Stimulation of dendritic cells (DC) is considered critical in cancer
immunotherapy. BATF-3-dependent subsets, that express in humans CD141 (BDCA-3),
promote CD8 T-cell cross-priming against tumor antigens. Here, we evaluate two
clinical-grade stimuli for peripheral blood CD141 myeloid dendritic cells
(mDCs), a rare DC subset that is currently being explored for use in
immunotherapy. In contrast to routine evaluation methods, which focus on
predefined maturation markers on the surface or factors released from the
activated cells, we applied an unbiased transcriptome-based method using both
RNA-sequencing (RNA-seq) and microarrays. Specifically, we analyzed the mRNA of
CD141 mDCs from five human donors upon activation with two clinical-grade
adjuvants, Hiltonol (poly-ICLC, a TLR3 ligand) and protamine RNA (pRNA, a TLR7/8
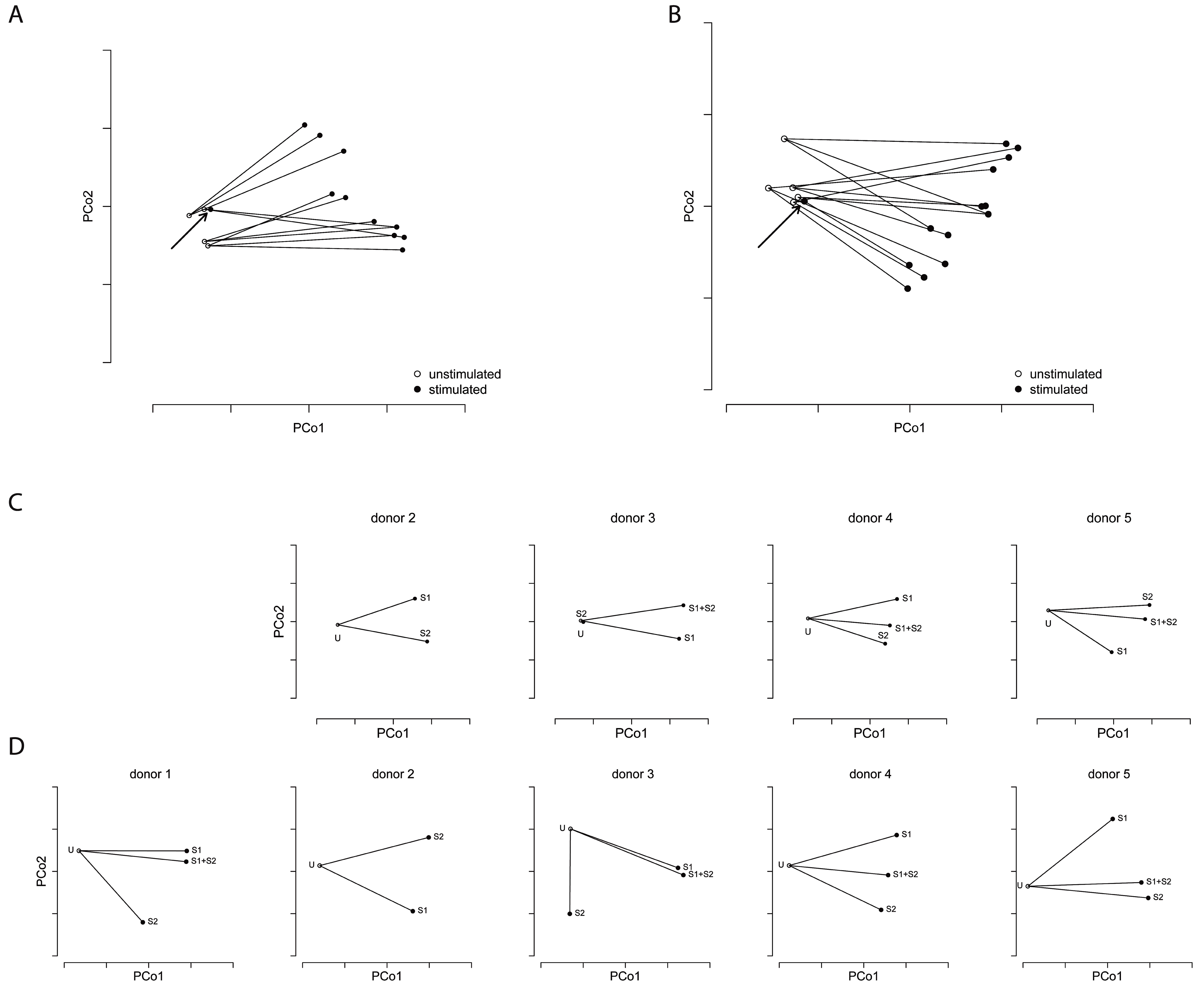
ligand), and compared these samples to unstimulated counterparts. Both methods,
RNA-seq, and microarray showed that Hiltonol and pRNA lead to almost identical
changes in the transcriptome of CD141 mDCs. A gene ontology (GO) term
analysis suggested that these changes were mainly related to activation and
maturation pathways, including induction of type I IFN and IL-12 transcription,
while pathways related to adverse effects or cell damage were not strongly
affected. The combination of both reagents in the DC cultures gave a very similar
result as compared to either stimulus alone, suggesting no synergistic effect.
Furthermore, our analysis demonstrates that microarray and RNA-seq analysis gave
similar conclusions about the activation status of these cells. Importantly,
microarray analyses instead of the advantages of RNA sequencing may still be
suitable for studying the activation of rare cell types that are minimally
represented or in very low frequency in the organism. Together, our results
indicate that both stimuli are potent clinical grade adjuvants with comparable
effects to mature CD141 mDCs in short-term cultures to be used in
immunotherapy.