1 Department of Neurology, Shanghai Fifth People’s Hospital Affiliated to Fudan University, 200240 Shanghai, China
2 Department of Neurology, Taizhou Clinical Medical School of Nanjing Medical University, Jiangsu Taizhou People’s Hospital, 225300 Taizhou, Jiangsu, China
Abstract
There are inherent risks associated with intravenous thrombolysis (IVT) therapy in patients with acute ischemic stroke (AIS). The atherogenic index of plasma (AIP), defined as log (triglyceride [TG]/high-density lipoprotein cholesterol [HDL-C]), has recently been associated with the prognosis. We aimed to gauge AIP prognostic value in AIS patients receiving IVT.
We retrospectively collected data from 183 AIS patients who underwent IVT. We grouped modified Rankin Scale scores of 0–2 and 3–6 as good and poor outcomes at 1 year, respectively. Multivariate logistic regression, receiver operating characteristic (ROC) curve and restricted cubic spline (RCS) analyses were used to investigate the underlying link between the AIP and 1-year functional outcomes.
In this study, 67 patients (36.6%) exhibited poor 1-year outcomes. An optimal AIP cut-off of 0.188 was used to divide the patients into low and high AIP levels. Our results showed that continuous AIP (odds ratio [OR] = 25.10, 95% confidence interval [CI]: 4.86–129.68, p < 0.001) was associated with poor 1-year outcome; when AIP was as a categorical variable, OR (95% CI) for the prognosis in the high AIP group was 27.86 (9.33–83.25) compared with the low AIP group. ROC analyses revealed that the area under the ROC curve for the AIP was 0.694 (0.603–0.785), with a sensitivity of 87.1% and a specificity of 61.2%. In the fully adjusted RCS, we found a positive but non-linear trend between the AIP and prognosis.
High AIP may offer potential value as a novel target for predicting 1-year outcomes in patients receiving IVT.
Keywords
- atherogenic index of plasma
- acute ischemic stroke
- intravenous thrombolysis
- risk factor
Acute ischemic stroke (AIS) is a debilitating and devastating disease, with a high global burden that is continuously increasing [1]. Although intravenous thrombolysis (IVT) with recombinant tissue-type plasminogen activator (rt-PA) has continued to occupy a pivotal position in the treatment of AIS, there are inherent risks associated with the process of IVT [2]. It is crucial to develop simple, non-invasive, and affordable biomarkers that could help assess prognosis and guide decision-making for eligible patients.
Atherosclerosis is a progressive disorder of arterial vessels. It is marked by the accumulation of lipids in the inner layer of the artery wall, increasing the incidence of AIS and cardiovascular disease (CVD) [3, 4]. Dyslipidemia is a key contributor to atherosclerosis, and is characterized by abnormal triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels [5, 6]. These findings have spurred further interest in the prognostic relevance of lipid profiles in AIS. The atherogenic index of plasma (AIP) is defined as log (TG/HDL-C) and is negatively correlated with LDL-C levels [7, 8]. Therefore, the AIP serves as a metric to assess the severity of dyslipidemia in patients. Notably, several studies have observed an association between AIP and AIS prognosis [9, 10]. High AIP was correlated with the 3-month clinical outcomes in patients with AIS. However, data regarding the long-term prognosis of patients undergoing IVT are scarce. Therefore, we aimed to further explore the ability of the AIP to predict 1-year functional outcomes in a population of AIS patients receiving IVT by building on previous research [11].
To enhance data consistency and minimize loss to follow-up, 236 patients who underwent IVT and were admitted to Shanghai Fifth People’s Hospital (129 cases) and Taizhou People’s Hospital (107 cases) between January and December 2023 were enrolled between January and December 2023. Furthermore, all patients received standard statin therapy according to the guidelines [12]. Standardized telephone surveys were used to collect follow-up information.
The inclusion criteria for patients: (1) Aged
We collected demographic characteristics from the hospital records, including age, sex, body mass index (BMI), blood pressure, current smoking and drinking status, and medical history (including stroke or transient ischemic attack, coronary heart disease, atrial fibrillation, hypertension, and diabetes mellitus type 2). Neurological function was assessed using the NIH Stroke Scale (NIHSS) scores [13]. The Trial of ORG 10172 in Acute Stroke Treatment (TOAST) criteria were applied to categorize stroke subtypes [14].
We gathered laboratory data, such as white blood cell (WBC), red blood cells (RBC), platelets (PLT), fasting plasma glucose (FPG), glycated haemoglobin A1c (HbA1c), TC, TG, HDL-C, and LDL-C. The AIP parameter was calculated as log(TG/HDL-C) [7].
This study assessed the patients’ neurological function at 1 year. We classified mRS scores of 0–2 and 3–6 as indicating good and poor outcomes, respectively [15].
All statistical analyses were performed using R software (version 4.4.1; R
Foundation for Statistical Computing, Vienna, Austria). For categorical
variables, the Chi-square test was applied. As for non-normally distributed
continuous and ordinal variables, the Kruskal-Wallis test was employed.
Multivariate logistic regression models were performed to explore the
associations between continuous and categorical AIP and the 1-year functional
outcomes. The best AIP cut-off value of 0.188 was determined corresponding the
maximum Youden index (sensitivity – [1–specificity]) by the receiver operating
characteristic (ROC) curve, with AIP divided into low and high levels. The crude
model was a univariable analysis. Model 2 was adjusted for age, sex, BMI. In
Model 3, we further adjusted for diastolic blood pressure (DBP), admission NIHSS,
WBC, RBC, PLT, FPG, HbA1c, TC and LDL-C. In addition, we used ROC models to
assess the predictive abilities of AIP and related lipid profiles. A fully
adjusted restricted cubic spline (RCS) was applied to assess the associations of
the continuous AIP with 1-year functional outcomes. Statistical significance was
set at p
After excluding a total of 53 patients (42 who received bridging therapy; 5 with concomitant aneurysm and/or arteriovenous malformation; 3 with intracranial tumor; 3 without complete data), 183 patients were finally selected. The flowchart is presented in Fig. 1.
 Fig. 1.
Fig. 1.
Flowchart of the current study.
The characteristics of subjects are presented in Table 1. The included
participants had a mean age of 67.16 years, and 65.03% were men. In total, 67
patients (36.6%) had poor outcomes, whereas 73.4% had good outcomes. The AIP
was significantly higher in the poor outcome group (0.15
| Total | Good outcome | Poor outcome | p value | ||
| (n = 116) | (n = 67) | ||||
| Demographics | |||||
| Age, years | 67.16 |
67.31 |
66.90 |
0.432 | |
| Male, n (%) | 119 (65.03) | 67 (56.30) | 52 (43.70) | 0.007 | |
| Body Mass Index, kg/m2 | 23.31 (22.20, 25.60) | 23.31 (22.20, 25.56) | 23.26 (22.20, 25.54) | 0.832 | |
| Clinical assessment | |||||
| Systolic blood pressure, mmHg | 150.00 (136.00, 163.00) | 149.00 (136.00, 162.25) | 150.00 (136.00, 163.00) | 0.808 | |
| Diastolic blood pressure, mmHg | 82.00 (75.00, 94.00) | 81.00 (73.50, 91.25) | 86.00 (79.00, 97.50) | 0.007 | |
| Admission NIHSS | 4.00 (3.00, 8.00) | 4.00 (2.00, 7.00) | 5.00 (3.00, 9.00) | 0.030 | |
| Time to admission, hours | 3.00 (2.00, 4.00) | 3.00 (2.00, 4.00) | 3.00 (2.00, 4.50) | 0.910 | |
| Medical history, n (%) | |||||
| Smoking | 57 (31.15) | 32 (56.14) | 25 (43.86) | 0.171 | |
| Drinking | 37 (20.22) | 24 (64.86) | 13 (35.14) | 0.835 | |
| Hypertension | 121 (66.12) | 78 (64.46) | 43 (35.54) | 0.673 | |
| Diabetes mellitus type 2 | 49 (26.78) | 28 (57.14) | 21 (42.86) | 0.289 | |
| Coronary heart disease | 11 (6.01) | 6 (54.55) | 5 (45.45) | 0.760 | |
| Atrial fibrillation | 28 (15.30) | 19 (67.86) | 9 (32.14) | 0.594 | |
| Stroke or Transient ischemic attack | 19 (10.38) | 10 (8.62) | 9 (13.43) | 0.304 | |
| Stroke subtype, n (%) | 0.444 | ||||
| Small-vessel | 89 (48.63) | 61 (68.54) | 28 (31.46) | ||
| Large artery atherosclerosis | 49 (26.78) | 27 (55.10) | 22 (44.90) | ||
| Cardioembolic | 23 (12.57) | 15 (65.22) | 8 (34.78) | ||
| Undetermined or others | 22 (12.02) | 13 (59.09) | 9 (40.91) | ||
| Laboratory data | |||||
| White blood cell, 109/L | 6.82 (5.50, 8.66) | 6.64 (5.44, 8.05) | 7.44 (5.66, 9.75) | 0.026 | |
| Red blood cell, 109/L | 4.40 (4.09, 4.85) | 4.33 (3.98, 4.82) | 4.64 (4.22, 4.95) | 0.017 | |
| Platelet, 109/L | 192.26 |
187.17 |
201.09 |
0.125 | |
| Fasting plasma glucose, mmol/L | 5.90 (4.99, 7.38) | 5.61 (4.93, 6.88) | 6.20 (5.46, 8.00) | 0.015 | |
| HbA1c, (%) | 5.90 (5.50, 6.70) | 5.90 (5.50, 6.50) | 6.00 (5.60, 7.40) | 0.046 | |
| TC, mmol/L | 4.40 (3.60, 5.17) | 4.20 (3.27, 5.08) | 4.60 (3.90, 5.29) | 0.027 | |
| TG, mmol/L | 1.15 (0.87, 1.68) | 1.10 (0.80, 1.40) | 1.49 (1.02, 2.15) | ||
| HDL-C, mmol/L | 1.13 (0.93, 1.34) | 1.21 (1.01, 1.36) | 0.97 (0.86, 1.25) | ||
| LDL-C, mmol/L | 2.78 (2.05, 3.35) | 2.61 (1.89, 3.29) | 2.91 (2.34, 3.38) | 0.039 | |
| Atherogenic index of plasma | 0.03 |
–0.03 |
0.15 |
||
Normally distributed continuous variables are presented as means
NIHSS, National Institutes of Health Stroke Scale; HbA1c, glycated haemoglobin A1c; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
In the fully adjusted regression models, AIP as a continuous variable (odds ratio [OR]: 25.10; 95% confidence interval [CI]: 4.86–129.68) was associated with 1-year poor outcomes. When AIP was set as a categorical variable, ORs (95% CI) with the high AIP were 27.86 (9.33–83.25) for the prognosis compared with the low AIP (Table 2). ROC analyses showed that the best cut-off AIP value was 0.188. The sensitivity was 87.1%, the specificity was 61.2%, and the area under the ROC curve (AUC) of the AIP was 0.694 (0.603–0.785), which was preferable to other related lipid profiles (0.598 for TC, 0.663 for TG, 0.656 for HDL-C, and 0.592 for LDL-C) (Table 3 and Fig. 2). We also applied adjusted RCS plots to reveal the potential dose-manner associations between the AIP and poor 1-year outcome (Fig. 3). Here, we observed that high AIP was associated with a higher risk of 1-year outcomes, and a positive but non-linear trend was observed.
| Model 1 | Model 2 | Model 3 | ||||
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| AIP | 12.85 (3.70 |
15.06 (3.90 |
25.10 (4.86 |
|||
| Low | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | |||
| High | 10.62 (5.11 |
14.32 (6.23 |
27.86 (9.33 |
|||
Multivariate logistic regression results are presented as ORs and 95% CIs.
Model 1: Crude.
Model 2: Adjusted for age, sex, BMI.
Model 3: Adjusted for age, sex, BMI, and DBP, admission NIHSS, WBC, RBC, PLT, FPG, HbA1c, TC, LDL-C.
OR, Odds Ratio; CI, Confidence Interval; AIP, atherogenic index of plasma.
| AUC | 95% CI | Sensitivity | Specificity | p value | |
| AIP | 0.694 | (0.603–0.785) | 0.871 | 0.612 | |
| TC | 0.598 | (0.516–0.681) | 0.250 | 0.955 | 0.053 |
| TG | 0.663 | (0.576–0.750) | 0.784 | 0.537 | |
| HDL-C | 0.656 | (0.567–0.746) | 0.707 | 0.642 | 0.008 |
| LDL-C | 0.592 | (0.509–0.674) | 0.293 | 0.925 | 0.065 |
Receiver operating characteristic curve results are presented as AUC with 95% CI, sensitivity, and specificity.
AUC, Area Under the Curve; CI, Confidence Interval; AIP, atherogenic index of plasma.
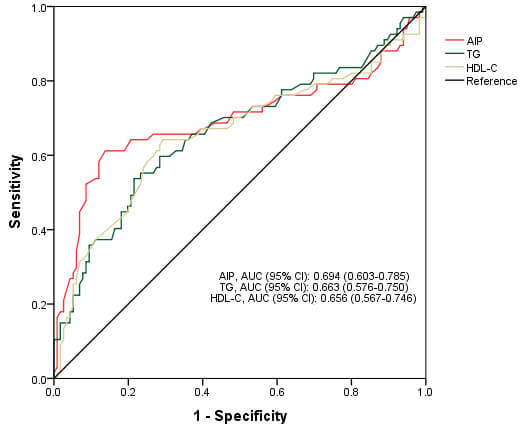 Fig. 2.
Fig. 2.
Receiver operating characteristic curve of AIP for predicting 1-year functional outcome in patients with acute ischemic stroke receiving intravenous thrombolysis.
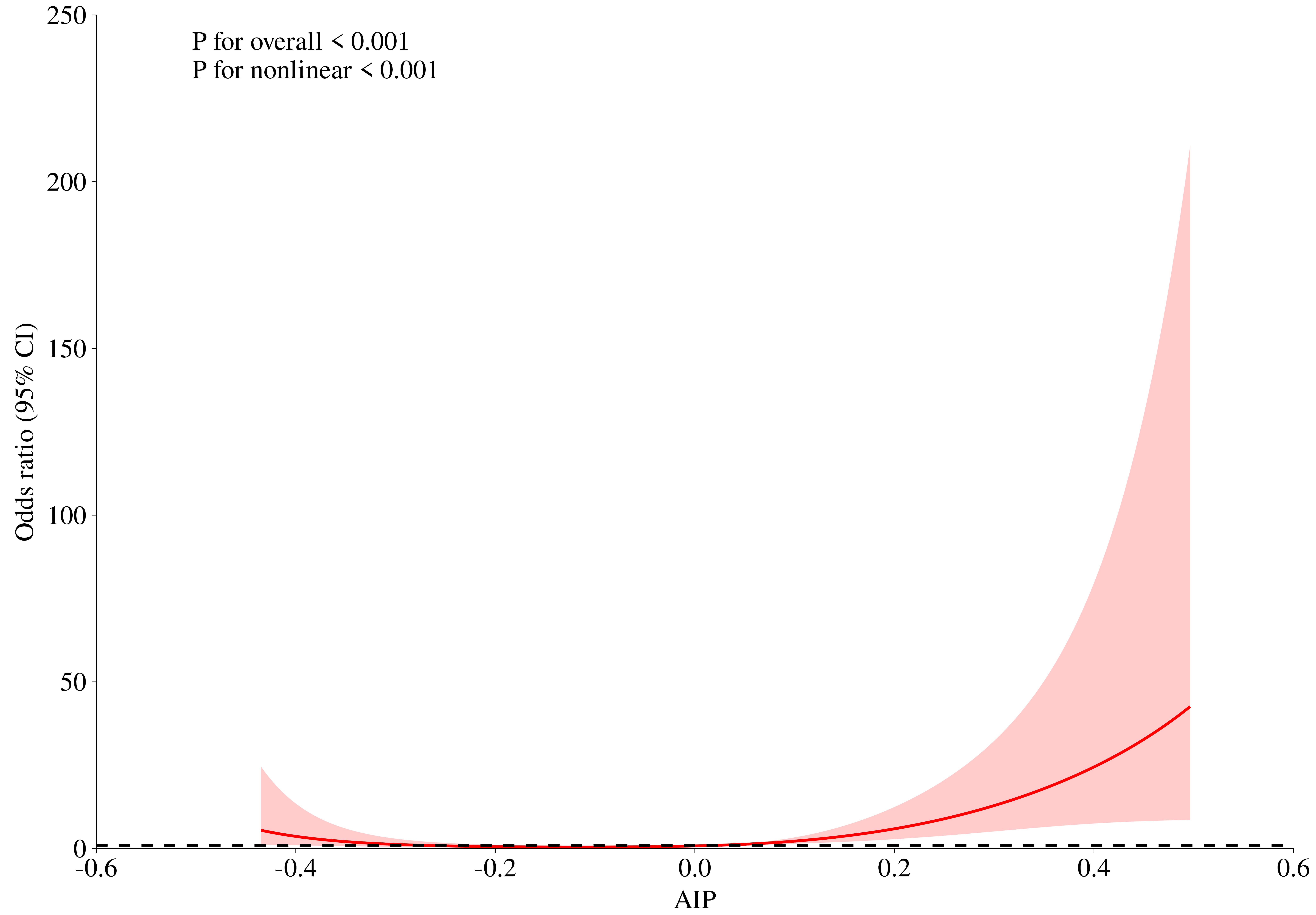 Fig. 3.
Fig. 3.
Association between AIP and 1-year functional outcome in patients with acute ischemic stroke receiving intravenous thrombolysis using restricted cubic spline analysis.
This is the first research focusing on AIP-related differences in the prognosis of AIS patients receiving IVT. High AIP was associated with poor 1-year functional outcomes. Additionally, we observed a positive but non-linear relationship between AIP and prognosis. These findings underscore the importance and need to consider the AIP levels when making medical decisions for AIS patients, consistent with the growing data suggesting that atherosclerosis plays a crucial role in AIS [16].
Atherosclerosis is the key cause of AIS and CVD [3, 4, 5, 6]. Thus, numerous lipid profiles have been used to evaluate the functional outcomes of AIS. However, traditional single index (TC, TG, HDL-C, LDL-C) as the evaluation of AIS was still limited and exhibited low predictive value. Small dense LDL-C (sdLDL-C) is significantly high associated with atherosclerosis, as is the case with AIS [17]. Furthermore, sdLDL-C detection is both difficult and costly, creating a need for an inexpensive and reliable tool to assess the degree of atherosclerosis in given patients. The AIP is a routine index that can indirectly reflect sdLDL-C levels [18]. Importantly, AIP can be easily computed using TG and HDL-C values, making it an inexpensive and reliable tool. The prognostic ability of the AIP has been suggested in CVD and the instability of carotid plaque [19, 20]. A 2024 study confirmed the associations of AIP with the 3-month outcomes of AIS [21]. However, no studies have examined the potential relationship between the AIP and long-term prognosis of AIS patients, regardless of receiving IVT. Our findings could extend the explanation, as we revealed that high AIP was linked to the 1-year poor outcome, with a positive but non-linear trend seen. When patients had high AIP, they had a higher risk of 1-year poor outcome compared with those with low AIP. Our study suggests that high AIP may offer greater predictive value than other related conventional lipid profiles. Given its ease of measurement and high predictive value, the AIP serves as an ideal tool for assessing patients with AIS, helping to better predict functional outcomes before IVT.
Despite the unclear mechanisms, several possible explanations may be proposed. High TG levels have been implicated in vascular subclinical atherosclerosis and might intensify the inflammatory reactions in both smooth muscle cells and vascular endothelial cells [22]. Conversely, HDL-C could exert multiple vasoprotective effects, including reducing apoptosis, mitigating inflammation, and protecting against oxidative stress [23]. Accordingly, the fact that AIP values offer simultaneous information obtained from patients’ TG and HDL-C levels could reflect pro-inflammatory and atherosclerotic effects modulated by high TG levels and the reduced anti-inflammatory HDL-C responses. Participants with high AIP tended to have higher BMI and HbA1c levels and were more likely to be smokers or drinkers, all of which led to AIS.
Our study, however, has several limitations. As a two-center, single-year investigation, its relatively small sample size may have limited the statistical power. Moreover, we did not assess the dynamic changes in AIP during hospital stay, an aspect that warrants further investigation in future studies.
In summary, high AIP was associated with poor 1-year functional outcomes in AIS patients receiving IVT, with a positive but non-linear relationship between AIP and prognosis. Future larger-scale studies are needed to clarify its clinical application.
The datasets are available from the corresponding author on reasonable request.
RS: conceptualization, investigation, formal analysis, writing. ZW: designing the research study, performing the research, writing. Both authors read and approved the final manuscript. Both authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
This study was approved by the ethics committee of Taizhou Clinical Medical School of Nanjing Medical University (KY2024-124-01) and the Shanghai Fifth People’s Hospital (2024-096). Informed consent was waived because of the retrospective nature. The study was carried out in accordance with the guidelines of the Declaration of Helsinki.
Not applicable.
This study was funded by Minhang District natural science research project funding (2022MHZ039).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.



