (89).
Several fungal pathogens may produce mycotoxins interfering with the sphingolipid metabolism of the host, as reported below.
Some plant necrotrophic pathogens can control the host CerS through mycotoxins. Alternaria alternata f. sp. lycopersici, tomato’s pathogen, produce the AAL toxin (90). This toxin is structurally similar to sphingosine and act by inhibiting the CerS of the host; this leads accumulating LCB into tomato cell sap (90). The accumulation of LCBs triggers programmed cell death (PCD) in plant (91). Similarly, to AAL toxin, fumonisins (FBs) toxins produced by the Fusarium genus, in particular by F. verticillioides and F. proliferatum can inhibit the CerS activity in mammals and plants inducing cell death (92-93). AAL and FB, are therefore able inducing PCD by inhibiting the CerS; this mechanism can be seen as a virulence strategy of necrotrophic pathogens suggesting a close link between sphingolipid metabolism and plant PCD.
Moreover, sphingolipids are important molecules being as main components of the lipid rafts (LRs). In the eukaryotic plasma membranes, these structures are rich in sterols and sphingolipids; in the mammal, the main sterol is the cholesterol. In fungal LRs, the main components are the glycol-sphingolipids and ergosterol; these membrane subdomains have a significant role in the secretory pathway. In the yeast, functional LRs are composed by glycosphingolipids and ergosterol (94). LRs play important roles in the membrane dynamic processes, including protein sorting, cell polarity, and signal transduction. In the budding yeast S. cerevisiae, LRs are detected with filipin staining on the hyphal tips of the cells induced with mating pheromone (95-96). Several works show that LRs are transiently present at sites of polarized morphogenesis in the fungal pathogen Cryptococcus neoformans and Aspergillus nidulans (97). Sphingolipids, particularly the ceramides, can stabilize the LR formation (98); this may have important physiological consequences. The ability of ceramides to participate in LR formation, stability, organization is important in cell signalling, as proposed in several studies (99-100). In general, LRs are characterized by a reduced lateral mobility, a change of the membrane thickness and, in particular, a composition different from other parts of the membrane. The latter feature seems of particular importance in allowing these domains to function in sorting proteins. Several studies in the last years indicate that a change in the composition of rafts and membrane domains results in the re-organization of membrane proteins (101); these domains might be the preferential interaction sites between a variety of toxins, bacteria and the target cell (102).
The LRs in pathogenic fungi may play a role in pathogenesis by mediating the presentation of virulence factors and by influencing the biophysical properties of the plasma membrane.
Glycosylphosphatidylinositol (GPI)-anchored proteins function as virulence factors in C. albicans during the (pathogenic) hyphal growth. These factors belong to the adhesin protein family, which mediate adhesion to the host cells and biofilm formation; alternatively, they may be members of the secreted aspartyl protease family, which are needed for fungal virulence (103-104-105). Similarly, it has been suggested that special membrane domains in C. neoformans regulate membrane localization and release of the virulence factors phospholipase B1 and superoxide dismutase (106).
These evidence show that the LRs ceramide-enriched can be defined as focal areas during the infection of fungal pathogens.
The puzzle composing lipid-mediated signalling in fungi is still incomplete. Nevertheless, the area of lipid-mediated signal communication is new and rapidly growing with widespread implications ranging from quorum sensing in bacteria to microbe niche interactions, insect–plant communication and mammalian disease defences.
FAs can act as modulators of several signal transduction pathways. They can be oxidized and form oxylipins able mediating the cross-talk between a host and a pathogen or being incorporated into sphingolipids. These latter are crucial in cell signalling, membrane shaping and lipid rafts formation allowing cells to perceive stimuli and trigger responses. Thus, lipids play a role that goes further beyond being mere structural components of membranes. Their chameleonic nature made these molecules fabulous drivers of information exchange. In relation to this, lipids act as shapers of the host-pathogen interaction, allowing to each contenders to develop fine-tuned strategies of defence or virulence.
Mass spectrometry analysis has been carried out by using an Agilent 6420 QQQ acquired through funding for medium devices provided by Sapienza, grant number C26G14XYEX.