1 Department of Obstetrics and Gynecology, Nanfang Hospital, Southern Medical University, 510515 Guangzhou, Guangdong, China
2 Department of Radiology, Nanfang Hospital, Southern Medical University, 510515 Guangzhou, Guangdong, China
Academic Editor: Emanuele Perrone
Abstract
Background: To evaluate the preoperative diagnostic efficacy of 3D-MRI
for the depth of myometrial invasion of endometrial carcinoma. Methods:
A total of 116 patients with endometrial carcinoma who had undergone pelvic MRI
before surgery were included. Mimics 21.0 (Materialize Co., Belgium) software was
used to reconstruct three-dimensional MRI models (3D-MRI). The tumor volume and
surface area, uterine volume and surface area were measured, and the tumor volume
ratio was calculated. TVR (Tumor Volume Ratio) = tumor volume/uterine volume, TAR
(Tumor Area Ratio) = tumor surface area/uterine surface area. Postoperative
pathology was used as the gold standard to compare the accuracy, sensitivity,
specificity, negative predictive value, and positive predictive value of
conventional MRI and 3D-MRI in preoperative assessment of endometrial carcinoma
myometrial invasion. Results: The accuracy and specificity of 3D-MRI in
the diagnosis of deep myometrial invasion were better than conventional MRI
(p
Keywords
- endometrial carcinoma
- 3D-MRI
- preoperative
- tumor volume
- tumor surface
Endometrial carcinoma is one of the three major malignant tumors in women, accounting for 20%–30% of malignant tumors in the female reproductive tract. In recent years, the incidence rate has been increasing worldwide, mostly in women over 50 years old, and the peak age of onset is 50 to 59 years old [1]. Early stage patients have a better prognosis, with a 5-year survival rate of more than 95%, compared with 17% for stage IV patients [2].
Preoperative accurate staging of endometrial carcinoma is very important for surgical decision and prognosis. The high-risk factors related to prognosis mainly include: pathological type, histological grade, deep myometrial invasion, vascular space invasion, cervical interstitial invasion, lymphatic metastasis, and extrauterine metastasis. Among them, the depth of myometrial invasion is included in the FIGO 2009 staging. The staging standard is divided into IA stage and IB stage according to the depth of tumor invasion. MRI has become an important imaging method for preoperative evaluation due to its high resolution of soft tissues. However, the accuracy of conventional T2W1 for the depth of invasion is still controversial. Some studies believe that the accuracy is 70–80%, and other study is 55–77% [3]. For postmenopausal women, the uterine atrophy, the obscuration of the junction zone, and the thinning of the myometrium layer also make it difficult to evaluate. When the cancer is small, located in the corner of the uterus, or combined with special factors such as uterine fibroids, the value of conventional MRI for judging myometrial invasion is limited. Moreover, a single layer of a two-dimensional MRI image cannot fully reflect the relationship between the lesion and the myometrium layer, because the scan is only a section in a certain direction, and the largest or smallest diameter of the lesion and the uterus may not be synchronized at the same layer.
In recent years, domestic and foreign scholars have tried to further improve the accuracy of preoperative staging through various new imaging methods such as DWI, DCE-MRI, FLASH-3D [4, 5, 6]. However, the above-mentioned studies require injection of contrast agents. Some patients are unacceptable, sensitive to contrast agents, expensive, and scan time increased significantly. 3D-MRI uses software to reconstruct conventional MRI image data into three-dimensional visualization models of tumors and uterus, simulating the real three-dimensional structure of space, and combining two-dimensional images on the basis of the three-dimensional model to determine myometrial invasion. Currently research in endometrial carcinoma is limited.
Therefore, our study intends to perform preoperative visualization of endometrial carcinoma with three-dimensional reconstruction based on conventional MRI data, to measure the volume and surface area of the tumor and uterus, and to explore the value of 3D-MRI in the preoperative diagnosis of myometrial invasion in early endometrial carcinoma.
This study retrospectively included a total of 222 endometrial carcinoma
patients confirmed by histology in our hospital from January 1, 2011 to December
30, 2020, and excluded complicated uterine fibroids with diameter
Scans were performed on a 3.0-Tesla MRI scanner (Philips, Achieva 3.0T TX) using
an abdominal coil. The examiner took a supine position, with the median sagittal
plane perpendicular to the bed surface, holding the head with both hands, and
stretching the legs together. First, routine coronal, sagittal and transverse
scans were performed, and then fast spin echo sequence T2-weighted imaging
(T2WI-TSE) axial thin-slice scans were performed. The parameters were as follows:
TR/TE, 4920/85 ms; NSA, 2; flip angle, 90; FOV, 280 mm
Import the DICOM format MRI data into Mimics 21.0 (Materialize Co., Belgium) software, and outline ROI area of the images at all levels, including the uterus and lesions, and reconstruct a complete three-dimensional model. Obtain the volume and surface area of the tumor and the uterus, and calculate: TVR = tumor volume/uterine volume, TAR = tumor surface area/uterine surface area. Considering that we only analyze myometrial invasion of endometrial carcinoma, the cervix is not counted.
Two senior imaging physicians and one obstetrician and gynecologist jointly made the evaluation. If the binding zone is visible and the endometrial surface is smooth and continuous, then there is no myometrial invasion. If the junction zone between the inner membrane and the myometrium layer is irregular or incomplete, the myometrium layer is considered to have been invasion. If the signal intensity of the tumor at the T2W1 level is greater than half, it is considered to be a deep myometrial invasion [7]. 3D-MRI’s transparency, contrast, rotation, measurement and other functions, combined with two-dimensional images, to make evaluations together as shown in Figs. 1,2.
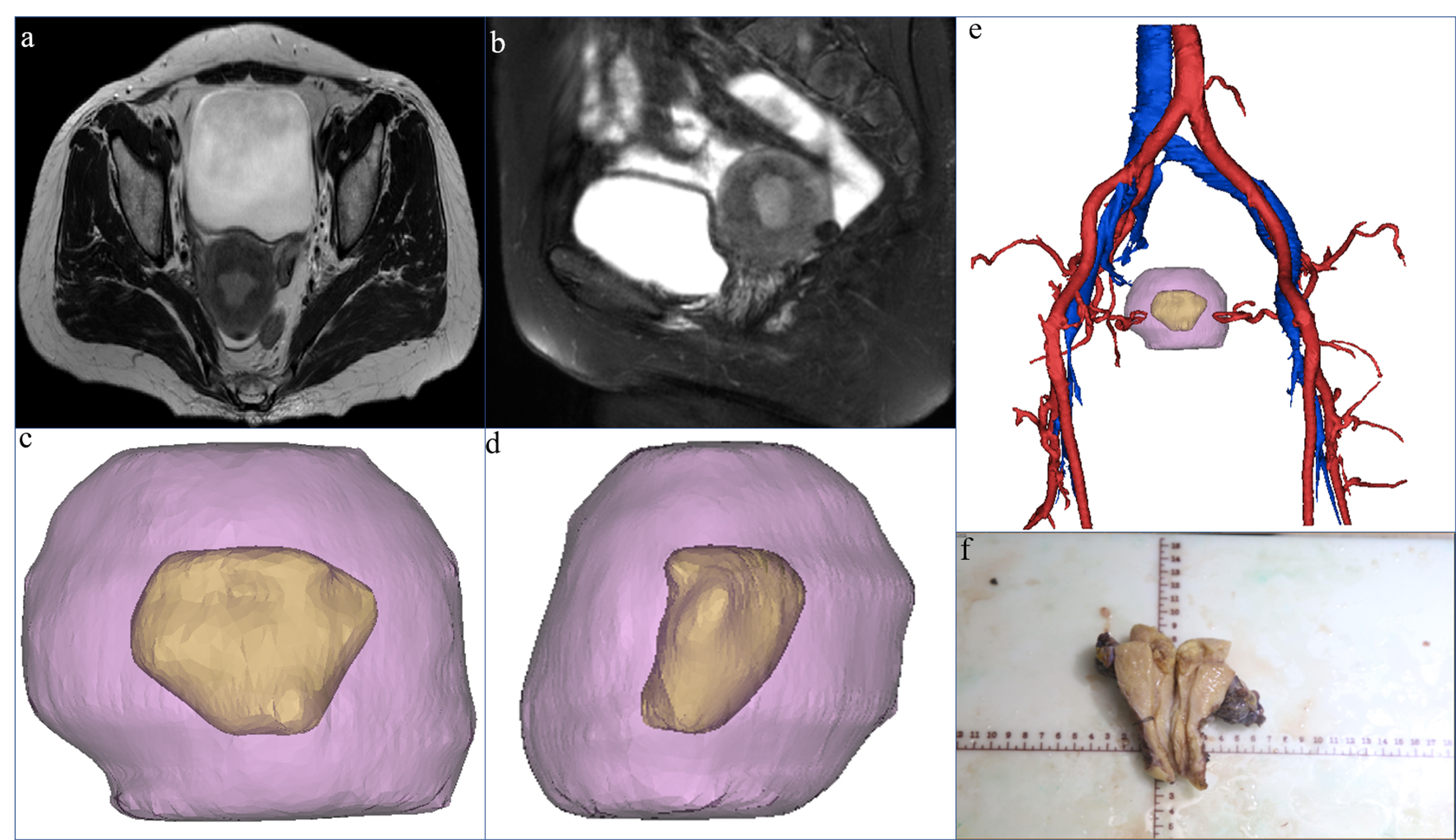 Fig. 1.
Fig. 1.A 53-year-old female patient, preoperative MRI showed both axial
(a) and sagittal (b) deep myometrial invasion. The picture (c,d,e), pink purple
represents the uterus, yellow represents the lesion, red represents the arterial
blood vessel, and blue represents the venous blood vessel. 3D-MRI indicated
superficial myometrial invasion, and showed the three-dimensional positional
relationship between the lesion and the uterus and blood vessels. The uterine
volume was 84462.09 mm
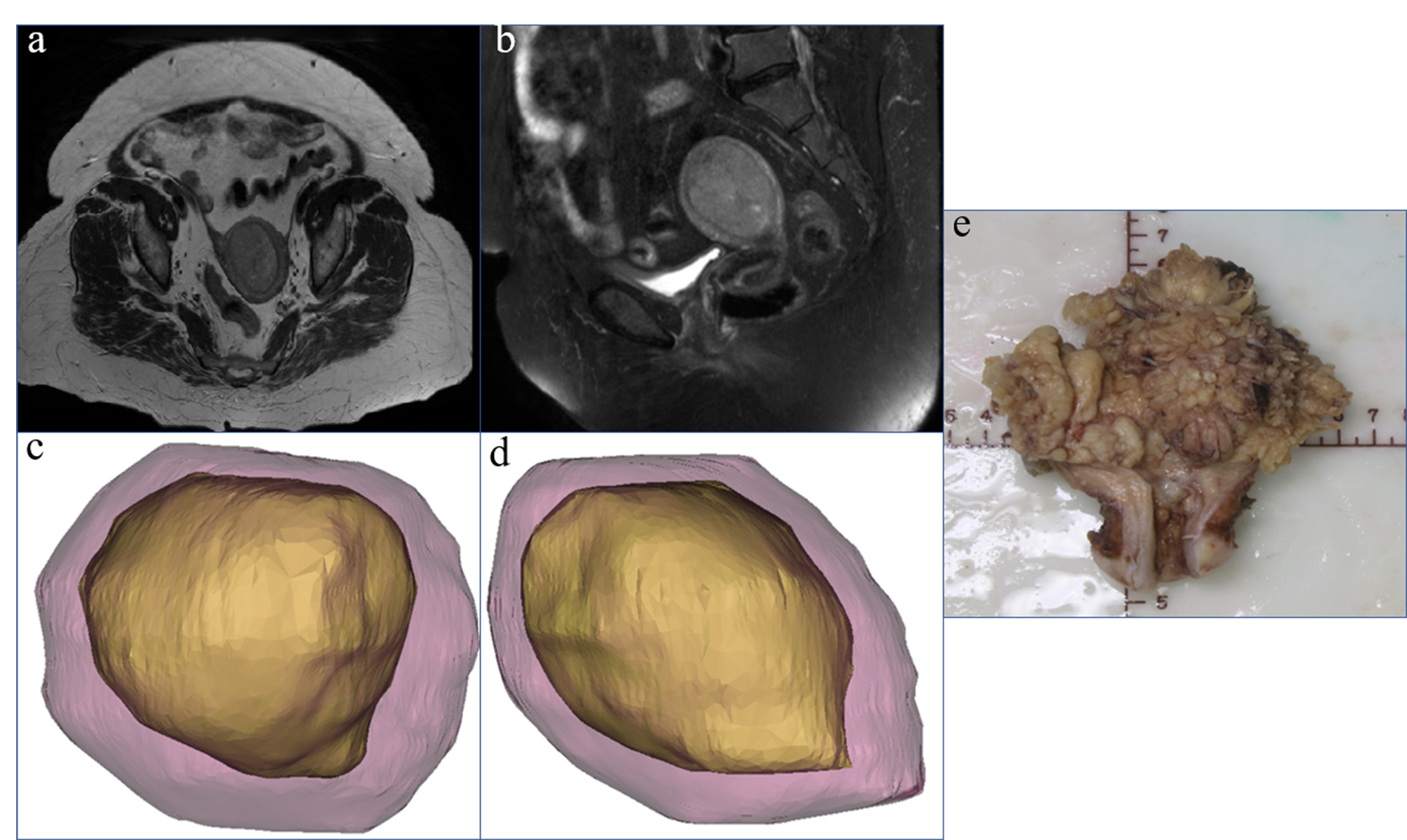 Fig. 2.
Fig. 2.A 53-year-old female patient, preoperative MRI showed both axial
(a) and sagittal (b) deep myometrial invasion. 3D-MRI showed deep myometrial
invasion (c,d). The uterine volume was 113623.07 mm
SPSS 23.0 software (version 23.0, USA) was used for statistical analysis.
Quantitative data are expressed as the mean
A total of 116 patients were identified in the analysis (Fig. 3). The
characteristics of general cases are shown in Table 1. 93 cases were in stage IA
and 23 cases were in stage IB. The mean age of the patients was 53.90
 Fig. 3.
Fig. 3.Flow diagram.
| General characteristics | Mean | |
| Age, years (mean |
53.90 | |
| High risk factors | ||
| Hypertension | 33 (28.4) | |
| Diabetes | 9 (0.7) | |
| BMI | 25.04 | |
| Menopausal status | ||
| Not menopausal | 54 (46.6) | |
| Menopausal | 62 (53.4) | |
| FIGO stage | ||
| IA | 93 (80.2) | |
| IB | 23 (19.8) | |
| Histologic subtype | ||
| Endometrioid adenocarcinoma | 109 (94.0) | |
| Serous carcinoma | 2 (1.7) | |
| Clear cell carcinoma | 1 (0.9) | |
| Mixed carcinoma | 4 (3.4) | |
| Pathological grade | ||
| 1 | 54 (46.5) | |
| 2 | 43 (37.1) | |
| 3 | 19 (16.4) | |
| BMI, Body Mass Index. | ||
Taking the postoperative pathological results as the gold standard, comparing
the evaluation results of conventional MRI and 3D-MRI, the results showed that
the accuracy and specificity of 3D-MRI in diagnosing deep myometrial invasion
were better than conventional MRI (p
| TP (n) | TN (n) | FP (n) | FN (n) | Accuracy (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
| MRI | 17 | 78 | 15 | 6 | 81.9 | 73.9 | 83.8 | 53.1 | 92.8 |
| 3DMRI | 20 | 87 | 6 | 3 | 92.2 | 86.9 | 93.5 | 76.9 | 96.6 |
| TP, True positive; TN, True negative; FP, False positive; FN, False negative; PPV, positive predictive value; NPV, negative predictive value. | |||||||||
The tumor volume, uterine volume, tumor surface area, and uterine surface area
were measured by 3D-MRI, calculated TVR and TAR. The results showed (Table 3)
that there was no statistically significant difference in tumor volume or tumor
surface area between stage IA and stage IB (p
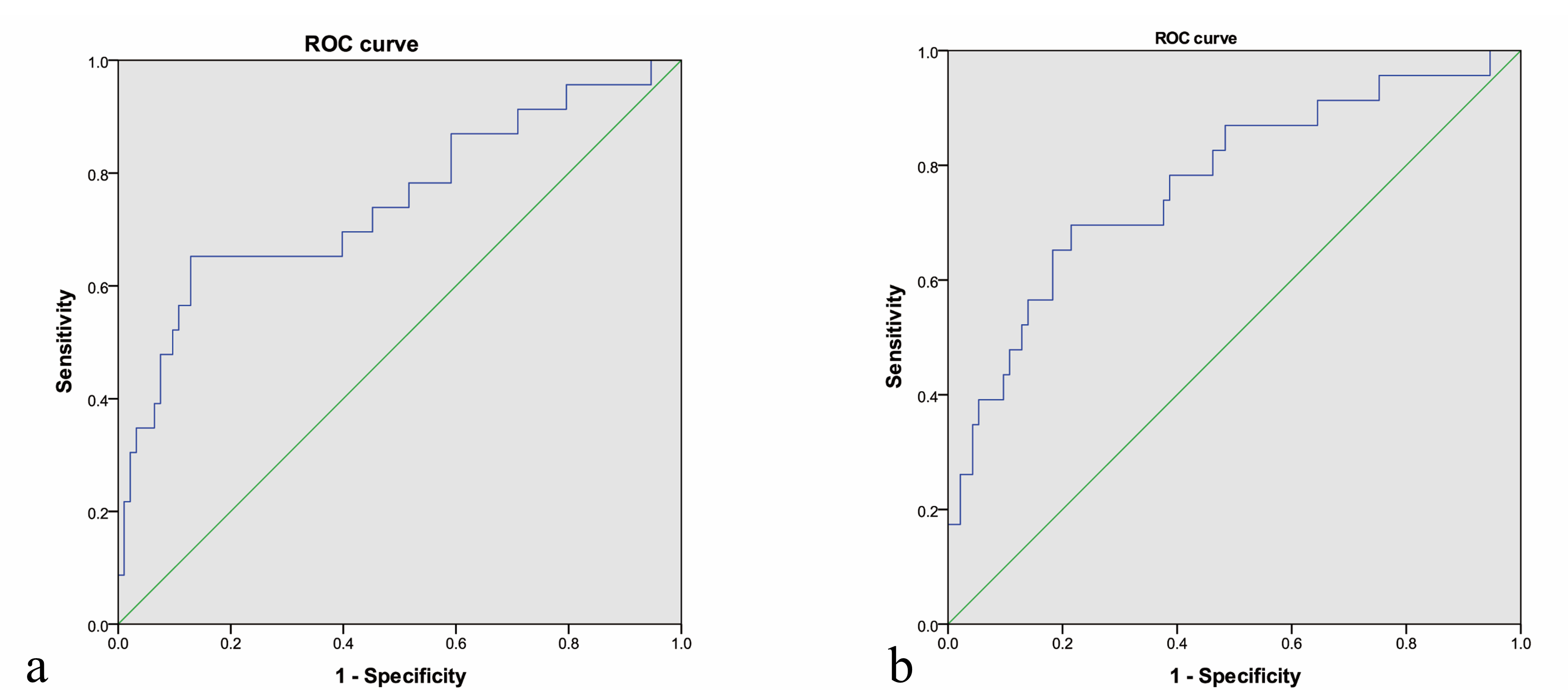 Fig. 4.
Fig. 4.ROC curve of TVR (a) and TAR (b). The picture a showed the area under the ROC curve of TVR was 0.738, and the Youden’s index was 13.59%. The picture b showed the area under the ROC curve of TAR is 0.770, and the Youden’s index is 27.41%.
| IA (n = 93) | IB (n = 23) | p value | |
| Tumor volume (mm |
10111.87 |
37532.96 |
0.158 |
| TVR (%) | 8.05 |
19.12 |
0.002 |
| Tumor surface area (mm |
2895.49 |
5503.00 |
0.084 |
| TAR (%) | 21.38 |
35.15 |
0.001 |
| TVR, Tumor Volume Ratio = tumor volume/uterine volume; TAR, Tumor Area Ratio = tumor surface area/uterine surface area. | |||
The study reconstruct three-dimensional, visualized three-dimensional models on
the basis of conventional MRI, simulate the real three-dimensional structure of
space, and combine two-dimensional images on the basis of the three-dimensional
model to evaluate tumor invasion. The results confirmed that the accuracy and
specificity of 3D-MRI in the diagnosis of early endometrial cancer myometrial
invasion is better than conventional MRI (p
The depth of myometrial invasion of endometrial cancer has always been the focus
of clinicians, and it is also an important factor in determining FIGO staging and
prognosis. For young patients who want to preserve fertility, no myometrium
invasion and, at most, a superficial myometrial invasion [9]. Studies have shown
that on conventional MRI sequences [10], when the largest tumor diameter is
greater than 2 cm, the probability of deep myometrial invasion increases by 10.04
times (95% CI 3.34–30.17, p
There are also scholars who had used the new imaging technology to study this
problem. Liu , Reyes-Pérez and other scholars calculated the apparent
diffusion coefficient (ADC) of diffusion-weighted imaging (DWI), and they all
believed that the ADC value was related to the tumor histological grade and the
depth of myometrium invasion [11, 12]. Yamada found that diffusion tensor imaging
(DTI) is useful for evaluating the depth of tumor invasion and histological
grading in EMC patients. The results of the study showed that the FA map of all
patients clearly identified the junction area as a high FA area (0.864
Similar to our study, Yan discussed the application value of TAR in predicting the deep myometrial invasion and tumor grading of stage I endometrioid adenocarcinoma [16]. This study measured the maximum tumor diameter in three orthogonal planes. Obtain the tumor volume and area, and the results show that TVR and TAR values can be used to distinguish deep and superficial myometrial invasion (p = 0.000). However, the study calculated the volume and area under the assumption that the tumor is a spherical shape. This is obviously inconsistent with the real situation. Whether it is a uterus or a tumor, it is often presented in an irregular three-dimensional shape. The study measured volume obviously exceeds the real tumor volume.
Therefore, in our research, we reconstruct a three-dimensional model close to the real state after ROI outline, and calculate the volume by voxel measurement on the software. The result is more accurate and can reflect the myometrial invasion. The depth of tumor invasion and anatomical structure changes can also be intuitively judged by adjusting the blurred 3D model.
Our research has the following limitations. First, there is a certain degree of subjectivity in the delineation of the tumor ROI area, even for experienced radiologists, there are certain errors; second, the use of software requires a time curve. This requires a process for the popular use of older doctors, but skilled users generally only need 5–10 minutes.
In conclusion, preoperative 3D-MRI can effectively assess the myometrial invasion of early endometrial carcinoma. The best cut-off value is 13.59%, and the best cut-off value of TAR is 27.41%.
TVR, Tumor Volume Ratio; TAR, Tumor Area Ratio; 3D, Three-dimensional; MRI, Magnetic resonance imaging; FIGO, International Federation of Gynecology and Obstetrics.
CC and PL conceptualized,designed the research study. JF performed the research. JF, YC, LX, PS and XL collected data and analyzed the data. JF wrote, reviewed the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
This study was approved by the Ethics Committee of Nanfang Hospital of Southern Medical University, and the ethics number is [2013] Review (032).
Not applicable.
The study was supported by the National Natural Science Foundation of China (grant no. 81571422).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.




