1 Department of Cardiology, The Second Affiliated Hospital, School of Medicine, Zhejiang University, 310009 Hangzhou, Zhejiang, China
2 State Key Laboratory of Transvascular Implantation Devices, 310009 Hangzhou, Zhejiang, China
3 Heart Regeneration and Repair Key Laboratory of Zhejiang Province, 310009 Hangzhou, Zhejiang, China
Abstract
Heart failure (HF) is a heterogeneous clinical syndrome, the prevalence of which is increasing among younger adults, promoting global concern due to its significant morbidity and mortality. Therefore, predicting the occurrence of HF using risk-related biomarkers is essential for screening and prevention. Retinol-binding protein 4 (RBP4) is a 21 kDa secreted factor produced by the liver and adipose tissue. Elevated serum RBP4 levels are consistently observed in HF patients and are associated with different New York Heart Association (NYHA) class and left ventricular dysfunction. In addition to its role in retinol transport, emerging evidence suggests that RBP4 contributes to the pathogenesis of HF by inducing insulin resistance, triggering chronic inflammation, and directly injuring cardiomyocytes. Studies have found that RBP4 is a potential diagnostic biomarker for HF; however, its clinical relevance is limited due to a paucity of clinical studies and basic science research. This article reviews the current clinical and experimental evidence regarding the pathophysiological effects of RBP4 related to its role in the progression of HF.
Keywords
- retinol-binding protein 4
- heart failure
- inflammation
- oxidative stress
Heart failure (HF) impacts approximately 56 million individuals globally and is increasing due to an aging population with increased risk factors. Moreover, young adults (15–44 years) are experiencing an increase in mortality (age-adjusted rate: 3.16 in 2019) compared to older adults (75+ years). Meanwhile, data from Europe and North America indicate a significant rise in HF with preserved ejection fraction (HFpEF), especially among women [1, 2]. Currently, the diagnosis of HF primarily relies on a Clinical Triad as reflected in several well-established diagnostic systems, such as the Framingham criteria, which may not consistently provide appropriate sensitivity and specificity data [3]. The European Society of Cardiology (ESC) guidelines are more accurate and are established on evidence-based medicine (e.g., integration of N-terminal pro-brain natriuretic peptide and imaging criteria). However, these guidelines are limited in providing explicit recommendations for the clinical utilization of emerging biomarkers [4, 5].
Retinol-binding protein 4 (RBP4), a plasma secreted protein, is primarily released by the liver and adipose tissue. RBP4 is recognized mainly for its crucial role in transporting vitamin A, which is essential for maintaining vision, immunity, skin health, and cellular processes [6]. Additionally, the involvement of RBP4 in insulin resistance and systemic inflammation has been extensively documented. Recent research has highlighted that RBP4 exhibits cardiac-specific effects, including myocardial fibrosis and hypertrophy, which are mediated by the Toll-like receptor 4 (TLR4)/myeloid differentiation primary response 88 (MYD88) pathway [7, 8]. Furthermore, RBP4 levels have been shown to increase significantly, by 2–3 fold, in the serum of HF patients compared to healthy controls [9], and are negatively correlated with left ventricular shortening fraction (LVFS) and ejection fraction (LVEF) (p
RBP4 belongs to the lipocalin family and is a 21 kDa plasma protein first described by Masamitsu Kanai in 1968 [12]. Kanai et al. [12] conducted a study examining the plasma of patients who had received radiolabeled retinol by injection and were able to isolate and purify the protein bound to the labeled retinol, which was named RBP4, and is a single polypeptide chain consisting of 201 amino acids and three disulfide bridges in humans [13]. RBP4 performs as a lipocalin-fold tertiary structure, which contains a N-terminal loop, a core
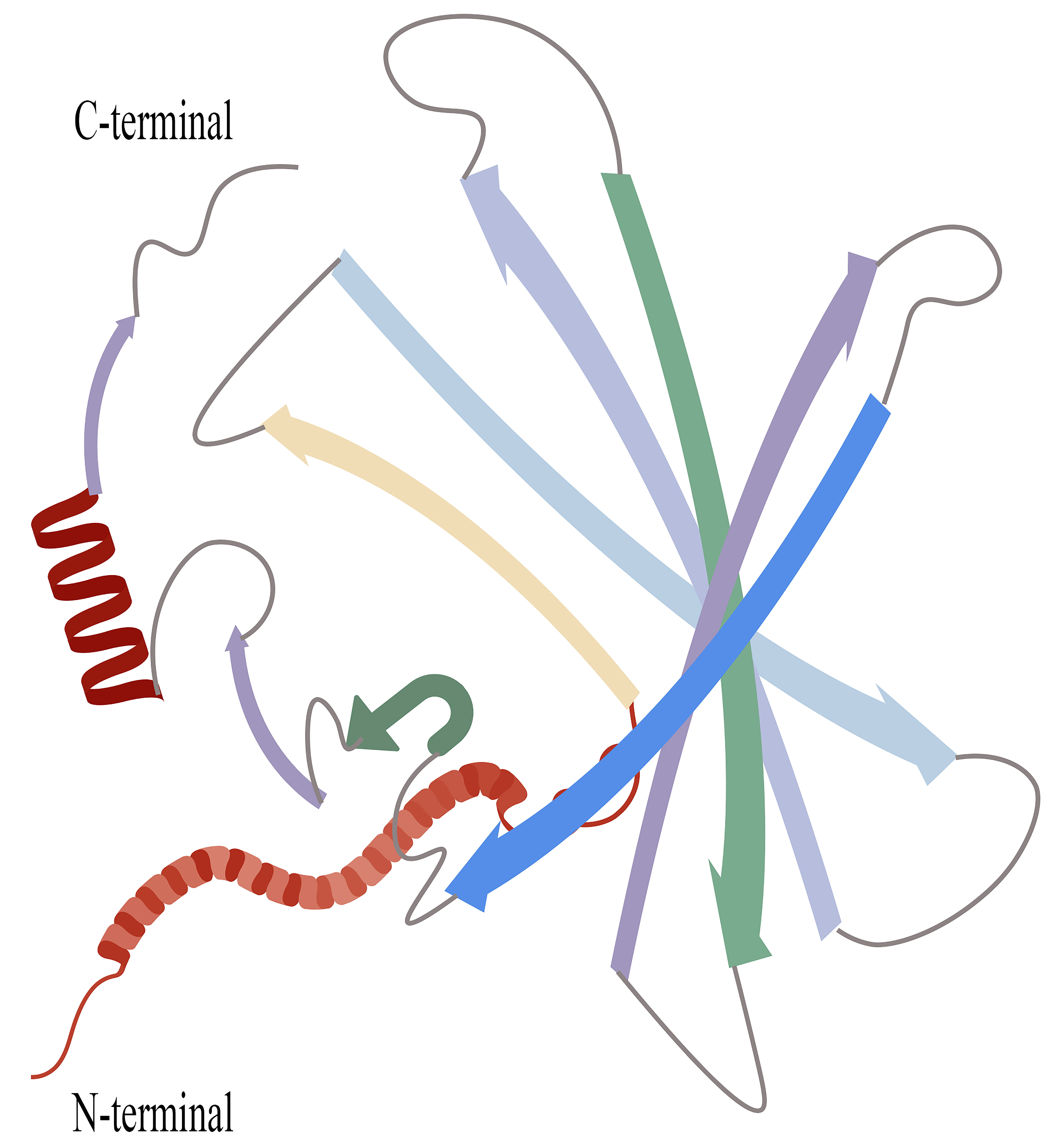 Fig. 1.
Fig. 1. Structure of RBP4. Created with BioGDP.com. The eight arrows on the right side of the diagram represent the eight antiparallel
The circulating RPB4 concentration is ~2–3 µM in humans and ~1 µM in mice [17]. The hepatocytes are the primary site responsible for the synthesis of RBP4, and mature adipocytes are the secondary site, secreting approximately 20% [18]. Mice with hepatic RBP4 gene-specific knockout have been shown to possess undetectable serum levels of RBP4, proving that adipocyte RBP4 is not a significant source of circulating RBP4 in mice, even in the setting of insulin resistance [19]. In addition, human kidneys, retinal pigment epithelial cells, peritubular cells of the testis, Sertoli cells, spleen, brain, stomach, small intestine, pancreas, and choroid plexus have different levels of RBP4 mRNA [20, 21].
RBP4 has a critically important role in retinol homeostasis in vivo, whereby RBP4 is responsible for transporting retinol from the liver to peripheral tissues; furthermore, circulating levels of RBP4 are positively correlated with the concentration of retinol [22]. There are two main forms of RBP4 involved in retinol transport in the blood. The one bound to retinol is called holo-RBP4, and is the main form of RBP4 found under normal physiological conditions. Holo-RBP4 is highly lipophilic and is responsible for transporting vitamin A to peripheral tissues, especially retinal and epithelial tissues, after its synthesis in the liver. The form not bound to retinol is called apo-RBP4 and normally accounts for only 13–17% of the total RBP4. The ratio of apo-RBP4 in the blood may be increased in the presence of reduced retinol levels (e.g., vitamin A deficiency) or disturbed RBP4 metabolism [16]. When RBP4 function is impaired, the uptake of LPL-mediated lipoprotein-derived retinyl esters may be an important alternative source of cellular retinoids [23].
RBP4 homeostasis relies on binding to the plasma thyroxine transport protein (TTR) in the endoplasmic reticulum (ER) endomorphism [24, 25]. The TTR monomer also features eight antiparallel
There are two main RBP4 receptors involved in retinol metabolism: those stimulated by retinoic acid 6 (STRA6) and the others stimulated by retinoic acid 6-like (STRA6L). STRA6 is mainly expressed in extrahepatic tissues, with the highest expression in the retinal pigment epithelium (RPE) [32, 33]. The function of STRA6 in bidirectional transport is mediated by esterification via lecithin: retinol acyltransferase (LRAT) [34]. When binding with RBP4, STRA6 can facilitate the uptake of extrahepatic cells and transport retinol from holo-RBP4, where intracellular retinol binds to cellular retinol-binding protein 1 (CRBP1) for storage and metabolism. The prerequisite is that holo-RBP4 must be isolated from the TTR to allow the assembly with the STRA6 dimer, which subsequently enters the lipophilic cleft [35, 36]. Mutations in the STRA6 gene can lead to the Matthew-Wood syndrome, the main features of which include microphthalmia, pulmonary hypoplasia [2], heart defects, and diaphragmatic hernias [37]. STRA6 can also impair insulin signaling by enabling tyrosine phosphorylation to activate Janus kinase 2 (JAK2)/signal transducer and the activator of transcription 5 (STAT5) signaling pathway. STRA6L, also known as retinol-binding protein 4 receptor 2 (RBP4R2), represents the main receptor in the liver and intestine, and shares 20% homology with STRA6. Notably, STRA6L is mainly responsible for the retinol uptake by hepatocytes. Meanwhile, studies have shown a possible negative feedback mechanism in regulating STRA6L expression through hepatic retinol storage capacity [38, 39]. SYL residues (S294, Y295, and L296) in mouse RBPR2 have been previously shown to be important for RBP4–retinol (ROL) binding and retinol uptake using in vitro and CRISPR mutant zebrafish models [40]. Both receptors regulate the abundance of retinoids in vivo, indirectly affecting the activation of the retinoic acid receptor (RAR)/retinoid X receptor (RXR) complex and influencing their gene transcription and expression, which subsequently modulate the effects of retinoids on cell proliferation, differentiation, and immune responses [28]. However, the effect of retinol status on RBP4 levels has not been uniformly verified, particularly regarding its species-specific effects. Soprano et al. [41] confirmed in animal experiments that hepatic RBP4 levels did not differ significantly in rats with altered retinol concentrations. In contrast, a study by Hermsdorff et al. [42] in non-obese Spanish women showed a positive correlation between vitamin A intake and RBP4 concentrations. Therefore, further studies are warranted to elucidate the interaction mechanisms among retinol, RBP4, and their cognate receptors.
The RBP4 gene is located at 10q23.33, with a full length of 10,050 bp, and contains six exons and five introns in its mRNA, which is approximately 1070 bp in length [43]. The regulation of RBP4 gene expression is a multilevel process.
At the transcriptional level, hepatocyte nuclear factor 1 alpha (HNF1A) is an important transcription factor in the synthesis of RBP4 in hepatocytes. HNF1A binds to a specific site in the 5′ side region of the RBP4 gene responsible for the high hepatic transcriptional promoter region, and recruits other co-activators and RNA polymerases that activate RBP4 gene transcription [44]. In a study by Munkhtulga et al. [45], rs3758539, i.e., -803 G
There are several post-translational modifications (PTMs) of RBP4. Indeed, methylation of RBP4 was first reported in an esophageal cancer model, where treatment with the demethylating agent 5-aza-2′-deoxycytidine (aza-dC) restored RBP4 expression, confirming its role in epigenetic silencing [52]. Phosphorylation of RBP4 also plays an important role in several biological processes. Notably, phosphorylated RBP4 has been shown to promote denervation-induced muscle atrophy and correlates with the elevated expression of muscle atrophy markers such as atrogin-1 and MuRF1, via the STRA6/JAK2/STAT3 pathway [53]. These coordinated mechanisms ensure precise spatiotemporal control of RBP4 production.
Hormonal and adipokine regulation also significantly influences RBP4 levels. A well-established bidirectional relationship exists between insulin resistance and elevated RBP4 levels [54]. Furthermore, adipose-derived hormones such as leptin and lipocalin modulate RBP4 levels. Multivariate regression analyses have identified leptin as a positive predictor of RBP4 in both subcutaneous and visceral adipose tissue in women; meanwhile, lipocalin serves as a predictive factor for RBP4 specifically in male visceral adipose tissue [55]. Conversely, experimental evidence demonstrates that atrial natriuretic peptide (ANP) directly modulates the secretory activity of adipose tissue, thereby reducing the generation of RBP4 [56]. Nonetheless, the specific molecular pathways through which these neuroendocrine factors regulate RBP4 gene expression await further investigation.
There are numerous SNPs associated with a high risk of cardiovascular disease (CVD) in different regions of the RBP4 gene. In a study among Spanish children, rs3758538, located in the 5′ flanking region of the first promoter, and rs12265684, located in the non-coding region between exons 4 and 5, were shown to be associated with blood pressure. Mean arterial pressure was higher in minor allele C carriers than in major allele G carriers [57]. In another study, rs7094671, another non-coding region located between exons 4 and 5, was confirmed to be associated with an increased risk of developing CAD. The A allele was more suggestive than the G allele, but this SNP was not confirmed to be related to the severity of CAD in this study [58, 59]. Some SNPs may be associated with the protection of patients with CVD, but the mechanisms underlying this association remain incompletely understood. In a study of a Chinese Han population, the minor C allele rs3758538 was significantly associated with a lower risk of hypertriglyceridemia. The Shanghai subgroup with minor G allele rs17108993 showed a lower risk of hypertensive disease [60].
Several studies have demonstrated that RBP4 is a promising biomarker for predicting HF and related adverse events. In a prospective cohort study in older adults with chronic heart failure (CHF), serum RBP4 concentration showed a positive correlation with New York Heart Association (NYHA) classification guidelines and a negative correlation with LVEF (p
Recent research indicates that the circulating levels of RBP4 are substantially influenced by modifiable lifestyle factors, which may, in turn, affect the risk of HF. Elevated RBP4 concentrations have been consistently shown to correlate with several metabolic and behavioral risk factors. Specifically, obesity and proinflammatory dietary patterns, particularly high-fat/high-carbohydrate Western diets, have been demonstrated to elevate RBP4 concentrations significantly in clinical models [62]. Additionally, research conducted by Gao et al. [63] involving normoglycemic healthy males, as well as a cross-sectional study by Hong et al. [64], indicated that smoking tobacco and chronic alcohol consumption are independent behavioral risk factors linked to elevated RBP4 levels. This diet-induced dysregulation of RBP4 exacerbates insulin resistance and systemic inflammatory responses, ultimately accelerating the pathophysiology and progression of HF [65].
Sleep and mental disorders have also been linked to raised RBP4 levels, which are positively correlated with a heightened incidence of HF. Obstructive sleep apnea syndrome (OSAS) is a chronic inflammatory condition that results in upper airway obstruction during sleep. Untreated OSAS causes HF and other CVDs [66]. RBP4 was found to exhibit a positive correlation with the apnea–hypopnea index (AHI) in a study of OSAS patients (r = 0.47; p
In addition to the aforementioned risk factors, several studies have demonstrated that RBP4 levels are also significantly associated with various cardioprotective behaviors [71], as detailed in Table 1 (Ref. [63, 64, 67, 68, 70, 72, 73, 74, 75, 76, 77, 78, 79]). Resistance training and high total physical activity levels have been shown to reduce circulating RBP4 concentrations [72]. Similarly, specific dietary patterns, such as the Mediterranean and ketogenic diets [73], demonstrate comparable effects. These findings not only support the role of RBP4 as a potential biomarker for HF risk but also suggest its utility as a modifiable preventive target, highlighting its dual function in lifestyle intervention strategies.
| Factors or condition | Year (Ref) | Study design | Study population | Correlation | Study size | Analysis | |
| Risk factors | Cigarette smoking | 2012 [63] | Cross-sectional analysis | Healthy male subjects with normal glucose tolerance | + | 136 | Correlation |
| Chronic alcohol intake | 2022 [64] | Cross-Sectional Study | Han Chinese adults aged | + | 2075 | Correlation | |
| Sedentary lifestyle | 2018 [74] | Cross-sectional study | Sedentary T2D patients | + | 106 | Multivariate regression | |
| High salt intake | 2021 [75] | Cross-sectional study | Healthy Chinese subjects | + | 42 | Correlation | |
| Low AHI | 2023 [76] | Case–control study | OSAS group and HC | + | 171 | Correlation | |
| Irregular sleep habits | 2023 [67] | Cross-sectional study | Workers from the OHSPIW cohort | + | 1499 | Multivariate regression | |
| Shorter sleep duration | 2017 [68] | Cross-sectional study | School-aged children | + | 3166 | Multivariate regression | |
| Mental disorder | 2020 [70] | Case–control study | MDD patients and HC | + | 285 | Correlation | |
| Obesity | 2022 [77] | Cohort study | Non-diabetic participants aged | + | 784 | Correlation | |
| Protective factors | Resistance exercise | 2010 [72] | Cohort study | Female patients with T2D | – | 44 | Correlation |
| High levels of total physical activity | 2009 [78] | Cross-sectional study | Chinese people aged 50–70 years | – | 3289 | Multivariate regression | |
| Ketogenic diet | 2023 [73] | Cohort study | Middle-aged male patients with metabolic syndrome | – | 40 | Correlation | |
| Low-fat diet | 2016 [79] | Cross-sectional study | Patients with hypertriglyceridemia | – | 46 | Correlation | |
+, positive correlation; –, negative correlation; T2D, type 2 diabetes; OSAS, obstructive sleep apnea syndrome; AHI, apnea–hypopnea index; MDD, major depressive disorder; HC, health control.
Beyond the prognostic value of RBP4, these studies have shown its significant associations with biological risk factors and heart diseases related to HF, as detailed in Table 2 (Ref. [9, 10, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90]).
| Factors or condition | Year (Ref) | Study design | Study population | Positive correlation event | Study size | Analysis |
| CHF | 2020 [80] | Cohort study | CHF patients aged | Cardiovascular mortality re-hospitalization | 1072 | Multivariate regression |
| 2018 [9] | Cross-sectional analysis | participants of the PolSenior study aged | Renal function | 2826 | Correlation | |
| Hypertension | 2022 [81] | Case–cohort study | EPIC-Potsdam cohort | Cardiometabolic risk | 27,548 | Multivariate regression |
| 2024 [82] | Cross-sectional study | EH with T2D and HC | Disease state | 119 | Correlation | |
| 2019 [10] | Cross-sectional study | EH and HC | E/A on echocardiogram | 120 | Correlation | |
| Hyperlipidemia | 2018 [83] | Cross-sectional study | Participants in the PolSenior study aged | Hypertriglyceridemia, hypertension | 3038 | Multivariate regression |
| 2018 [84] | Cohort study | School-aged children | Triglyceride concentration, insulin resistance | 352 | Multivariate regression | |
| Diabetes mellitus | 2022 [85] | Case–control study | DCM and HC | Disease state | 347 | Multivariate analysis |
| Coronary artery disease | 2020 [86] | Case–control study | ACS patients and non-CAD patients | CAD diagnosis | 240 | Multivariate regression |
| 2023 [87] | Cross-sectional study | T2D patients with/without CHD | Coronary artery wall elastic | 130 | Multivariate regression | |
| 2022 [88] | Cohort study | Patients with stable CAD | MACEs | 840 | Multivariate regression | |
| 2021 [89] | Case–control study | ACS patients and patients with cardiovascular risk factors but normal angiography | CAD severity | 98 | Correlation | |
| Cardiomyopathy | 2017 [90] | Cohort study | Patients with ATTR | ATTR diagnosis | 111 | Multivariate regression |
CHF, chronic heart failure; HC, health control; EPIC-Potsdam cohort, European Prospective Investigation into Cancer and Nutrition-Potsdam cohort; EH, essential hypertension; T2D, type 2 diabetes; DCM, diabetic cardiomyopathy; ACS, acute coronary syndrome; CHD, coronary heart disease; CAD, coronary artery disease; MACEs, major adverse cardiovascular events; ATTR, amyloid transthyretin; OHSPIW cohort, Occupational Health Study of Petroleum Industry Workers cohort.
3.1.2.1 Components of Metabolic Syndrome
Metabolic syndrome (MetS) is a collection of interconnected metabolic disorders (including obesity, hypertension, dyslipidemia, and insulin resistance) that collectively raise the risk of CVD and type 2 diabetes mellitus (T2DM) [91]. RBP4 was observed to be associated with multiple components of MetS, and elevated RBP4 levels in childhood are also good predictors of their cardiometabolic risk in adults [84].
RBP4 exhibits different characteristics in hypertensive patients of different genders, disease stages, and therapy. Plasma RBP4 concentration was significantly higher in the male hypertension population than in normotensive patients (median concentration [95% CI]: 43.4 [30.4–64.8] vs. 38.1 [27.1–54.4] ng/mL, respectively; p
In patients with diabetic cardiomyopathy, both RBP4 levels showed a positive linear association with the risk of diabetic DCM (odds ratio (OR) = 16.87 (6.5, 43.23); p
3.1.2.2 Vascular Disease
Among patients with CAD, the RBP4 concentration showed a positive correlation with small, dense low-density lipoprotein (sd-LDL) levels (r = 0.273; p = 0.001) and oxidized low-density lipoprotein (ox-LDL) levels (r = 0.167; p = 0.043). This suggests that RBP4 may play an important role in atherosclerosis, particularly in the formation of sd-LDL [59]. Additionally, RBP4 has been linked to vascular function and clinical prognosis. In patients with coronary heart disease (CHD) and T2DM, RBP4 is an independent risk factor for the coronary artery elasticity parameter
3.1.2.3 Cardiomyopathy
Amyloid transthyretin (ATTR) cardiomyopathy is a cause of HF in older adults that has been attributed to mutant TTR proteins or RBP4. Indeed, RBP4 was shown to be a predictor of ATTR in conjunction with LVEF, interventricular septal wall thickness, and mean limb lead voltage in a cohort study, with a threshold value of
RBP4 has been found to play a crucial role in the energy metabolism abnormalities associated with HF by regulating fatty acid metabolism, glucose utilization, and insulin signaling pathways through autocrine or paracrine signaling [96].
3.2.1.1 RBP4 Mediates Systemic Insulin Resistance Leading to Heart Failure
Optimal cardiac function requires a consistent supply of adenosine triphosphate (ATP) from two primary sources: mitochondrial oxidative phosphorylation and glycolysis, which require coordination between cardiomyocytes and the circulatory system [97]. Under normal conditions, the main source of energy for the myocardium is fatty acids (
RBP4-mediated insulin resistance can be categorized into two pathways: retinol-dependent and retinol-independent. The retinol–RBP4 complex mediates insulin resistance mainly through interaction with the STRA6 receptor, subsequently activating the JAK2/STAT5 suppressor of cytokine signaling 3 (SOCS3) signaling pathway. SOCS3 specifically inhibits the binding of Phosphoinositide 3-kinase (PI3K) to insulin receptor substrate (IRS)-1 by increasing its serine phosphorylation in the PI3K/Protein kinase B (AKT) pathway, leading to IR, which is mainly found in adipose tissues [61]. In the retinoid-independent mechanism, RBP4 increases the hepatic expression of phosphoenolpyruvate carboxykinase (PEPCK), which catalyzes the conversion of oxaloacetate to phosphoenolpyruvate, thereby increasing glucose production by gluconeogenesis in the liver. The chronic hyperglycemic state stimulates pancreatic
Glucose transporter 4 (GLUT4) expression is decreased in adipocytes in nearly all insulin-resistant states in humans and rodents. In a study by Yang et al. [7], adipose-specific deletion of glucose transporter-4 (adipose-GLUT4-/-) mice exhibited increased levels of RBP4 mRNA and serum protein, which increased PI3K activity by 80% in muscle tissue and interrupted insulin signaling. Insulin signaling in the heart is crucial for regulating myocardial metabolism of oxidative substrates, specifically glucose and fatty acids. Insulin resistance leads to a reduction in glucose oxidation in myocardial cells, either by decreasing glucose uptake or by directly inhibiting mitochondrial pyruvate dehydrogenase (PDH) activity [102]. This results in decreased myocardial glucose metabolism (MrGlu) and ATP production from glucose metabolism, subsequently leading to myocardial contractile dysfunction, resulting in decreased myocardial mechanical energy efficiency (MEEi), ultimately leading to myocardial hypertrophy and diastolic dysfunction. This is the primary mechanism through which RBP4 mediates HFpEF via insulin resistance [103]. During HFrEF, there is uncoupling between glycolysis and glucose oxidation, causing acidosis, which worsens contractile dysfunction in the failing heart by desensitizing contractile proteins to Ca2+, slowing the inward Ca2+ current, and redirecting cardiac ATP to ionic homeostasis instead of contractility [104]. Myocardial compensation mechanisms are dependent on excess fatty acid oxidation to maintain ATP production [105]. However, a study demonstrated that liver-specific RBP4 overexpression did not impair glucose homeostasis and whole-body energy metabolism in mice. This finding differs from other prior studies, which showed impairment of glucose homeostasis in mice with muscle-specific and adipose-specific overexpression of RBP4 [106, 107]. Thus, it was hypothesized that endogenous RBP4 protein levels may exhibit a distinct expression or secretion pattern, as well as a varying degree of retinol binding, compared to the overexpressed protein. The RBP4 secreted by the liver does not affect local RBP4 functions in adipose tissue and, therefore, fails to affect glucose homeostasis. Thus, the mechanism through which RBP4 mediates HF through insulin resistance requires further clarification [108].
In addition, RBP4 also enhances insulin-induced proliferation of RASMCs and the expression of p-ERK1/2 and p-JAK2, which can be inhibited by ERK1/2 and vitamin D inhibitors but not by JAK2 inhibitors [109, 110]. This mechanism may also result in HF.
3.2.1.2 RBP4-Mediated Heart Failure via Lipid Metabolism Disorders
In HF, while fatty acid oxidation (FAO) remains the primary source of ATP, a discrepancy can occur between lipid uptake and utilization in cardiomyocytes [111]. The production of malonyl-CoA is elevated, functioning as an allosteric inhibitor of carnitine palmitoyl transferase in various cell types, including cardiomyocytes, thereby restricting the transfer of fatty acids into mitochondria [112]. PPARs serve as the principal regulators of cardiac fatty acid metabolism, with PPAR
Intracellular lipids accumulate in HF patients primarily in the form of triglycerides (TGs), diacylglycerols (DAGs), ceramides, cholesterol, and its derivatives. Among these, ceramides and DAGs function as lipotoxic mediators, playing a role in cardiac lipotoxicity [116]. These two lipids change the structure of cell membranes, which directly leads to the death of cardiomyocytes and HFrEF [117]. RBP4 interferes with the anti-lipolytic function of insulin, increasing basal lipolysis and leading to the excessive release of free fatty acids (FFAs), which can be converted into DAG [118]. These DAGs are also associated with the acute induction of insulin resistance by temporally activating protein kinase (PKC)
Hypercholesterolemia has also been shown to be associated with a worse prognosis in HF by promoting hypertrophy and fibrosis [120], as demonstrated in hypertensive mouse models where lipoprotein lipase inhibitor P-407 worsened diastolic dysfunction [121]. Additionally, hypercholesterolemia disrupts the liver–heart crosstalk, increasing systemic metabolic dysfunction. Elevated RBP4 levels are associated with increased production of apolipoprotein B (a component of very low-density lipoprotein (VLDL)) [122, 123]. An independent association was observed between RBP4 and the percentage of small HDL particles, as well as between RBP4 and LDL-C, HDL-C, and TGs, suggesting that RBP4 may contribute to the formation of small HDL particles and altered lipoprotein profiles [124]. Elevated RBP4 results in increased cholesterol uptake in macrophages, primarily by influencing scavenger receptors such as CD36 and SR-A1. These receptors facilitate the internalization of ox-LDL, thereby enhancing lipid uptake in macrophages and promoting the formation of foam cells. This process contributes to inflammation in atherosclerotic lesions, which contributes to HF [125].
Several studies have investigated the impact of inflammatory factors on RBP4 concentrations. Bobbert et al. [126] demonstrated that IL-8 enhances adipocyte RBP4 expression in a dose-dependent manner. A positive correlation has also been found between adipose RBP4 and the inflammatory marker CD68 [100]. Though basic research on myocardial injury directly caused by RBP4 remains limited, it has been shown that RBP4 can trigger a decline in cardiac function and ultimately lead to HF by promoting myocardial inflammation, pyroptosis, and cardiomyocyte hypertrophy.
3.2.2.1 RBP4 Induces Myocardial Pyroptosis via the NLRP3/Caspase-1/GSDMD Axis
In a study by Zhang et al. [127], using a mouse model of AMI induced by left anterior descending coronary artery ligation, it was found that in the border zone of infarcted myocardium as well as in ischemia/hypoxia (I/H) -treated mouse primary heart cardiomyocytes, there was a marked increase in the expression of RBP4, and RBP4 activated caspase-1 cleavage through a direct interaction with NOD-like receptor family pyrin domain-containing 3 (NLRP3), which, in turn, induced a gasdermin D (GSDMD) dependent pyroptosis pathway. This process led to cardiomyocyte death and further deterioration of cardiac function. Knockdown of the RBP4 gene using an adenovirus was found to significantly attenuate the ischemia–hypoxia-induced cardiomyocyte injury and pyroptosis, suggesting a critical role of RBP4 in cardiomyocyte death [127]. CHF after myocardial pyroptosis is commonly the result of prolonged neurohormonal activation and sustained remodeling, in which necrotic tissue is replaced by scar tissue after myocardial infarction resulting in ventricular remodeling, with thinning of the infarcted myocardial wall and enlargement of the left ventricular cavity leading to loss of systolic function and increased wall stress [128], This remodeling process is also exacerbated by the activation of signaling pathways by elevated levels of catecholamines and angiotensin II (Ang II), as shown in Fig. 2 [129, 130].
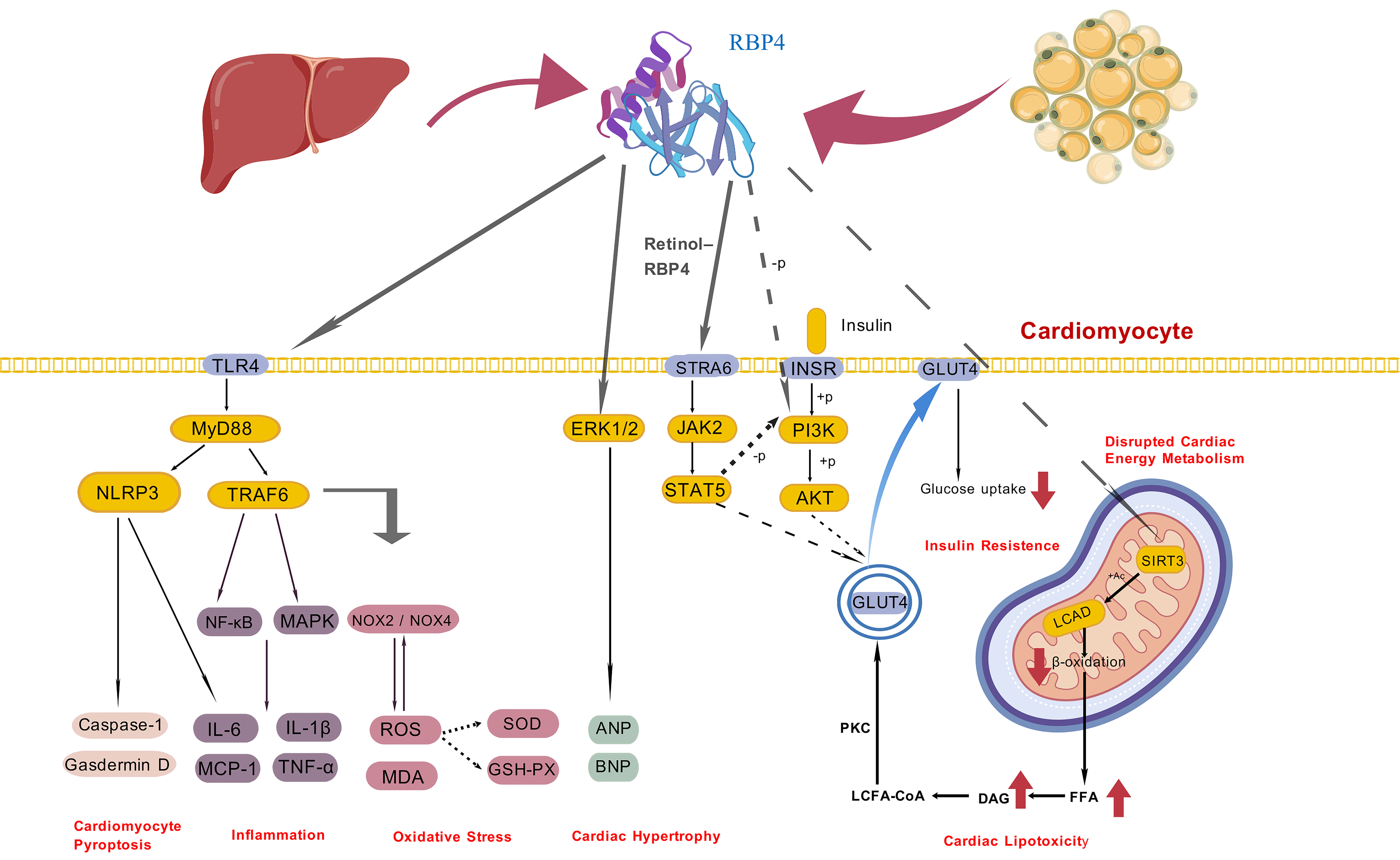 Fig. 2.
Fig. 2. Mechanism of RBP4 in heart failure. Created with BioGDP.com. This schematic diagram illustrates the molecular mechanisms through which retinol-binding protein 4 (RBP4) contributes to heart failure pathogenesis through: (1) activation of cardiomyocyte pyroptosis via the TLR/NLRP3 pathway, (2) activation of the cardiomyocyte inflammation via the TLR4/MYD88 pathway, (3) enhanced oxidative stress and generation of ROS, (4) promotion of cardiomyocyte hypertrophy via the MAPK/ERK pathway, (5) exacerbation of insulin resistance by the JAK2/STAT5 and PI3K/AKT pathway, (6) promotion of energy and metabolic lipid disorders. RBP4, retinol-binding protein 4; TLR4, Toll-like receptor 4; STRA6, stimulated by retinoic acid 6; INSR, insulin receptor; GLUT4, glucose transporter type 4; MYD88, myeloid differentiation primary response 88; NLRP3, NOD-like receptor family pyrin domain-containing 3; TRAF6, TNF receptor-associated factor 6; ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; ERK, extracellular regulated protein kinases; IL, interleukin; MCP-1, monocyte chemoattractant protein-1; NOX, NADPH oxidase; ROS, reactive oxygen species; MDA, malondialdehyde; SOD, superoxide dismutase; DAG, diacylglycerol; LCFA-CoA, long-chain fatty acyl-coenzyme A; PKC, protein kinase C; LCAD, long-chain Acyl-CoA dehydrogenase; FFAs, free fatty acids; SIRT3, sirtuin 3.
3.2.2.2 RBP4 Promotes Myocardial Inflammation and Hypertrophy via the TLR4/MYD88 Pathway
In a study by Gao et al. [131], a model of cardiac hypertrophy induced by transverse aortic constriction (TAC) and Ang II infusion was constructed in mice. RBP4 levels were found to be significantly higher in the serum TAC group than in the control group. RBP4 mRNA was selectively increased in white adipose tissue (WAT). In the Ang II group, serum RBP4 levels increased and were positively correlated with Ang II. In vitro experiments with RBP4-stimulated cardiomyocytes also showed a dose-dependent increase in cell volume and the level of RBP4, in addition to enhanced expression of inflammatory factors (e.g., TNF-
Increased oxidative stress is a key factor in the pathogenesis of HF, leading to inflammation, activation of the renin-angiotensin–aldosterone system and the sympathetic nervous system, as well as vascular remodeling and damage to cellular components, which contribute to myocardial dysfunction and the progression of HF [135]. In a study by Wang et al. [136], RBP4 levels showed a positive correlation with the oxidative stress marker 8-iso-PGF2
In view of the role of RBP4 in facilitating cardiac metabolism, oxidative stress, and myocardial injury, known critical contributors to HF, targeting RBP4 has become a promising therapeutic approach. The following paragraphs summarize the effectiveness of current pharmacological RBP4 inhibitors, metabolic medications, and direct RBP4 antagonists in alleviating the progression of HF.
RBP4 plays a significant role in cardiac metabolism in HF. Consequently, metabolic drugs that alter RBP4 levels may provide valuable therapeutic options for the management of HF.
Oral antidiabetic drugs, such as pioglitazone, a PPAR
In a study of CAD patients, rosuvastatin significantly reduced serum RBP4 levels and mitigated disease progression [143]. Wu and colleagues found that in studies on rats and 3T3-L1 adipocytes, fenofibrate decreased adipocyte RBP4 mRNA levels and improved insulin sensitivity. Treatment with fenofibrate for 8 weeks resulted in a 30% reduction in serum RBP4 concentrations in insulin-resistant and dyslipidemic males [144].
A natural compound, resveratrol, has been shown to protect the heart by decreasing oxidative stress and apoptosis in HF by activating Foxo3a [145]. Meanwhile, resveratrol has also been found to lower RBP4 expression and enhance cardiovascular function in rats [146].
Fenretinide, a synthetic retinol derivative, can enter the central cavity of the
Metabolic drugs may help to decrease RBP4 levels, whereas direct antagonists might protect the heart via other mechanisms. In the future, RBP4-targeted treatments may play an important therapeutic role in HF patients with RBP4-associated pathology.
Although RBP4 is now considered to be associated with HF, current research remains limited. The following points need to be clearly addressed in future studies: First, RBP4 lacks a clear threshold as a biomarker for HF. Large-scale clinical trials are required in the future to establish a standard threshold for RBP4. Second, multiple factors, including nutrition, hormones, and drugs, can affect circulating RBP4 levels. Most existing studies only consider a small portion of these factors, and future clinical research must strictly and comprehensively control for these confounding factors to minimize experimental error and bias. Third, while prior research has elucidated several potential mechanisms through which RBP4 may contribute to HF, the mechanism through which it affects the myocardium remains unclear. Hence, future studies are needed to determine the mechanism for these actions.
RBP4, a retinol transporter protein in the lipid transport protein family, plays an important role in the diagnosis, prognostic assessment, and pathogenesis of HF. Clinical studies have shown that high RBP4 levels correlate with risk factors and adverse cardiovascular events of HF, revealing its potential value for diagnosis and disease prognosis. The role of RBP4 in the pathogenesis of HF has been studied in metabolic disorders, inflammation, direct myocardial injury, and oxidative stress, which together exacerbate cardiac function. Metabolic drugs and direct RBP4 antagonists also provide some guidance for the treatment of HF. Future research will further elucidate the intricate mechanisms through which RBP4 functions, potentially leading to favorable alterations in the diagnosis and treatment of HF.
JYL was responsible for literature retrieval, manuscript drafting, and revisions, while YPW contributed to topic selection and final version approval. Both authors contributed to conception and critical revision of the manuscript for important intellectual content. Both authors read and approved the final manuscript. Both authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This study was supported by the Medicine and Health Science and Technology Project of Zhejiang Province (No.2022KY805 and No.2024KY1071 to Yaping Wang).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.


