1 Hematology, Wexham Park Hospital, SL2 4HL Slough Berkshire, UK
2 Haematology and Blood Transfusion, Basingstoke and North Hampshire Hospital, RG24 9NA Basingstoke, UK
3 Biomedical Sciences Research Institute, Ulster University, BT52 1SA Coleraine, UK
Abstract
Dextran is an exopolysaccharide synthesized in reactions catalyzed by enzymes obtained from microbial agents of specific species and strains. Products of dextran polysaccharides with different molecular weights are suitable for diverse pharmaceutical and clinical uses. Dextran solutions have multiple characteristics, including viscosity, solubility, rheological, and thermal properties; hence, dextran has been studied for its commercial applications in several sectors. Certain bacteria can produce extracellular polysaccharide dextran of different molecular weights and configurations. Dextran products of diverse molecular weights have been used in several industries, including medicine, cosmetics, and food. This article aims to provide an overview of the reports on dextran applications in blood transfusion and clinical studies and its biosynthesis. Information has been summarized on enzyme-catalyzed reactions for dextran biosynthesis from sucrose and on the bio-transformation process of high molecular weight dextran molecules to obtain preparations of diverse molecular weights and configurations.
Keywords
- biomolecule
- blood
- clinical
- dextran
- dextran–sucrase
- dextranase
- enzymes
- glucan–sucrase
- pharmaceutical
- sucrose
- transfusion
Dextrans are exopolysaccharide biomolecules synthesized by bacterial species.
Dextran is a polysaccharide of glucose units that are
Dextrans, particularly those with low molecular weights (MWs), are required for clinical use and as products for research analytical studies [2]. Fluids prepared using dextran of specific MWs are used in blood transfusions, where they function as an anticoagulant, osmotic agent, volume extender, and antithrombotic and intravenous plasma-lubricant. Dextran-40 solution is used to develop blood volume by injecting it through a vein directly into the bloodstream. Specifically, it is used when blood transfusions of specific matching types are not immediately available, and there is a deficit in circulating blood volume, for instance, in the condition of shock initiated by burns, surgery, and hemorrhage. In such cases of blood deficit, hemoglobin concentration is not considered the only therapeutic guide, the treatment is focussed on restoring the intravascular volume and adequate hemodynamic parameters [3].
In addition, functional polymers based on dextran are used as cryo-preservative agents for vaccines and organs [4]. Dextran has also been used in the cosmetic industry as a moisturizing and thickening ingredient in some cosmetic products. In pharmaceutical applications, the reducing property of dextran allows its use in products formulated as an anti-aging agent. In the research and development sector, dextran is used for its convenience in creating matrices for chromatography, immobilization of entities on biosensors, generating nanoparticles, and preparing different emulsions. The adaptability of dextran, due to its multifunctional contributions to medicine, cosmetics, and food, has been the subject of research for decades [5].
This article aims to review reports published on the uses of dextran for clinical and pharmaceutical applications and then on the synthesis of dextrans of different MWs. Information has been presented on enzyme-catalyzed biosynthesis and the bio-transformation of high-MW dextrans to yield low-MW molecules with different branching configuration ratios.
Dextran polysaccharides contain
Dextran has been used as an expander of plasma volume for several years. Dextran-70 and 40 preparations, with molecular weights of 70 and 40 kDa, were recommended to manage shock or impending shock caused by hemorrhage, burns, and trauma [2, 3, 6]. Kam et al. [7] used dextran-40 in vitro to evaluate the effect of hemodilution on the coagulation profile, where the thromboelastometry and multiple electrode aggregometry methods measured the test samples. The use of albumin as an alternative might be beneficial in patients suffering from septic shock; however, the cost of clinical-grade albumin is high. Therefore, Bentzer et al. [8] investigated the effects of using dextran-70 on organ failure or mortality in patients suffering from severe sepsis or septic shock. Researchers performed their study using a group of patients with severe sepsis or septic shock admitted to a hospital intensive care unit between 2007 and 2015 and administered dextran-70 for resuscitation. The report concluded that no evidence could be detected to support an unfavorable effect of the use of dextran-70 on organ failures and or mortality in patients with septic shock or severe sepsis [8].
For plasma expansion, alternatives to albumin include crystalloids (normal saline 0.9% sodium chloride and Ringer’s lactate), plasma protein fraction (alternate protein colloids), and dextran (nonprotein colloids). Although nonprotein colloids and crystalloids have not exhibited a benefit over using albumin, these are low-cost options. Dextrans are added to normal saline as an isotonic medium when used as an element in colloid solutions. Winkler mentioned in a previous article on albumin and related products that Rescueflow, a hypertonic mixture, is limited to its prehospital use in treating hypotension caused by bleeding, although its benefit remains uncertain [9]. Warden discussed in his article on fluid resuscitation and early management that in young pediatric burn patients with major burn injuries, colloid replacement is frequently required; thus, the serum protein concentration decreases rapidly during burn shock. In such cases, dextran colloid is routinely used during the resuscitation of children with major burn injuries [10]. Important types of dextran have been summarized in Table 1 (Ref. [11, 12]), indicating their molecular weights and applications in blood transfusions.
| Product | Application | Product Molecular Weight (MW, Dalton, Da) |
| Dextran-70 | (1) a plasma substitute | Average: 70,000 |
| (2) to prevent deep venous thrombosis | ||
| Dextran-40 (D-40) | (1) Plasma expander | Average: 40,000 |
| (2) It is used to enhance blood flow in ischemic limbs | ||
| (3) D-40 improves the micro-circulatory flow by decreasing red cell aggregation | ||
| Macrodex, | Expands plasma to the same volume as the infused amount; solution exists in the plasma for up to 4 hours | Average: 70,000 |
| Dextran-70 (6%) | ||
| Dextran-70 (32%) | Sometimes administered intraperitoneally to reduce the adhesions after reproductive surgery | Average: 70,000 |
| Rheomacrodex, | This product is used for its excellent rheologic properties during vascular surgeries. It has efficacy of a larger plasma volume expansion with a shorter half-life than that of 6% Dextran-70 | Average: 40,000 |
| 10% Dextran-40 | ||
| Hapten, Dextran-1—a low-MW dextran | Applied as a desensitizer to avoid allergic reactions to large molecular weight dextrans, before the main dextran solution is infused | A fraction of dextran polysaccharide with a low molecular weight of 1000 Da |
Dextrans are used as volume expanders, as they have an effect of inhibition on the aggregation of thrombocyte and coagulation factors. The dextrans infusion can direct to anaphylactic reactions in mothers, which can make the embryo vulnerable. It has been suggested that the anaphylactic reactions can be lowered by administering Hapten–Dextran (Dextran-1 of a low molecular weight preparation of specifically 1.0 kDa) immediately before an infusion using main dextran. The binding of dextran-reactive antibodies to dextran-1 prevents the occurrence of large immune complexes and, subsequently, an immune response [13].
Researchers have investigated the characteristics of dextran polysaccharides in studies relevant to hematology. Dextran-40 has been tested for use in hemodilution compared with normal saline. Kam et al. [7] reported the in vitro effect of hemodilution evaluation on the coagulation profile by dextran-40, which was determined by thromboelastometry and multiple-electrode aggregometry. Researchers used blood samples taken from the veins of 20 volunteers who were in good health. Samples were diluted using dextran-40, or 5%, 10%, and 15% saline solutions; the results of this study for the coagulation profile did not show any platelet dysfunction. Clot formation was significantly impaired by hemodilution with dextran-40 compared to hemodilution with saline and in blood samples without dilution. At these concentrations of hemodilution performed in vitro to exhibit the clinical use of dextran infusions, no considerable fibrinolysis or platelet inhibitions were observed [7].
Studies have been performed to compare the effect of dextran and albumin on blood coagulation in patients undergoing gynecological surgeries. Their platelet count, standard coagulation tests based on plasma, and whole blood viscoelastic clot structure were reported to be highly affected by dextran-70 (6%) compared to albumin (5%); however, the aggregation of platelets did not change. Sigurjonsson et al. [14] reported that the Von Willebrand function was not deranged by isovolumic substitution of dextran-70 more than by albumin (5%) because dextran is hyper-oncotic compared to the 5% solution of albumin and, therefore, it induces a greater expansion in plasma volume and produces a higher dilutional coagulopathy.
A study on dextran deposition in tissues revealed that infused dextran was
mainly unchanged when eliminated at a rate of 50% by the kidney within 24 hours
of infusion and another 20% in the next 48 hours. Some of the residual 30%
dextrans are metabolized by dextranase (6-
The clinical performance of dextran was studied in a control test group of 11 hemodialyzed patients receiving alternative plasma expanders instead of dextran. In the other 11 patients in the dextran test group, who were given the maximum dosage of 0.38 g/kg per week of dextran-40, particles were detected in the macrophage cytoplasm of their various organs. These particles presented positive results in the periodic acid–Schiff (PAS) staining method, which detects dextran. None of the intracellular dextran particles were tested in the control group patients (receiver of an alternative for dextran), while no inclusions were also found in those patients who received a lower dose of dextran-40 below 0.08 g/kg per week. A linear relation was observed between the dextran-40 dosage and the number of inclusion particles. This study demonstrated that the material verified in the macrophages was dextran, structurally modified by macrophage activity to a water-insoluble particle form. Hence, dextran-40 at a lower dose is equally effective without its particle inclusion as other plasma-extender options [11].
Products of dextran polysaccharides with different molecular specifications have established several applications in the pharmaceutical industry. Dextrans of analytical quality are available commercially as active pharmaceutical ingredients (API). They are applied as excipients for the final formulation of clinical drug products, for medical devices, and for the production of solutions used in injections and infusions. Dextran is an inert material formulated with an active drug ingredient for the long-term stabilization of a medication. Dextran is also used as a bulking agent to increase the mass of those specific solid medicine formulations that contain the main active ingredients in very small measures in the ensemble weight of the product [15]. The role of dextran in pharmaceutical products is to act as a diluent—a filler—and to deliberate a medicinal improvement in the final dosage form of an active component in a pharmaceutical preparation.
Dextran is used as a critical constituent of hydrogels for biomedical applications, including in biosensor materials, drug delivery devices, and tissue engineering scaffolds. Dextran has applications as a pro-drug and in many classes of drug delivery vehicles, such as microspheres and nanocarriers. Injectable hydrophilic dextran/AgNP nanocomposite products have been prepared as white light-active biomolecules for their application as an antitumor agent [16].
Dextrans have established their varied applications for analytical purposes, e.g., in the gel filtration process used to separate pharmaceutical compounds [5, 17]. Dextran polymers can be easily cross-linked and fabricated to generate beads, which are required for their use in columns prepared for gel filtration. A commercialized filtration gel based on dextran is Sephadex®, which is available in many bead sizes and porosities, possessing characteristic ranges for molecular weight fractionation. Such filtration gels have multiple applications in analytical examinations, e.g., Sephadex® G-200, with the highest porosity, is suitable for fractionating proteins in the molecular weight range of 4.0 kDa to 80 kDa. In contrast, Sephadex G-25 is specifically useful for separating peptides with molecular weights lower than 5 kDa. Other than neutral dextran gels, some dextran derivatives, such as quaternized and carboxymethyl dextrans, are also commercially available, which are required for anion and cation-exchange chromatography [17].
Dextrans, with their hydrophilic features, have been applied in the conjugation of bioactive substances (such as enzymes, drugs, antibodies, and hormones) to extend their circulation lifetime, increase stability in vivo, or depress their antigenicity. Dextran nanoparticles have been applied in conjugation with insulin for oral insulin administration. Dextran nanocarriers protect insulin from degradation in the gut and modulate its release profiles. In addition to being a hydrophilic and functional polymer, dextrans are extensively used to modify surfaces to fabricate a biosensor. A dextran layer on sensor chips helps minimize the nonspecific adsorption of analytes and facilitates surface immobilization of ligands, thereby successively improving the biosensors’ sensitivity.
Derivatives of different polymer lengths of dextran [18] have many biological applications, such as an antithrombotic agent, in MRI contrast components and drug delivery systems due to its unique benefits of solubility and non-immunogenicity. Several dextran-based drug delivery systems with required design properties, such as nanocarriers and self-assembled micelles, have been created. Because of the high solubility of dextran nanocarriers in water, the solubilization and permeation of drugs are facilitated across the gastrointestinal membrane. Dextran polysaccharide molecules also protect drug molecules against their chemical or enzymatic degradation in the gastrointestinal tract, meaning they can only be depolymerized by a specific enzyme, dextranase. The neutral nature of dextran proves favorable in its protection from the mucus layer, which is negatively charged [19].
Two commonly co-administered drugs for chemotherapy in a wide range of cancers are doxorubicin (DOX) and cisplatin (CIS). Dextran-based self-assembled nanovesicles were prepared to conjugate anticancer drugs DOX and CIS in their core and layer [20]. Further, responsive biopolymer-based microgels/nanogels have been suggested for drug delivery applications [21]. The stability of nanovesicles against the glutathione detoxification was due to dextran vesicular geometry. These had adequate entrapment efficiency and were submitted for breakdown by lysosomal esterase enzyme for the delivery of drugs. In another study, dextran was modified with a 3-pentadecyl phenol to add stimuli-responsive characteristics for pH and enzyme. The anticancer drug doxorubicin could be effectively delivered under acidic pH conditions with the assistance of self-assembled and dual-responsive characteristics of dextran nanovesicles. A different type of derivative, curcumin-functionalized poly (lactic-co-glycolic acid)-dextran nano-micelles, has been synthesized, which exhibited the self-assembled behavior and natural antibacterial activity of curcumin [22]. Table 2 summarizes some commercially available dextrans as active pharmaceutical ingredients for human vaccine applications, lyophilization, organ preservation, and blood volume expanders.
| Pharmaceutical dextran | Product specification | Product molecular weight (Da) |
| Dextran-500 | European Pharmacopoeia (EP), United States Pharmacopoeia (USP) | 500,000 |
| Dextran-250 | EP; USP | 250,000 |
| Dextran-150 | EP; USP | 150,000 |
| Dextran-110 | EP; USP | 110,000 |
| Dextran-70 | EP, USP, Japanese Pharmacopoeia (JP) | 70,000 |
| Dextran-60 | EP; USP | 60,000 |
| Dextran-40 | EP, USP, Japanese Pharmacopoeia (JP) | 40,000 |
| Dextran-25 | EP, USP | 25,000 |
| Dextran-20 | EP, USP | 20,000 |
| Dextran-10 | EP, USP | 10,000 |
| Dextran-5 | EP, USP | 5000 |
| Dextran-3.5 | EP; USP | 3500 |
| Dextran-1.5 | EP; USP | 1500 |
| Dextran-1 | EP; USP | 1000 |
* Information sourced from https://www.dextran.com/; accessed on 22/07/2023.
Dextran has many applications in nanomedicine. Dextran nanoparticles have better water solubility, higher load capacity, and inherent viscosity. Thus, these features of dextran made it a favorable polysaccharide material for applications in nano-drug carriers, cell imaging systems, and the fabrication of nano biosensors [23]. For several in vivo applications, dextran is one of the most effectively used polysaccharide-based polymers. Dextran-coated superparamagnetic iron oxide nanoparticles are clinically applied as contrast agents in magnetic resonance imaging, and they also possess cancer nodal staging capabilities. The introduction of a carboxymethyl group can upgrade the functionality and stability of dextran-coated magnetic particles cross-linked to epichlorohydrin to obtain cross-linked iron oxide particles, which have better stability than dextran-coated superparamagnetic iron oxide nanoparticles of the same dimensions [24].
Dextran of different molecular weights and branching configurations has become of commercial and pharmaceutical importance for such a wide range of applications. Therefore, several researchers have studied its biosynthesis using enzymes obtained from specifically selected microbial agents. Some of the methods studied for its enzymatic biosynthesis are discussed in Sections 4 and 5.
Dextran is a generic name given to a few types of glucans, which are formed by
the polymerization of the
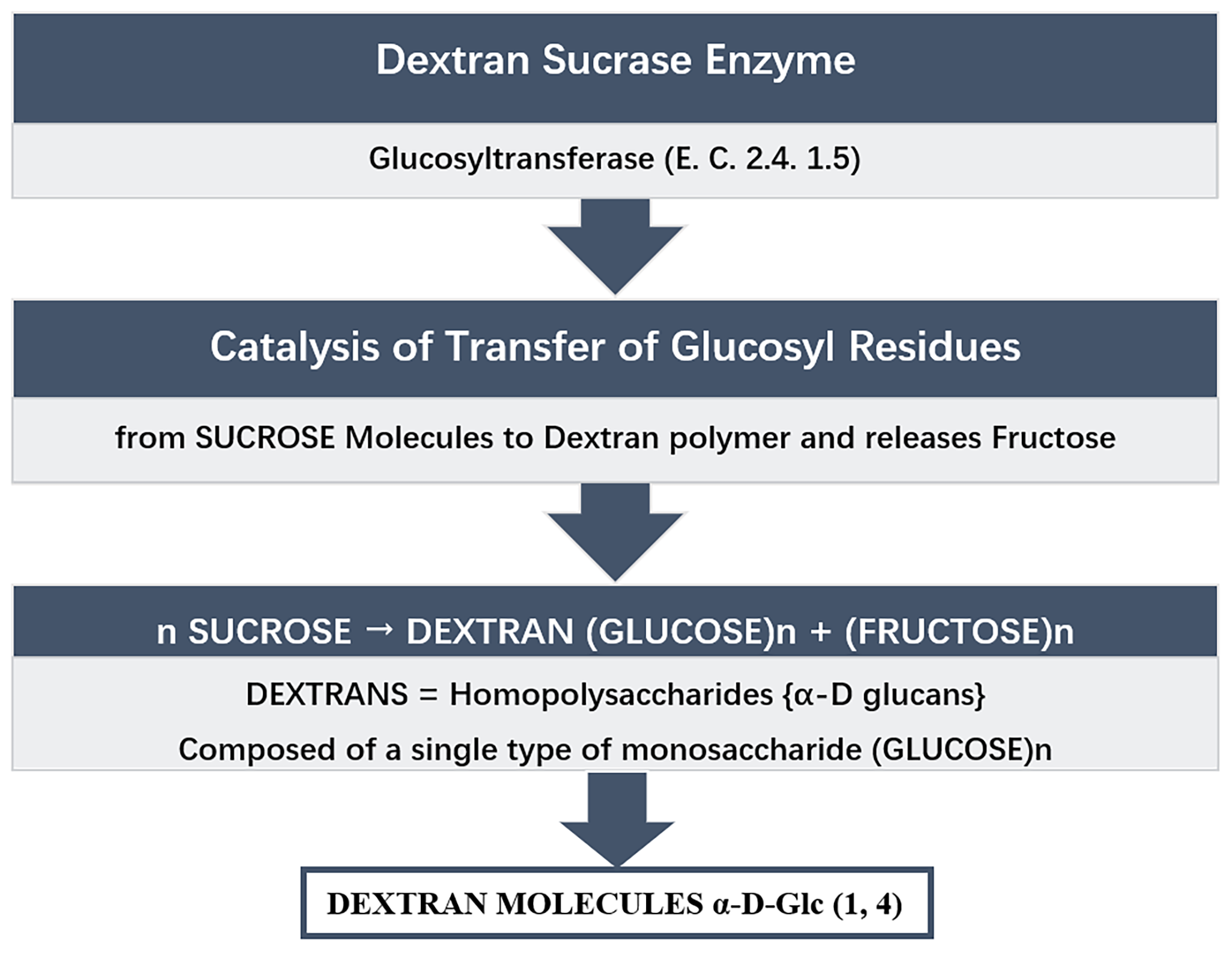 Fig. 1.
Fig. 1.Synthesis of dextran from sucrose catalysed by enzyme dextran sucrase.
Dextran polysaccharides contain
A commercial strain, Leuconostoc mesenteroides NRRL B-512, is used for dextran production. Since the enzyme catalysis is a very effective method for the synthesis of bioactive molecules for their applications in the pharmaceutical industry [26], dextran also can be synthesized in an enzyme-catalyzed process, which is free from the direct cultivation of microorganisms. Several such molecules prepared from microbial strains have been studied for medicinal and therapeutic applications [27]. Biomolecules with special characteristics for industrial biotechnological applications have been prepared using enzymes obtained from purposely selected microbial sources [28]. Considering the productive biosynthesis process of suitable enzyme catalysts, a similar strategy was applied to dextran synthesis in enzyme-catalyzed reactions.
Table 3 (Ref. [29, 30, 31, 32, 33]) summarizes some of the processes where dextran has been synthesized from sugars by enzymes dextran–sucrase and glucan–sucrase. These reactions were catalyzed by natural, mutated, or engineered bacterial enzymes.
| Synthesized product specification | Substrate and mixture of substrates used in enzyme-catalytic synthesis of product | Enzymes from original, engineered, or mutated strains used as biocatalyst in reaction | Enzyme sourced from a bioagent | Reference |
| 178 kDa | 5%, w/v sucrose | Dextransucrase | Weissella confusa Cab3 | [29] |
| 97% |
WcCab3 from original strain | |||
| 3% |
||||
| 10–20 kDa | 10%, w/v sucrose | Dextransucrase | Escherichia coli, | [30] |
| (DE3)/pET28- | Penicillium | |||
| dexYG-dextranase | aculeatum | |||
| from engineered strains | BL21 | |||
| 5 kDa | 10%, w/v sucrose | Dextransucrase | Escherichia coli BL21 | [30] |
| (DE3)/pET28- | ||||
| dexYG from engineered strains | ||||
| 400 mM sucrose | Dextransucrase | Leuconostoc | [31] | |
| 98% |
FT045B-dextranase from original strains | mesenteroides, Penicillium | ||
| 2% |
FT045Bsp. | |||
| 1645 kDa | 1000 mM sucrose | Dextransucrase | Leuconostoc | [32] |
| B-512FMC from mutated strains | mesenteroides B-512FMC | |||
| 126–787 kDa | 200 mM sucrose | Dextransucrase | Leuconostoc | [32] |
| B-512FMC from mutated strains | mesenteroides B-512FMC | |||
| 63–514 kDa | 100 mM sucrose | Dextransucrase | Leuconostoc | [32] |
| B-512FMC from mutated strain | mesenteroides B-512FMC | |||
| 49–431 kDa | 50 mM sucrose | Dextransucrase | Leuconostoc | [32] |
| B-512FMC | mesenteroides B-512FMC | |||
| from mutated strain | ||||
| 20–341 kDa | 20 mM sucrose | Dextransucrase | Leuconostoc | [32] |
| B-512FMC | mesenteroides B-512FMC | |||
| from mutated strain | ||||
| Maltose 100 mM + sucrose 100 mM | Glucansucrase | Leuconostoc | [33] | |
| 100% of | L940W | reuteri 180 | ||
| from mutated strain | ||||
| Maltose 100 mM + sucrose 100 mM | Glucansucrase | Leuconostoc | [33] | |
| 93% |
L940F | reuteri 180 | ||
| 7% |
from mutated strains | |||
| Maltose 100 mM + sucrose 100 mM | Glucansucrase | Leuconostoc | [33] | |
| 73% |
L940E | reuteri 180 | ||
| 27% |
from mutated strain | |||
| Maltose 100 mM + sucrose 100 mM | Glucansucrase | Leuconostoc | [33] | |
| 72% |
L940M | reuteri 180 | ||
| 28% |
from mutated strain | |||
| Maltose 100 mM + sucrose 100 mM | Glucansucrase | Leuconostoc | [33] | |
| 84% |
L940S | reuteri 180 | ||
| 16% |
from mutated strain | |||
| Maltose 100 mM + sucrose 100 mM | Glucansucrase | Leuconostoc | [33] | |
| 84% |
L940A | reuteri 180 | ||
| 16% |
from mutated strain | |||
| Maltose 100 mM + sucrose 100 mM | Glucansucrase | Leuconostoc | [33] | |
| 74% |
L940C | reuteri 180 | ||
| 26% |
from mutated strain | |||
| Maltose 100 mM + sucrose 100 mM | Glucansucrase | Leuconostoc | [33] | |
| 85% |
L940G | reuteri 180 | ||
| 15% |
from mutated strain | |||
| Maltose 100 mM + sucrose 100 mM | Glucansucrase | Leuconostoc | [33] | |
| 78% |
GTF180 | reuteri 180 | ||
| 22% |
from mutated strain |
Water-soluble dextran fractions of low molecular weight have multiple applications in pharmaceuticals, including being a carrier for drug delivery. The enzymatic transformation was used to obtain such dextran preparations since the enzymes as biocatalysts have proved useful in the biotransformation of compounds into their pharmaceutically active isomers [34]. Similarly, enzymatic transformation studies produced dextrans of different configurations and MWs. Enzymatic synthesis of dextran was conducted under controlled conditions to produce clinically suitable molecular weight products [35]. The biotransformation of dextran into several molecular weight products has been performed using trans-glucosidase and dextranase enzymes (Table 4, Ref. [36, 37, 38, 39, 40, 41, 42, 43]).
| MW of products synthesized | Product configuration–glycosidic linkages | Substrates used for bio-transformation | Enzymes used in the reaction | Reference |
| 2000 kDa | 64% of |
70 kDa dextran + 292 mM sucrose | Transglucosidase | [36] |
| 36% of |
GBD–CD2 cloned | |||
| 528 kDa | Not applicable (NA) | 892 kDa high molecular weight dextran | Dextranase produced by Leuconostoc mesenteroides KIBGE-IB26 | [37] |
| 70 kDa | 81–88% of |
70 kDa dextran + 292 mM sucrose | Transglucosidase | [36] |
| 12–19% of |
GBD–CD2 | |||
| 62–67% of |
cloned | |||
| 33–38% of |
||||
| 40 kDa | 63% of |
70 kDa dextran + 292 mM sucrose | Transglucosidase | [36] |
| 37% of |
GBD–CD2 | |||
| cloned | ||||
| 10 kDa | 62% of |
70 kDa dextran + 292 mM sucrose | Transglucosidase | [36] |
| 38% of |
GBD–CD2 | |||
| cloned | ||||
| 1.0 kDa | 68% of |
70 kDa dextran | Engineered | [38] |
| 32% of |
||||
| transglucosidase | ||||
| 0.5 kDa | 75% of |
70 kDa dextran | Engineered |
[38] |
| 25% of |
transglucosidase | |||
| 5–8 kDa | NA | 40 kDa dextran | Dextranase original from Penicillium Sp | [39] |
| Low MW fractions | NA | Dextran fractions with high molecular masses of 1500 to 2800 kDa | Dextranase (Sugazym DX L, SternEnzym) from a non-genetically modified strain Chaetomium gracile | [40] |
| Low molar mass dextran L-Dextran, 3000–5000 Da | Average MW of 3951 Da, | 20%, w/v sucrose in sodium acetate buffer (50 mM) | Dextransucrase enzyme DSR-MΔ2 Q634A and its variants | [41] |
| 86.0% | ||||
| 14.0% | ||||
| Three different fractions of oligodextran | NMWCO of 10 kDa | Substrates (20–50 g/liter) and enzyme (6250–62,500 Units/liter) | Endo-dextranase (l,6-( |
[42] |
| Low-molecular weight dextran (LMWD) | 0.1–4.0 M sucrose | dextransucrase from Leuconostoc mesenteroides B-512FMCM | [43] | |
| branching 5% to 16.60% |
A synergistic catalytic process performed by two enzymes, dextranase and dextransucrase, was applied to synthesize oligo dextrans of different molecular weights [30]. A new dextran was structurally characterized as having a low branching produced by a dextran–sucrase enzyme sourced from Leuconostoc mesenteroides FT045B [31]. In another study, the main parameter affecting the enzymatic biosynthesis of dextrans was optimized by varying concentrations of enzyme dextransucrase, which was sourced from a different strain B-512FMC of L. mesenteroides, with two other factors—the concentration of sucrose used as the main carbon source in the process and the reaction temperature [32]. The biosynthesis of dextran molecules with exact ratios of alpha-1,2 linkages using transglucosidase GBD-CD2 has been studied by Brison et al. [36]. Low molecular weight dextran molecules with alpha-1,2 branching were prepared using an engineered alpha-1,2 transglucosidase enzyme [38]. The method of directing filtration to narrow the molecular weight distribution of oligo-dextran has been tried by Su et al. [39] in an enzymatic membrane reactor.
The prospects for dextran applications have increased since the clinical importance of dextran biomolecules was first identified a few decades previous in an early report where a dextran with a low molecular weight was used in 85 cases of vascular surgery to prevent early thrombosis after arterial reconstruction [44]. As a result of ongoing research, dextran polymers of different MWs are now commercially available for use in studies researching drug development [15]. Drug delivery products are prepared as bioparticles made of acid, aldehyde, amine, thiol, sulfate, and vinyl sulfone groups modified dextran polymer [45]. Dextran use is widely accepted as an important component of an aqueous two-phase system, which is a decisive technique for extracting and filtration biomolecules, including proteins, membranes, viruses, enzymes, nucleic acids, and others. Hence, the role of dextrans as an important component in aqueous two-phase systems has established its ever-increasing prospects in the pharmaceutical industry [46]. Therefore, researchers focused on the biosynthesis of dextran for large-scale production using bacterial strains of Leuconostoc, Lactobacillus, and Weissella, which secrete extracellular metabolites in the culture medium in the form of a polysaccharide characterized as dextran [47, 48].
Consequently, in the last decade, different methods have been reported to produce various dextran products, either low or higher MW dextrans, as required for specific applications. Such possibilities have increased their wider applications in several industries, including pharmaceutical, cosmetics, and food, as well as dextran as a polysaccharide, which is also used in analytical materials and procedures for research. Owing to various important applications of dextran in pharmaceutical industries, a draft document on dextrans by the European Medicines Agency supported the revision of the annex to the guideline on “Excipients in the labelling and package leaflet of medicinal products for human use” concerning the applications of dextrans [49].
The detailed characterization of each type of prepared dextran is important so that any new dextran obtained from a biocatalytic process [50, 51] could be as useful as a commercial dextran synthesized through a microbial process. However, dextran products produced via enzyme-catalyzed reactions could be of clinical importance for specific applications compared to those produced in microbial fermentation employing actively growing bacterial cells. Enzymatically produced dextran molecules will be free from any contamination of microorganisms and can be easily characterized for their chemical configurations. Although, low MW fractions of high MW dextrans could also be prepared in a shorter time using a chemical process of acid hydrolysis compared to longer enzymatic hydrolysis. However, low MW fractions obtained in the acid process would require further steps for their purification, which would increase the overall cost of their production process. Therefore, a cost-effective method is preferred; for that, there is the prospect of optimizing enzymatic hydrolysis to obtain the required MW dextran molecules.
DD and PSN conceptualized the design of this review article, drafted and wrote the manuscript for first submission. Both authors contributed to editorial revisions and changes suggested by reviewers in the manuscript. Both authors read and approved the final manuscript. Both authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to its accuracy or integrity.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

