1 Laboratory of Biodiversity of Microorganisms, Institute of Microbiology, Academy of Sciences of the Republic of Uzbekistan, 100128 Tashkent, Uzbekistan
2 Department of Biochemistry, National University of Uzbekistan, 100174 Tashkent, Uzbekistan
Abstract
Pesticides spread into the air, contaminate soil and water, and can affect various objects, contributing to secondary pollution regardless of the employed type or application method. Currently, organophosphorus pesticides (OPs) are widely utilized in agriculture, forestry, and livestock farming worldwide. These chemicals enter the body through multiple exposure routes and can harm the nervous system, endocrine system, and other organs. Owing to the environmental persistence and elevated toxicity exhibited by these pesticides, certain OPs are difficult to break down biologically, thus posing serious threats to human health and ecosystems. Disinfection or destruction of those pesticides remaining in the environment represents one of the important tasks scientists face. This review presents information on OPs, some of their properties, environmental impacts, and mechanisms for the effective decomposition of these pesticide residues by microorganisms. Bacteria and fungi isolated from samples contaminated with various OPs were analyzed. New metabolites formed during OP degradation by these microorganisms, as well as microbial enzymes involved in OP degradation and the molecular mechanisms of the process, are presented. The methods used in these studies and recommendations for future research are also detailed.
Graphical Abstract
Keywords
- organophosphate pesticides
- biodegradation
- bacteria
- fungi
- enzymes
- molecular mechanisms
Pesticides (pestis - =infection, caedo - =kill) are chemical substances applied to prevent, destroy, or reduce damage from various pests [1]. Pests can be bacteria, fungi, some insects, shellfish, birds, mammals, nematodes (roundworms), and other harmful organisms that spread or help transmit diseases [2]. Meanwhile, pesticides have been used in modern times and in ancient times. Indeed, the history of pesticide use dates back to ancient civilizations, with some of the earliest known applications occurring in Ancient Egypt [3]. While the exact origins and the first individuals to use pesticides are unknown, this practice likely dates back several centuries, with farmers seeking to protect their crops from pests believed to have been the first to use such substances [4]. Ancient people often relied on botanical solutions to control insect pests, with Dalmatian pyrethrum being one of the most notable examples. This flower contains up to 1.5% pyrethrin, a compound first used as an insecticide in Ancient China and later in Persia during the Middle Ages [4, 5]. Notably, pesticide usage became more widespread in the mid-19th century, with Paris green being successfully used in 1871 to tackle the Colorado potato beetle [6]. Moreover, Paris green was also widely employed across the globe until the mid-20th century to control malaria-carrying Anopheles mosquitoes [7]. The World Health Organization (WHO) categorizes pesticides into four groups based on their toxicity to humans and warm-blooded animals [8]: extremely toxic, highly toxic, moderately toxic, and slightly toxic [9]. Moreover, pesticides are divided into different groups. Currently, pesticides are classified based on the chemical structure, mode of action, and use or target pests [9]. Fig. 1 details the pesticide classifications.
 Fig. 1.
Fig. 1. Classification of pesticides.
Organochlorine pesticides: These pesticides are specifically designed to kill certain living organisms. OPs, unlike many other chemical pollutants, are deliberately introduced into the environment. These substances are specifically designed to be toxic, interfering with the nervous system of pests, resulting in death [10]. Organochlorines are categorized as persistent organic pollutants (POPs) and are characterized by the presence of chlorine, polar functional groups, and a cyclic structure, which may be aromatic. These pesticides are positively stable in the environment and are difficult to decompose in soil. The representatives of this class of insecticides include dichlorodiphenyltrichloroethane (DDT), dichlorodiphenyldichloroethane (DDE), lindane, aldrin, and dieldrin [11]. These insecticides effectively combat mosquitoes, fleas, other blood-sucking organisms, locusts, cotton, and forest pests. However, due to their persistence and toxicity, POPs are banned or limited in most countries around the world [12].
Organophosphate pesticides: These pesticides have been extensively used globally since the 1960s [13]. Compared to organochlorine pesticides, organophosphate pesticides exhibit less resistance to environmental influences [14]. Moreover, organophosphate pesticides contain a central phosphate molecular group. In the case of organophosphorus esters, these pesticides have three ester bonds with alkyl or aromatic substituents [15, 16]. Organophosphorus pesticides (OPs) disrupt the activity of acetylcholinesterase, an enzyme vital for the proper functioning of the nervous system in insects, animals, and humans [11]. These groups of insecticides are used to protect agricultural plants from pests. However, not all of these pesticides reach the pests. Indeed, when used, these pesticides also pollute the environment, causing loss of soil fertility and biodiversity [17]. Chlorpyrifos, parathion, dichlorophos, dimethoate, fenthion, malathion, methyl parathion, and diazinon represent organophosphorus pesticides [18].
Carbamates: These are a class of pesticides that derive from carbamic acid; their mechanism of action is similar to organophosphorus pesticides, whereby carbamates affect the transmission of nerve impulses through nerve cells, causing poisoning and death [19]. Carbamate-based pesticides are commonly used as insect, fungal, and weed control agents. Examples of carbamate insecticides include carbofuran, carbosulfan, furatiocarb, carbaryl, and pyromicarb [20]. These pesticides are typically grouped into N-methylcarbamate insecticides and N-allylcarbamate herbicides based on their chemical structure and biological effects [20, 21]. Due to their mechanism of action and stability, carbamates are closely related to organophosphates and act as acetylcholinesterase inhibitors, causing very similar symptoms [20].
Synthetic pyrethroids are frequently applied as insecticides in agricultural practices [22]. These insecticides are derived from natural pyrethrins in dried chrysanthemum flowers [23]. Furthermore, pyrethroids are very toxic to bedbugs and cockroaches. Pyrethroids are divided into two groups: Type I includes allethrin, permethrin, tefluthrin, tetramethrin, and possesses no cyano group; Type II pyrethroids include cypermethrin, deltamethrin, cyfluthrin, fenvalerate, and tralomethrin, which contain a cyano group, and can cause choreoathetosis and salivation [23].
Biological pesticides (biopesticides) consist of naturally occurring organisms or substances that inhibit the growth and reproduction of pest populations through various mechanisms of action [24, 25, 26, 27]. Biopesticides include natural substances or substances produced by organisms, including microorganisms, plants, etc. [28, 29, 30]. Biopesticides are cost-effective, environmentally friendly, promote a specific effect, and leave no trace residues. Although biopesticides have been used since the late 19th century, this class of pesticide currently comprises a very small part of the global pesticide market. However, production volumes have increased significantly recently [31, 32, 33], with international organizations and institutions increasingly using these pesticides. Therefore, the biopesticide market has recently grown by 10–15% annually [34].
In 1850, Moshenin synthesized tetraethyl pyrophosphate (TEPP), marking the recognition of organophosphorus pesticides. Shortly after, Long and Roger Vaughan conducted the first organophosphorus compound synthesis and identified the presence of the P–F bond [35].
OPs were first synthesized and used as chemical weapons during World War II before being repurposed as pesticides. The widespread use of these pesticides in agriculture and everyday life has resulted in significant environmental contamination and animal toxicity [36]. OPs function by inhibiting or entirely blocking acetylcholinesterase (AChE) activity, an enzyme essential for the nervous system in insects, animals, and humans to function properly [37]. OPs are some of the most widely used pesticides globally, representing 38% of the total pesticide consumption worldwide [37, 38]. These pesticides are used primarily as insecticides and can enter the body through the respiratory tract, skin, or oral ingestion, with ingestion being the most frequent route [39]. Once inside the body, OPs are metabolized into highly toxic compounds known as oxons via the activation of cytochrome P450 [40], which involves oxidative desulfurization—removing sulfur from the phosphorus and adding an oxygen atom. Gross [41] first reported the effectiveness of organophosphates as AChE inactivators in 1952. OPs inhibit the activity of AChE by phosphorylating the hydroxyl group of the serine in the active site, disrupting the normal function of breaking down the neurotransmitter acetylcholine (ACh). Subsequently, ACh accumulates at nerve synapses [42], leading to overstimulation of muscarinic (mAChR) and nicotinic (nAChR) receptors, and promoting uncontrolled transmission of nerve impulses, leading to the death of the insects [39, 42]. Notably, the toxic effects of OPs impact not only pests but also any living organism that possesses cholinergic systems, making them potentially susceptible to these effects [43, 44, 45]. Humans have a neural cholinergic system, meaning exposure to these substances, either accidentally or through occupational contact, can lead to acute and chronic effects. Acute effects typically appear within minutes or hours of exposure to OPs, promoting symptoms such as headaches, muscle weakness, diarrhea, excessive salivation, etc. [46]. In contrast, chronic effects are linked to long-term consequences that are challenging to attribute exclusively to pesticide exposure.
OP compounds are divided into two primary categories [36]. Organophosphate pesticides are degradable organic compounds derived from phosphoric or related acids. These pesticides usually exist as esters, amides, or thiols, and are typically connected to two organic groups with a side chain of cyanide, thiocyanate, or phenoxy groups [47]. Table 1 (Ref. [37, 38, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89]) lists the OPs and some of their characteristics.
| № | OP | Chemical formula | Chemical structure | Target organisms | The WHO (1990) guideline hazard classification | Half-life in soil (days) | References |
| 1 | Chlorpyrifos | C9H11Cl3NO3PS |  | Insecticide, nematicide, acaricide | II | 60–120 | [37, 47, 48] |
| 2 | Dichlorvos | C4H7Cl2O4P |  | Insecticide, acaracide | II | 16 | [49] |
| 3 | Dicrotophos | C8H16NO5P |  | Insecticide | Ib | 45–60 | [38, 47] |
| 4 | Diazinon | C12H21N2O3PS |  | Insecticide, acaricide | II | 11–21 | [50, 51] |
| 5 | Coumaphos | C14H16ClO5PS |  | Acaricide | Ia | 52 | [38, 47] |
| 6 | Ethoprophos | C8H19O2PS2 |  | Insecticide, nematicide | Ia | 30 | [52] |
| 7 | Methyl-parathion | (CH3O)2P(S)OC6H4NO2 |  | Insecticide | Ia | 50–130 | [53, 54] |
| 8 | Fenitrothion | C9H12NO5PS |  | Insecticide | II | 19–28 | [55, 56] |
| 9 | Dimethoate | C5H12NO3PS2 |  | Insecticide, acaricide | II | 200 | [57, 58, 59] |
| 10 | Ethion | C9H22O4P2S4 |  | Insecticide, acaricide, ovicide | II | 41–70 | [60] |
| 11 | Fenthion | C10H15O3PS2 |  | Insecticide | II | 40 | [61, 62, 63] |
| 12 | Monocrotophos | C7H14NO5P |  | Insecticide, acaricide | Ib | 42–70 | [64, 65, 66, 67] |
| 13 | Profenofos | C11H15BrClO3PS |  | Insecticide | II | 17 | [68, 69] |
| 14 | Triazofos | C12H16N3O3PS |  | Insecticide | Ib | 44 | [70, 71] |
| 15 | Phorate | C7H17O2PS3 |  | Insecticide | II | 2–15 | [72] |
| 16 | Acephate | C4H10NO3PS |  | Insecticide | II | 14–23 | [73, 74] |
| 17 | Glyphosate | C3H8NO5P |  | Herbicide | 50–170 | [75, 76] | |
| 18 | Quinalphos | C12H15N2O3PS |  | Insecticide, acaricide | II | 1.07–1.2 | [77] |
| 19 | Cadusafos | C10H23O2PS2 |  | Nematicide | Ib | 21 | [78, 79] |
| 20 | Fenamiphos | C13H22NO3PS |  | Nematicide, insecticide | Ib | 30–90 | [80, 81] |
| 21 | Malathion | C10H19O6PS2 |  | Insecticide | III | 3–17 | [82, 83, 84] |
| 22 | Terbufos | C9H21O2PS3 |  | Insecticide, nematicide | Ia | 14–30 | [85, 86] |
| 23 | Tetrachlor vinphos | C10H9Cl4O4P |  | Insecticide | 4 | [87, 88, 89] |
OP, organophosphorus pesticide.
AChE activity is an important biological indicator of organophosphate exposure [90]. Organophosphates can bind to cholinesterase enzymes, inhibiting their ability to break down acetylcholine, leading to acetylcholine accumulation and the onset of neurological symptoms [91]. While pesticides are primarily intended for target organisms, pesticides can also disperse into the environment, causing harm to beneficial species. Furthermore, these chemicals can react with nitrogen oxides in the air to create ozone, harming air quality. Moreover, pesticides are commonly found in both ground and surface waters, as well as in drinking water sources [92]. Pesticides that persist in the soil are not easily broken down by most microorganisms, reducing their decomposition and disrupting microbial activity. Toxicological research has demonstrated that these pesticides pose ongoing risks to the nervous, endocrine, and reproductive systems, and continue to threaten non-target organisms after accumulating over time [93]. Pesticides, such as glyphosate and chlorphoxime, cause oxidative stress and mitochondrial damage in nerve cells, resulting in DNA damage [94]. Meanwhile, OPs have been linked to Parkinson’s disease and autism, and the development of human neurological diseases represents the primary concern regarding contact with glyphosate and glyphosate-based herbicides (GBHs) [94]. Organophosphate insecticides accumulate in aquatic organisms and pollute the environment. Studies have identified that dimethoate and chlorpyrifos insecticides accumulate in juvenile Cyprinus carpio fish muscles. The contamination of these fish by insecticides and the subsequent retention of these chemicals in muscle tissue could pose a risk to human health. Prior research has shown that the accumulation of insecticides in muscles increases in parallel with the dosage [93, 94, 95]. These findings suggest that consuming contaminated fish could pose a potential risk. Additionally, the study emphasized the need for effective legislation to manage insecticides and protect aquatic ecosystems [95]. Meanwhile, air quality is deteriorating due to pesticide pollution, with drift being the main route of air pollution from pesticides. Spray drift for pesticides can occur through ground spraying or aerial application. However, regardless of the application methods, wind speed, temperature, and other factors may affect the release of pesticides into the air [96]. The presence of OPs in any form in the atmosphere raises the likelihood of non-occupational exposure to these pollutants, especially through inhalation [96]. Fig. 2 shows the use of OPs, their distribution in the environment, accumulation of residues, and impact on the biological cycle.
 Fig. 2.
Fig. 2. Use of OPs and the environmental impacts.
Environmental pesticide residue degradation is classified into biological and non-biological processes [92]. Non-biological degradation methods include chemical processes such as combustion [97], hydrolysis [98], oxidation [99], ultrasonic treatment [100], and photochemical degradation. These chemical degradation processes involve intense chemical reactions, which can have detrimental effects on the environment and lead to secondary pollution. For instance, combustion is unsuitable for pesticide decomposition due to the significant environmental pollution this process causes. Similarly, the hydrolysis of organophosphate pesticides results in the production of harmful compounds [92].
Biodegradation is mainly based on microorganisms and their biochemical potential. Microbiological pesticide degradation in the environment is a key pathway for eliminating these substances. This biodegradation process is usually complex and entails several biochemical reactions. Biodegradation has emerged as a viable alternative to conventional methods, offering an effective, cost-efficient, and environmentally friendly solution. Current knowledge of biodegradation processes means these can be utilized to enhance the bioremediation of pesticide-contaminated sites [101]. Our review includes information on the effective degradation of OPs by microorganisms, e.g., bacteria and fungi.
Research on microbial pesticide residue degradation began in the 1940s, and as environmental concerns increased, interest in the processes and mechanisms of organic pollutant degradation grew [102]. Local microorganisms primarily break down pesticides in the environment (such as in soil and water), a process known as biodegradation. This process involves the breakdown of the original substance into smaller, inert, and final products [103]. The rate at which decomposition occurs varies depending on the microorganism involved and environmental conditions, such as temperature, pH, light exposure, and moisture. In natural environments, most organophosphate pesticides are broken down by microbes, which use them as essential nutrients, primarily as sources of carbon or phosphorus that can limit their growth [104]. The primary reactions involved in the degradation process include hydrolysis, oxidative alkylation, and dealkylation. Microbial biodegradation of organophosphate pesticides mainly occurs through hydrolysis, which cleaves P–O bonds in alkyl and aryl groups, facilitated by enzymes such as alkaline phosphatase, phosphotriesterase [105, 106], and various hydrolases [107, 108]. Typically, microorganisms degrade organophosphate pesticides through the enzymatic hydrolysis of P–O–alkyl and aryl linkages, a process driven by enzymes, including App-phosphatase, phosphotriesterase, and hydrolase [108]. Microorganisms such as bacteria and fungi are crucial in the degradation of pesticides [37]. Table 2 (Ref. [57, 70, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128]) provides information on microorganisms capable of degrading OPs.
| № | Microbial strains | Pesticide | Detection of metabolites | Comments | References |
| 1 | Bacillus thuringiensis MB497 | Chlorpyrifos | 3,5,6-trichloro-2-pyridinol, diethylthiophosphate (DETP), and phosphorothioic acid | The bacterium was isolated from wheat/cotton fields in Pakistan, where OPs were widely used. The strain showed almost 99% degradation of CP (200 mg/L) added to the soil suspension in M-9 broth within 9 days. | [109] |
| 2 | Enterobacter strain: B-14 | Chlorpyrifos | DETP and TCP | Strain B-14 was isolated from soil and analyzed using various methods. The B-14 strain used the pesticide as the sole carbon and phosphorus source. | [112] |
| 3 | Starkeya novella YW6 | Monocrotophos | N-methylacetoacetamide and dimethylphosphate | Bacterial strain YW6 uses monocrotophos (MCP) as the sole carbon and nitrogen source for growth. The YW6 strain efficiently degraded 0.2 mM MCP within 36 hours. | [113] |
| 4 | Raoultella sp. X1 | Dimethoate | No data | The bacterium was identified using molecular methods. This strain showed the ability to degrade dimethoate through co-metabolism efficiently. | [114] |
| 5 | Pseudomonas putida | Malathion | Malaoxon, malathion monocarboxylic acid, and 2-mercaptosuccinic acid | The bacterial strain can degrade malathion (125 mg/L) by up to 72%. | [115] |
| 6 | Micrococcus sp. MAGK3 | Malathion | Malathion monocarboxylic acid, malathion dicarboxylic acid, dimethyl dithiophosphate, dimethyl thiophosphate, dimethyl phosphate, and thiophosphate | The strain was obtained from the soil of Pennisetum glaucum. The analysis showed that different pesticide concentrations were effectively degraded in a liquid medium. | [116] |
| 7 | Pseudomonas stutzeri smk | Dichlorvos | 2-chlorovinyl dimethyl phosphate, vinyl dimethyl phosphate, dimethyl phosphate, methylphosphate, and phosphate | This strain can decompose up to 80% of the pesticide within 7 days at a temperature of 30 °C. Intermediate metabolites are detected using various methods. | [117] |
| 8 | Ochrobactrum anthropi GPK | Glyphosate | Aminomethylphosphonic acid, glyoxylate, phosphonoformaldehyde, and formaldehyde | A glyphosate-degrading strain isolated from soil contaminated with glyphosate. The strain was characterized using the BLAST analysis of the 16S rRNA gene. | [118] |
| 9 | Bacillus cereus CB4 | Glyphosate | Aminomethylphosphonic acid, glyoxylate, sarcosine, glycine, and formaldehyde | Bacterial strain CB4 was isolated from soil. Determination of degradation was performed using HPLC. CB4 can decompose glyphosate by 94.47% under the following optimal conditions. The inoculum is introduced at 5% of the total volume, and the incubation period is set for 5 days. The microbial strain used can degrade glyphosate concentrations up to 12 g/L-1. | [119] |
| 10 | Four strains of Bacillus subtilis were used in the study | Profenofos | 4-bromo-2-chlorophenol | Four strains of Bacillus subtilis were isolated from the rhizosphere of grapes and studied based on the biodegradation of the insecticide profenofos. Each of the four strains enhanced the degradation of the pesticide. Among them, strain DR-39 proved to be the most efficient. | [120] |
| 11 | Four strains of Pseudomonas bacteria were used in the study | Acephate | Methamidophos, S-methyl O-hydrogen phosphorothioamidate, phosphenothioic S-acid, and phosphenamide | The ACP1, ACP2, and ACP3 strains were obtained from soils contaminated with acephate. The rate of pesticide degradation under the influence of humic acid and ions of the most common metals, Fe(III) and copper Cu(II), has been studied. During the study, 100 ppm of acephate was incubated with individual strains. This study revealed the mechanism of acephate degradation under different conditions. | [121] |
| 12 | Pseudomonas plecoglossicida strain: PF1 | Profenofos | 4-bromo-2-chlorophenol and 1,1-dimethylethylphenol | The study aimed to investigate the biodegradation and detoxification of profenofos under various conditions using the PF1 strain. The focus was on oxygen-free biodegradation in the presence of nitrates. Profenofos at concentrations from 10 to 150 mg/L decomposes with removal efficiencies of 38–55% under aerobic conditions and 27–45% under anaerobic conditions. | [122] |
| 13 | Aspergillus sydowii CBMAI 935 | Methyl parathion | Isoparathion, methyl paraoxon, trimethyl phosphate, O,O,O-trimethyl phosphorothioate, O,O,S-trimethyl phosphorothioate, 1-methoxy-4-nitrobenzene, and 4-nitrophenol | Using this strain, residue measurements of both the pesticide and its metabolite were performed. After 10, 20, and 30 days, biodegradation of methyl parathion was 58%, 70%, and 80%, respectively. | [110] |
| 14 | Aspergillus sydowii CBMAI 935 | Profenofos | 4-bromo-2-chlorophenol, 4-bromo-2-chloro-1-methoxybenzene, and O,O-diethyl S-propylphosphorothioate | It was found that after 10 days, profenofos (42 | [110] |
| 15 | Aspergillus sydowii CBMAI 935 | Chlorpyrifos | tetraethyl dithiodiphosphate, 3,5,6-trichloropyridin-2-ol, 2,3,5-trichloro-6-methoxypyridine, 3,5,6-trichloro-1-methylpyridin-2(1H)-one | The strain demonstrated 32% biodegradation of chlorpyrifos. Reductions in residual chlorpyrifos concentrations were 17%, 28%, and 32% after 10, 20, and 30 days, respectively. | [110] |
| 16 | Cladosporium cladosporioides Hu-01 | Chlorpyrifos | Diethylthiophosphoric acid (DETP) and 3,5,6-trichloro-2-pyridinol | The morphology and analysis of the 5.8S rDNA gene allowed us to isolate a new strain of the fungus. Strain Hu-01 used chlorpyrifos (50 mg/L) as the sole carbon source and completely degraded chlorpyrifos within 5 days. | [111] |
| 17 | Trichoderma sp. CBMAI 932 | Chlorpyrifos | O,O-diethyl-O-methyl phosphorothioate and 3,5,6-trichloro-2-pyridinol | Biodegradation reactions were performed in liquid media containing commercial chlorpyrifos over 10-, 20-, and 30-day period using Trichoderma sp. CBMAI 932. The microbial strain was introduced into the media, and the degradation of chlorpyrifos was monitored at various time intervals to evaluate the extent of degradation. The study provided valuable insights into the potential of Trichoderma sp. CBMAI 932 for bioremediation of chlorpyrifos-contaminated environments. | [123] |
| 18 | Aspergillus terreus JAS1 | Chlorpyrifos | 3,5,6-trichloro-2-pyridinol | The biodegradation of chlorpyrifos was studied using the newly isolated fungal strain from rice field soil. A total of 300 mg/L of chlorpyrifos and its metabolite TCP were completely degraded within 24 hours in a mineral medium. In soil, the strain degraded 300 mg/kg of chlorpyrifos in 24 hours and TCP in 48 hours, demonstrating its effectiveness in degrading both compounds under different conditions. | [124] |
| 19 | Acremonium sp. strain (GFRC-1) | Chlorpyrifos | 3,5,6-trichloropyridyl-2-phosphorothioate (desdiethyl chlorpyrifos) | The highest degradation of chlorpyrifos (83.9%) was observed when the strain was cultivated in a nutrient medium. The chlorpyrifos metabolite was identified using liquid chromatography–tandem mass spectrometry. | [125] |
| 20 | Rhizopus nodosus, Aspergillus fumigatus, Penciillium Citreonigum. | Diazinon | 2-isopropyl-4-methyl-6-hydroxypyrimidine (IMHP) | The degradation process of diazinon was studied for three types of fungi. These fungi were isolated from wastewater. Diazinon residues were determined during a 21-day incubation period in a liquid medium inoculated with each type of fungus. The pesticide and its metabolites extracted from the samples were analyzed using GC/MS. F1 (91.1%), F2 (76.4%), and F3 (72.2%) were found to be effective in removing diazinon. At the end of the study, it was found that the microbial strains reduced the degradation of the pesticide by several days compared to the control sample. | [126] |
| 21 | Escherichia coli IES-02 | Malathion | Malathion monocarboxylic acid (MMA), malathion dicarboxylic acid (MDA), succinic acid, mercapto, diethyle ester, S-ester with O, S-dimethyl phosphorodithioate, oxalic acid isobutyl nonyl, ethyl hydrogen fumarate, and diethyl maleate. | A bacterial strain isolated from a pesticide-contaminated sample showed significant efficiency in degrading malathion when used as the sole carbon source. Maximum degradation (99%) of the pesticide malathion by the strain IES-02 was observed within 4 hours at a malathion concentration of 50 ppm. | [127] |
| 22 | Stenotrophomonas maltophilia CAB5 | Monocrotophos | Dimethyl-phosphate, trimethyl phosphate, and cyclohexanone, 2-cyclohexylidene | The strain CAB5 showed tolerance to monocrotophos up to 1000 ppm. Metabolites were identified by Fourier transform infrared (FT-IR) spectrometry and LC–MS analyses. In addition, this strain has various plant growth-promoting properties. | [128] |
| 23 | Diaphorobacter sp. TPD-1 | Triazophos | 1-phenyl-3-hydroxy-1,2,4-triazole (PHT), O,O-diethyl phosphorothioic acid, 1-formyl-2-phenyldiazene, 2-phenylhydrazinecarboxylic acid, and phenylhydrazine | The bacterial strain was obtained from soil contaminated with triazophos. It degraded the pesticide (50 mg/L) to undetectable levels within 24 hours. | [70] |
| 24 | L. plantarum (CICC20261) | Dimethoate | Omethoate, O,O,S-trimethyl phosphorodithioate, O,O,S-trimethyl phosphorothioate, trimethylphosphate, and dimethyl phosphate | The mechanism of dimethoate degradation in contaminated food products (milk) was studied using the probiotic L. plantarum. At the end of the study, it was found that this probiotic can effectively destroy the initial concentration of dimethoate (up to 81.28%). | [57] |
Various microorganisms, such as bacteria, fungi, actinomycetes, and algae, can degrade pesticides. However, bacterial metabolism has been studied more extensively since bacteria are easier to grow. Therefore, most metabolic pathways have been explored in bacteria [85]. Bacteria can degrade pesticide residues cost-effectively and in an environmentally friendly way without causing secondary pollution. Several researchers have conducted in-depth studies on bacteria, gaining a clearer understanding of the degradation mechanisms of organic pesticides. Thus, several bacteria capable of decomposing and transforming pesticides have been identified [102]. Indeed, the initial bacterium capable of degrading OPs (Flavobacterium sp.) was obtained from Philippine soil in 1973. Subsequently, several strains have been identified that can utilize OP compounds as a source of carbon, nitrogen, or phosphorus [129]. One of the primary challenges in isolating pesticide-degrading bacteria is the chemical composition of the pesticides, which can frequently restrict biodegradability; bacteria use accessible organic compounds. Thus, by enhancing the solubility of hydrophobic substrates by applying surfactants or by dissolving the pesticide as its salt, the ability of the bacteria to degrade these hydrophobic compounds can be improved. After being isolated in a pure culture and presumptive identification, it was confirmed that bacteria can either utilize the pesticide as their only carbon source, or in some cases as their only nitrogen source, or co-metabolize it [103]. Bacteria are among the most accessible decomposers capable of degrading organophosphate pesticides [130]. The degradation of organophosphates by bacteria is associated with enzymatic activity that facilitates the hydrolysis of phosphodiester linkages [131]. While most microorganisms can degrade only a single OP or a narrow range of OP compounds [38], certain types of bacteria can also degrade other pesticides [132]. Various bacteria that degrade organophosphorus compounds have been isolated and studied, such as Bacillus stearothermophilus, Brevundimonas diminuta, Flavobacterium sp., Alteromonas sp., Nocardia sp., Escherichia coli, Arthrobacter, Burkholderia sp., and Corynebacterium glutamicum [35]. Most bacteria can destroy the original structure of the pesticide and its metabolites. A bacterial strain of Bacillus thuringiensis isolated from fields in Pakistan was able to degrade nearly 99% of chlorpyrifos (200 mg/L) in M-9 broth within 9 days. This strain could also degrade and transform 3,5,6-trichloro-2-pyridinol, an intermediate metabolite of chlorpyrifos. Additionally, gas chromatography–mass spectrometry (GC–MS) analyses showed that the MB497 strain degraded chlorpyrifos to 2-hydroxy-3,5,6-trichloropyridine and diethylthiophosphate (DETP) by organophosphorus phosphatase [109]. A native strain of Bacillus aryabhattai (VITNNDJ5) was obtained and used to degrade monocrotophos. The biodegradation results, analyzed by an ultraviolet (UV) spectrophotometer and high-performance liquid chromatography (HPLC), showed 93% degradation of MCP (1000 mg/L) within 5 days. Additionally, the decomposition products of this pesticide were identified using the GC–MS method, and a mechanism was proposed for the biodegradation of monocrotophos [65].
Fungi need a range of organic and inorganic nutrients to support their growth, the most important of which are carbon, nitrogen, oxygen, and phosphorus. Under such conditions, fungi may be a potential biocatalyst for the biodegradation of these pesticides [110]. Since the mid-1980s, fungi have been widely investigated for their potential in bioremediation due to their higher tolerance to high pollutant concentrations than bacteria. Nevertheless, most studies on fungi have been performed under laboratory conditions, and fungi might not consistently serve as the most effective agents for bioremediation in real-world environments. Comparatively, fungi in less-studied soils, such as those in tropical forests, may exhibit greater tolerance to environmental factors and be more capable of bioremediation than the temperate organisms currently studied [133]. Fungi are crucial in the biogeochemical cycle and contribute significantly to xenobiotic degradation. Indeed, the ability of fungi to form extensive mycelial networks, broaden enzyme specificity, and be independent from organic chemicals as growth substrates makes them suitable for bioremediation. However, the potential of fungi in organophosphate bioremediation remains underexplored [111]. The role of fungi in degradation processes is rarely quantified. However, fungi possess biochemical and environmental abilities to neutralize pollutants by modifying or altering chemical bioavailability. Nonetheless, this potential has not been adequately explored. Several fungal strains have been identified as capable of degrading different organophosphorus pesticides, but attempts to isolate pure fungal cultures that can fully mineralize pesticides have often been unsuccessful [134, 135]. Despite this, certain fungal strains that can degrade organophosphate pesticides have been successfully isolated and tested. For example, the Cladosporium cladosporioides strain Hu-01 was obtained from activated sludge samples at an aerobic wastewater treatment system designed for chlorpyrifos in Jiangmen, China [111]. This strain completely metabolized 50 mg/L chlorpyrifos under certain conditions (26.8 °C and pH 6.5). The Hu-01 strain supports the metabolic pathway involved in fully detoxifying chlorpyrifos and the hydrolysis product, TCP. These results suggest that this fungus could be a potential candidate for the bioremediation of water, soil, or crops contaminated with chlorpyrifos [111].
Enzyme-mediated reduction involves the use of enzymes isolated from various microorganisms. These enzymes, which are biocatalysts or globular proteins, facilitate biochemical reactions by accelerating the conversion of substrates into products. Under ideal conditions, enzymes enhance the reaction rate and speed up the transformation of substrates into desired products by reducing the activation energy required for the process. An enzyme may contain one or more catalytically active groups, which are part of the active sites through either covalent or non-covalent interactions [136]. Enzymes are effective disinfectants due to their biocatalytic properties, which can alter the structure and toxicity of contaminants, ultimately transforming them into harmless inorganic products [137]. Oxidoreductases and hydrolases are crucial in the metabolic and catabolic processes that transform pollutants [138].
Pesticides typically undergo biotransformation through a series of chemical reactions [136]. The toxicity of organophosphate compounds is reduced when one of the ester bonds to the main group is broken. The decomposition of organophosphate compounds involves mechanisms, such as oxidation or reduction, followed by hydrolysis. These degradation processes eventually lead to ring cleavage, which breaks open the OP molecule, releasing various metabolizable compounds through enzymatic catalysis. The resulting intermediates enter the tricarboxylic acid (TCA) cycle for full metabolic breakdown, producing CO2 and H2O as end products [137]. These products can integrate into a shared metabolic pathway [136]. Some of the most researched enzymes responsible for hydrolyzing and neutralizing OPs include organophosphate hydrolase (OpdA), diisopropyl fluorophosphatase (DFPase), phosphotriesterase (OP hydrolase or PTE), paraoxonase (PON1), organophosphoric acid anhydrolase (OPAA), and SsoPox [136, 139, 140, 141]. The enzymes involved in OP hydrolysis are listed in Table 3 (Ref. [112, 116, 127, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156]).
| № | Enzymes | Microorganisms | Degrading pesticides | References |
| 1 | Organophosphate hydrolase (OPH) | Pseudomonas nitroreducens AR-3 | Chlorpyrifos | [142, 143] |
| 2 | Chlorpyrifos hydrolase (CPD) | Paracoccus sp. TRP | Chlorpyrifos | [144] |
| 3 | Methyl parathion hydrolase (MPH) | Cupriavidus sp. DT-1 | Chlorpyrifos | [145] |
| 4 | Chlorpyrifos hydrolase (CPH) | Pseudomonas | Chlorpyrifos | [146] |
| putida JQ701740 | ||||
| 5 | Organophosphorus hydrolase (OPH) | Cupriavidus | Chlorpyrifos | [147] |
| taiwanensis X1 | ||||
| 6 | Phosphotriesterase (PTE) | Enterobacter sp. B-14 | Chlorpyrifos | [112] |
| 7 | Phosphotriesterase (PTE) | Agrobacterium | Chlorpyrifos | [148] |
| radiobacter P230 | ||||
| 8 | Chlorpyrifos hydrolase (CPH) | Cladosporium | Chlorpyrifos | [149] |
| Cladosporioides Hu-01 | ||||
| 9 | Carboxylesterase | Escherichia coli IES-02 | Malathion | [127] |
| 10 | Cutinase | Fusarium sp. | Malathion | [150] |
| 11 | Malathion carboxylesterase (MCE) | Bacillus licheniformis strain ML-1 | Malathion | [116] |
| 12 | Organophosphorus phosphatases (OPP) | Bacillus thuringiensis MB497 | Chlorpyrifos, Triazophos, Dimethoate | [151] |
| 13 | Organophosphorus hydrolase (OPH) | Pseudomonas diminuta | Methyl parathion | [152] |
| 14 | Organophosphorus hydrolase (OPH) | Brevundimonas diminuta | Methyl parathion | [153] |
| 15 | Organophosphorus hydrolase (OpdA); | Leuconostoc mesenteroides | Chlorpyrifos, Coumaphos, Diazinon, Methylparathion, Parathion | [154] |
| Organophosphorus hydrolase (OpdE) | WCP307 | |||
| 16 | Dimethoate-degrading enzyme | Aspergillus niger ZHY256 | Dimethoate, Malathion | [155] |
| 17 | Fenamiphos hydrolyzing enzyme (FHE) | Microbacterium esteraromaticum MM1 | Fenamiphos | [156] |
Enzyme catalytic mechanism. Phosphotriesterases (PTEs) catalyze the hydrolysis of organophosphates. There are three main types of PTEs: organophosphate hydrolases (OPH and OpdA), methyl parathion hydrolase (MPH), and organophosphorus acid anhydrolase (OPAA). These enzymes are predominantly found in microorganisms and other biological entities. OP-degrading enzymes promote the hydrolysis of various bonds, such as O–P, C–P, P–S, P–N, and P–F [157]. The OPH enzyme breaks down organophosphates through hydrolysis. Initially, the enzyme attaches to the organophosphate pesticide. In selecting the substrate, the OPH enzyme recognizes the organic group linked to the phosphorus atom. At this point, strong but temporary electrostatic and hydrophobic interactions form between the enzyme and the substrate. The OPH enzyme hydrolyzes the phosphate ester, detaching the phosphate group and converting the organophosphate into non-toxic products. After the organophosphate binds, a water molecule in the enzyme’s active site performs a nucleophilic attack on the phosphorus atom. This process is assisted by two divalent metal ions, a water molecule, and reactive amino acids in the enzyme’s active site [158]. During this phase, the water molecule attacks the phosphate group and cleaves its bond with the phosphate ester. This interaction leads to a transient mesomeric structure, which makes it easier to break down the organophosphate compound and form new substances. As a result, the toxic organophosphate pesticide is degraded, generating harmless products, such as aliphatic alcohols or acids. The hydrolysis of the phosphate group and degradation of harmful compounds occur through the water in the environment [158, 159, 160, 161].
OPH active site. The catalytic function of the OPH enzyme is largely dependent on its active site. This site contains various amino acids, including glutamate, histidine, and serine, which are vital for substrate recognition and the execution of the catalytic reaction. These amino acids in the active site contribute in the following ways: The serine or histidine residue performs a nucleophilic attack, which is necessary for the active binding of the phosphate group; glutamate and other basic amino acids aid in substrate recognition and facilitate the hydrolysis reaction; water molecules and metal ions can enhance the efficiency of certain OPH enzymes [162, 163].
Microbial degradation of pesticides is a widely recognized and effective biodegradation method, with various microorganisms involved in this process. Among these microorganisms, bacteria are the main participants. Many bacterial strains capable of degrading chlorpyrifos have been isolated and characterized [142]. Several researchers have proposed different mechanisms for the degradation of chlorpyrifos. Fig. 3 shows the mechanism involved in the microbial degradation of chlorpyrifos. The initial step in the degradation of chlorpyrifos involves its conversion to chlorpyrifos-oxon (CPO) by the enzyme oxidase [164]. CPO is an unstable intermediate that is formed through the oxidative desulfurization of chlorpyrifos. In alkaline soils, CPO is rapidly broken down into 3,5,6-trichloro-2-pyridinol (TCP) and diethylphosphate [142] (Fig. 3). The subsequent degradation of these compounds forms 3,5,6-trichloro-2-pyridinol and DETP [142]. TCP is considered the main intermediate in chlorpyrifos degradation and is only weakly adsorbed by soil particles, making it moderately mobile and stable within the soil environment [165]. TCP exhibits three times the toxicity of its parent compound [166]. The further breakdown of TCP results in 3,5,6-trichloro-2-methoxypyridine (TMP) [167]. In addition, 2,3-dihydroxypyridine is produced and undergoes hydrolysis to form 2-hydroxypyridine and 2,5-dihydroxypyridine. These metabolites are subsequently oxidized into smaller carbon fragments, aliphatic amines, and inorganic phosphate. Furthermore, 2,3-dihydroxypyridine can also be degraded into maleamic acid, which is oxidized into pyruvic acid, ultimately entering the Krebs cycle [142]. Further, DETP is hydrolyzed into thiophosphoric acid and ethanol, which microorganisms use as nutrients [168]. Enzymes, such as hydrolase, phosphotriesterase, phosphatase, catalase, and oxidase, are involved in the hydrolysis of chlorpyrifos by degrading P–O, P–F, and P–S bonds [169].
 Fig. 3.
Fig. 3. Biodegradation pathways of chlorpyrifos by microorganisms. CPO, chlorpyrifos-oxon; DTP, diethylphosphate; TMP, 3,5,6-trichloro-2-methoxypyridine.
Dimethoate is an acyclic aliphatic crystalline compound with a strong odor, widely used as an organophosphate insecticide. Although banned in many European countries, dimethoate is still partially used in Italy, Portugal, and Spain, and continues to be applied in several Asian, African, and American countries, posing potential risks to soil and water [59, 170, 171]. Dimethoate is an insecticide commonly used to manage insects and mites, acting on the central nervous system of pests, similar to other organophosphates [172]. It also combats numerous diseases in fruit trees, vegetables, and other plants [173]. Microorganisms, mainly bacteria, biodegrade dimethoate. The intermediate products of dimethoate biodegradation were discovered in prior microorganism studies, and two main biodegradation pathways were established [170, 171, 172, 173]. First, dimethoate is oxidized to omethoate (Fig. 4). Then, the phosphatase and carboxylamidase enzymes hydrolyze omethoate into phosphonic acid, propyl-O, S-dimethyl ester, and methyl diethanolamine. Phosphonothioic acid, a propyl-O, S-dimethyl ester, is a very unstable substance; O,O,O-trimethyl thiophosphate is formed following oxidation. This metabolite is broken down into phosphonic acid, propyl-O, S-dimethyl ether, and methyl diethanolamine by phosphatase and carboxylamidase enzymes. The propyl-O, S-dimethyl ether is highly unstable, and oxidation produces O,O,O-trimethyl thiophosphate. This compound then undergoes desulphurization and dephosphorization by phosphatase, providing the bacteria with carbon, sulfur, phosphorus, and nitrogen. The bacteria absorb methyl diethanolamine through a metabolic pathway [85, 174]. In a separate study, dimethoate undergoes initial hydrolysis by cleaving the amide bond, leading to dimethoate carboxylic acid (Fig. 4). This compound is then decarboxylated to produce O,O,S-trimethylthiophosphorodithioate. The resulting O,O, S-trimethylthiophosphorodithioate is subjected to oxidation, which forms O,O, S-trimethylphosphorothioate. The O, O, S-trimethylphosphorothioate can then be hydrolyzed at the C–O bond, releasing a CH3 group and forming O-methyl O, S-dihydrophosphorothioate. At the final stage of the metabolic pathway, O-methyl O,S-dihydrophosphorothioate is hydrolyzed, losing another CH3 group at the C–O bond, and producing phosphorothioic O, O, S acid [175].
 Fig. 4.
Fig. 4. Dimethoate degradation by microorganisms.
Malathion, a synthetic OP, is widely used to safeguard crops and livestock from pests [116]. The use of malathion in agricultural pest control has increased significantly, resulting in its residues being detected in various environmental media, including soil, water, vegetables, and even breast milk. The presence of organophosphates, such as malathion, in the environment raises global concerns due to their effects on the nervous system, which could pose serious risks to public health [84]. Additionally, the presence of malathion in the environment poses a significant threat to living organisms, as it can lead to mitogenic and cytogenetic effects at both low and high exposure levels [176]. Malathion is highly soluble, which in turn makes some traditional treatments ineffective. Therefore, new economical and effective technologies are needed to neutralize or completely remove malathion from contaminated environments [84].
The bioremediation method increases the rate of natural biodegradation of pollutants in the environment by introducing potential microorganisms into the contaminated environment [177]. Bacteria capable of degrading malathion were isolated from various samples. Microbial degradation of malathion in soil indicates that it is initially converted into malaoxon, malathion monoacid, diethyl fumarate, and trimethyl thiophosphate by a bacterial consortium comprising Micrococcus aloeverae, Bacillus cereus, and Bacillus paramycoides (Fig. 5A) [84]. Thus, introducing this bacterial consortium into the soil may result in the most efficient breakdown of pesticides, as it significantly reduces pesticide residues. This approach shows potential for degrading and detoxifying environments contaminated with malathion and other organophosphates. Alternatively, malathion is degraded into malathion monocarboxylic acid and malathion dicarboxylic acid through carboxylesterase. These metabolites are then converted by oxidoreductase to dimethyl dithiophosphate and dimethyl thiophosphate. The phosphorus moiety can then undergo demethylation by phosphoesterase to produce two further metabolites, dimethyl phosphate and thiophosphate (Fig. 5B) [84, 178, 179].
 Fig. 5.
Fig. 5. Malathion biodegradation pathway.
Acephate (O, S-dimethylacetylphosphoramidothioate) is a minimally toxic and highly effective OP commonly applied in pest management. Acephate acts as an insecticide and can disrupt nerve function by blocking acetylcholinesterase. Acephate residues are frequently found in water and soil samples [180]. This pesticide demonstrates neurotoxic, reproductive, and developmental effects on non-target species [181]. Acephate and its degradation products can also cause acute poisoning [182]. Microbial degradation is considered one of the most efficient methods for removing organic pollutants from the environment. Previous research has indicated that acephate undergoes an initial degradation process to form methamidophos (Fig. 6) or O-methyl-N-acetylphosphoramidate (Fig. 6) in both soil and water [180, 181, 182]. The formation of O-methyl-N-acetylphosphoramidate requires an enzyme such as phosphotriesterase, which is involved in the hydrolysis of P-S bonds. The enzyme carboxylesterase plays a role in forming methamidophos [183, 184]. Various compounds are also formed during the subsequent decomposition of methamidophos, with phosphoric acid being the final product. Phosphotriesterases (PTEs) are crucial in initiating the degradation of acephate, as the hydrolysis of P–S and P–O bonds in acephate and its metabolites largely relies on PTE-catalyzed reactions [73]. Acephate is hydrolyzed to S-methylphosphoramidate in the catalysis of PTE, which is then further catalyzed to form phosphoric acid, phosphoramides, or lower molecular weight phosphoric acids.
 Fig. 6.
Fig. 6. Acephate biodegradation pathway.
The environmental biodegradation of organophosphate pesticides presents several practical challenges. Notably, these pesticides are recognized for their significant toxicity and persistence in the environment over extended periods. Consequently, we will explore the key challenges involved in effectively managing the biodegradation processes of these pesticides.
1. Toxicity and risk to microorganisms: It is known that OP-based pesticides are extremely harmful to microorganisms and other living beings. The microorganisms responsible for breaking down these pesticides during biodegradation are especially vulnerable to these chemicals, which may damage them. This can ultimately slow down or even stop biodegradation [17, 185].
2. Environmental condition dependence: The biodegradation of organophosphate pesticides is highly influenced by environmental factors, such as temperature, moisture, pH levels, microbial activity in soil or water, and the composition of both mineral and organic compounds. In some environments, for example, in dry or hot conditions, the biodegradation process can significantly slow down, resulting in the prolonged presence of pesticides in the environment [138].
3. Diverse chemical composition: Organophosphate pesticides encompass a variety of compounds with different chemical structures, adding complexity to their biodegradation process. Certain organophosphates have exhibited significant resistance to biological degradation. Since these pesticides have distinct components, each type affects the biodegradation process differently [186].
4. Microorganism adaptation: Some microorganisms can degrade organophosphate pesticides, but this process typically requires specific environmental conditions or long-term pesticide exposure. Even though certain microorganisms can adapt to degrade these chemicals, the conditions needed to accelerate the degradation, such as the presence of particular bacteria, fungi, plants, or their enzymes [85, 187], may complicate the process and make it less cost-effective.
Microbial degradation is vital for bioremediation, where microorganisms degrade toxic organic pollutants into less harmful or harmless substances. Below, we provide some recommendations on how to implement microbial degradation of organic pollutants and accelerate bioremediation:
It is advisable to isolate such microorganisms primarily from samples (soil, water) from areas exposed to polluting chemicals, as microorganisms in these zones develop adaptations to toxic substances. Further contaminating these samples with toxicants under laboratory conditions and maintaining them in optimal conditions for a certain period can make it possible to isolate and select microorganisms resistant to higher pesticide concentrations. The isolated microorganisms can be identified using various classical and modern methods, including microorganism morphology [187], biochemical characteristics of bacteria [188], MALDI-TOF MS [189], and molecular genetic methods [111, 189, 190].
The biodegradation process can be made more efficient through in silico bioremediation. This method allows for identifying and predicting degradation enzymes and microorganisms through computer analyses [191, 192]. The effectiveness of the binding and degradation potentials of pesticides has been analyzed by in silico studies, such as evaluating the binding energy of insecticides with OpdA and Trichoderma harzianum paraoxonase 1 like (ThPON1-like) enzymes [191]. Molecular docking studies have emphasized the crucial role of OPH in degrading chlorpyrifos [192]. These methods help determine the interactions between pesticides and microorganisms, aiding in designing new microorganisms and enzymes, and enhancing the natural degradation of pesticides [191, 193, 194].
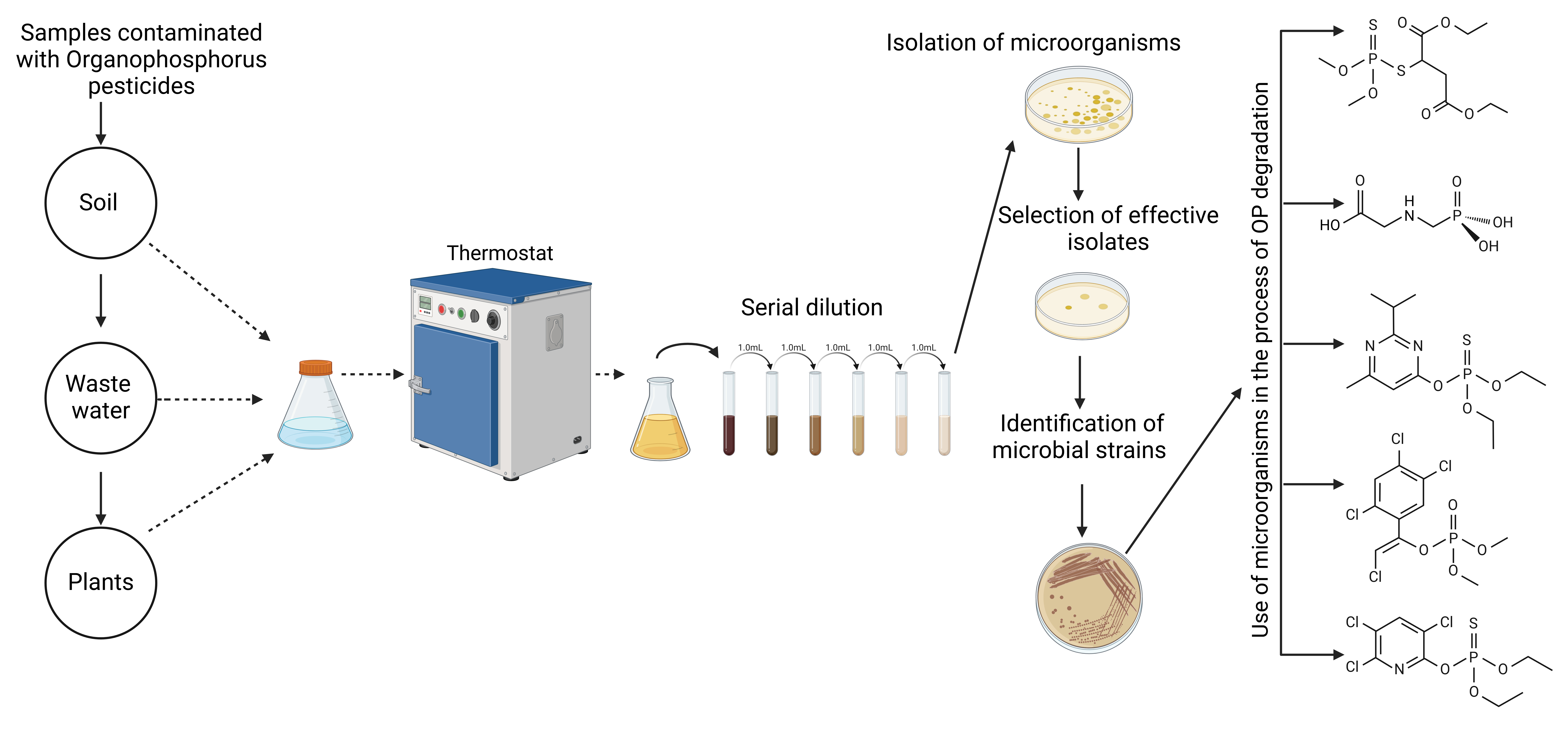
Various methods are used to determine the ability of microorganisms to decompose OPs. Initially, the capability of microorganisms to grow and develop on nutrient media is assessed with different concentrations of pesticides added to the medium. Although some microorganisms are resistant to pesticides, these organisms cannot necessarily use them as a source of energy. Pesticide degradation may be determined by various methods, including spectrophotometry [195], Thin Layer Chromatography (TLC) [196], Fourier Transform Infrared Spectroscopy (FTIR) [124, 197], GC [130], LC–MS/MS [198], GC–MS [199], and High Performance Liquid Chromatography (HPLC) [14]. The principal scheme for using microorganisms to degrade OPs is presented in Fig. 7.
 Fig. 7.
Fig. 7. Schematic diagram of the isolation and use of microorganisms in the degradation of OPs.
Degradation of organophosphorus compounds by microorganisms promotes the hydrolysis of ester bonds in phosphoric acid, typically assisted by enzymes such as organophosphorus hydrolases, which facilitate the conversion of OPs into less harmful metabolites. This microbial process leads to the restoration of contaminated sites. This bioremediation approach could become vital in addressing pesticide pollution and fostering sustainable agricultural practices through continued research and advancements. Additionally, adopting new strategies to improve biodegradation methods is expected to yield favorable results.
Genetic engineering makes it possible to change the biological properties of microorganisms and improve some of their characteristics [200, 201]. Genetically modified microorganisms have shown effectiveness in the bioremediation of pesticides, with studies focusing on the bioremediation of organophosphates [202]. Research has also investigated the potential of genetically modified bacteria to neutralize various stable substances [200].
Application of nanotechnologies. New materials can be developed with the aid of nanotechnology to accelerate and enhance the degradation of pesticides. Certain nanoparticles can catalyze the degradation process, increasing its efficiency. Since these nanoparticles are small and have a large surface area, they can quickly degrade substances and integrate easily with microorganisms, boosting their activity. Moreover, nanoparticles can adjust to varying environmental conditions while retaining their biodegradation capabilities, offering new prospects for environmental protection [203, 204, 205].
Using microbial consortia. Microbial consortia, composed of various microorganisms, are used together to degrade organophosphate pesticides and reduce their environmental risk effectively. These consortia comprise microbes that complement or enhance the activity of others during pesticide degradation. These microbes complement each other to degrade pesticides through multiple pathways. Each microorganism produces specific enzymes that help degrade certain components of the pesticides, while others are active in the later stages of the degradation process. These enzymes are crucial in metabolizing pesticides and converting them into products with lower toxicity [69].
Biostimulation. This process involves altering environmental factors to support and boost the performance of microorganisms or plants. This can be achieved by introducing certain fertilizers or chemical compounds that stimulate microbial growth and enhance their capacity to degrade harmful pollutants. By optimizing conditions for microbial activity, biostimulation can significantly speed up the natural process of bioremediation [206].
In addition, as mentioned above, utilizing modeling methods such as in silico studies and molecular docking can further enhance the effectiveness of bioremediation processes [191, 192].
In conclusion, although microbial degradation offers a promising and sustainable approach to mitigating the effects of organophosphorus pollution, further studies are necessary to optimize this natural process for effective bioremediation. Continued research into the genetic and biochemical pathways of microbial degradation of OPs may lead to their more efficient and widespread use in environmental remediation and reduced toxicity. The future of OP degradation using microorganisms is promising, and the information presented in this review can further optimization research in this field.
Original draft, writing, conceptualization DK; methodology RE, and AM; writing—DK; worked with figures and tables DK and SK; project administration DK. All authors contributed to editorial changes in the manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Not applicable.
We would like to express our gratitude to everyone who helped me write this manuscript.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.







