1 Department of Cardiovascular Biology and Medicine, Juntendo University Graduate School of Medicine, 113-8421 Tokyo, Japan
2 Cardiovascular Respiratory Sleep Medicine, Juntendo University Graduate School of Medicine, 113-8421 Tokyo, Japan
3 Sleep and Sleep-Disordered Breathing Center, Juntendo University Hospital, 113-8421 Tokyo, Japan
4 Department of Cardiovascular Management and Remote Monitoring, Juntendo University Graduate School of Medicine, 113-8421 Tokyo, Japan
Abstract
Limited data are available regarding the prevalence of sleep-disordered breathing (SDB), particularly Cheyne–Stokes respiration (CSR), in patients with atrial fibrillation (AF) and left ventricular (LV) systolic dysfunction. Thus, this study aimed to investigate the prevalence of SDB and CSR, as well as the factors associated with these conditions, in patients with AF without LV systolic dysfunction.
Patients with paroxysmal and non-paroxysmal AF underwent echocardiography and cardiorespiratory polygraphy. Multiple linear regression analysis was performed using the apnea–hypopnea index (AHI) and %CSR as the dependent variables.
A total of 462 patients were enrolled; 335 patients (72.5%) were diagnosed with SDB (AHI ≥5/h), with a median AHI of 10.3 events per hour (interquartile range, 4.7–20.8). CSR was observed in 107 patients (23.2%). Multiple linear regression analysis showed that age, sex, body mass index, and hypertension were independently correlated with AHI (p = 0.0188, 0.0002, <0.0001, and 0.0457, respectively). Conversely, age, diabetes mellitus (DM), and the plasma N-terminal prohormone of brain natriuretic peptide (NT-proBNP) level were independently correlated with %CSR (p < 0.0001, 0.0047, and 0.0095, respectively).
SDB and CSR were common in patients with AF. CSR was observed in older patients with DM and high NT-proBNP levels.
Keywords
- atrial fibrillation
- sleep-disordered breathing
- Cheyne-Stokes respiration
Atrial fibrillation (AF) affects approximately 2.5% of adults above 40 years [1] and is associated with an increased risk of ischemic stroke and heart failure (HF) [2, 3]. In general, diabetes mellitus (DM), hypertension, obesity, alcohol consumption, and sleep-disordered breathing (SDB) are important modifiable risk factors for AF [4]. In patients with obstructive sleep apnea (OSA), several pathophysiologic mechanisms, such as exaggerated negative intrathoracic pressure, sympathetic overactivity, and intermittent nocturnal hypoxia/reoxygenation, contribute to cardiac overload, increased left ventricular (LV) filling pressure, and electrical and structural remodeling of the atrium, all of which predispose to the development of an AF substrate [5, 6, 7, 8]. Autonomic dysregulation associated with each respiratory event has been linked to the development of incident AF [6]. Indeed, the prevalence of SDB has been reported as 40–57% in patients with AF [8]. Since patients with AF and SDB often do not report excessive daytime sleepiness or fatigue [9], and access to sleep testing is limited, SDB may be underdiagnosed in patients with AF [10]. Thus, knowledge regarding the prevalence and associated factors of SDB in patients with AF is needed.
Cheyne-Stokes respiration (CSR), characterized by repetitive apneas or hypopneas alternating with hyperventilation in a crescendo-decrescendo pattern of tidal volume [11], is generally associated with HF through pulmonary congestion and prolonged circulation time in association with low cardiac output [8]. Although several studies have reported on the prevalence of CSR in patients with LV systolic dysfunction—regardless of the presence or absence of AF [8, 12]—and while an association between AF and CSR has been suggested in HF patients with LV systolic dysfunction [12], specific data regarding CSR in AF patients without LV systolic dysfunction are unavailable. Furthermore, data regarding the correlates of CSR in this patient population remain unclear. Thus, in the present study, we investigated the prevalence of SDB and CSR, and factors associated with them in patients with AF without LV systolic dysfunction.
Patients with paroxysmal or non-paroxysmal AF without LV systolic dysfunction
were prospectively enrolled at Juntendo University Hospital between May 2017 and
March 2024. Paroxysmal AF was defined as an AF episode that terminated within
seven days of onset, either spontaneously or after the administration of
antiarrhythmic drugs. Non-paroxysmal AF was defined as an AF episode lasting for
more than seven days [13]. Patients with a previous diagnosis or treatment of
SDB, LV ejection fraction (LVEF)
For the sleep study, all patients underwent cardiorespiratory polygraphy
(ApneaLink Air; ResMed, Sydney, Australia). CSR determined by ApneaLink is
clinically acceptable, as it has been used in large-scale studies [14, 15, 16] and
because performing polysomnography in all AF patients is not considered feasible
[10]. Thus, we opted to use ApneaLink. Respiratory effort, airflow measured via
the pressure sensor, snoring, pulse, oxygen saturation, and percentage of CSR
patterns were recorded. This device provides a flow-based classifier for CSR.
Apnea was defined as a
Demographic and medical histories were obtained through clinical chart reviews and interviews. Blood tests and echocardiographic data were obtained within one month of the sleep study. Serum levels of N-terminal prohormone of brain natriuretic peptide (NT-proBNP) were measured, and the estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease equation with a Japanese coefficient [17]. Complete two-dimensional echocardiography was performed according to the American Society of Echocardiography guidelines [18]. Images were stored for at least three cardiac cycles, and the final values represent the average of at least three measurements. LVEF was calculated using the modified Simpson method. All echocardiographic studies were performed and interpreted by experienced cardiologists who were blinded to the clinical data.
Continuous variables are expressed as mean
A total of 462 patients underwent cardiorespiratory polygraphy; 37% had mild
(AHI
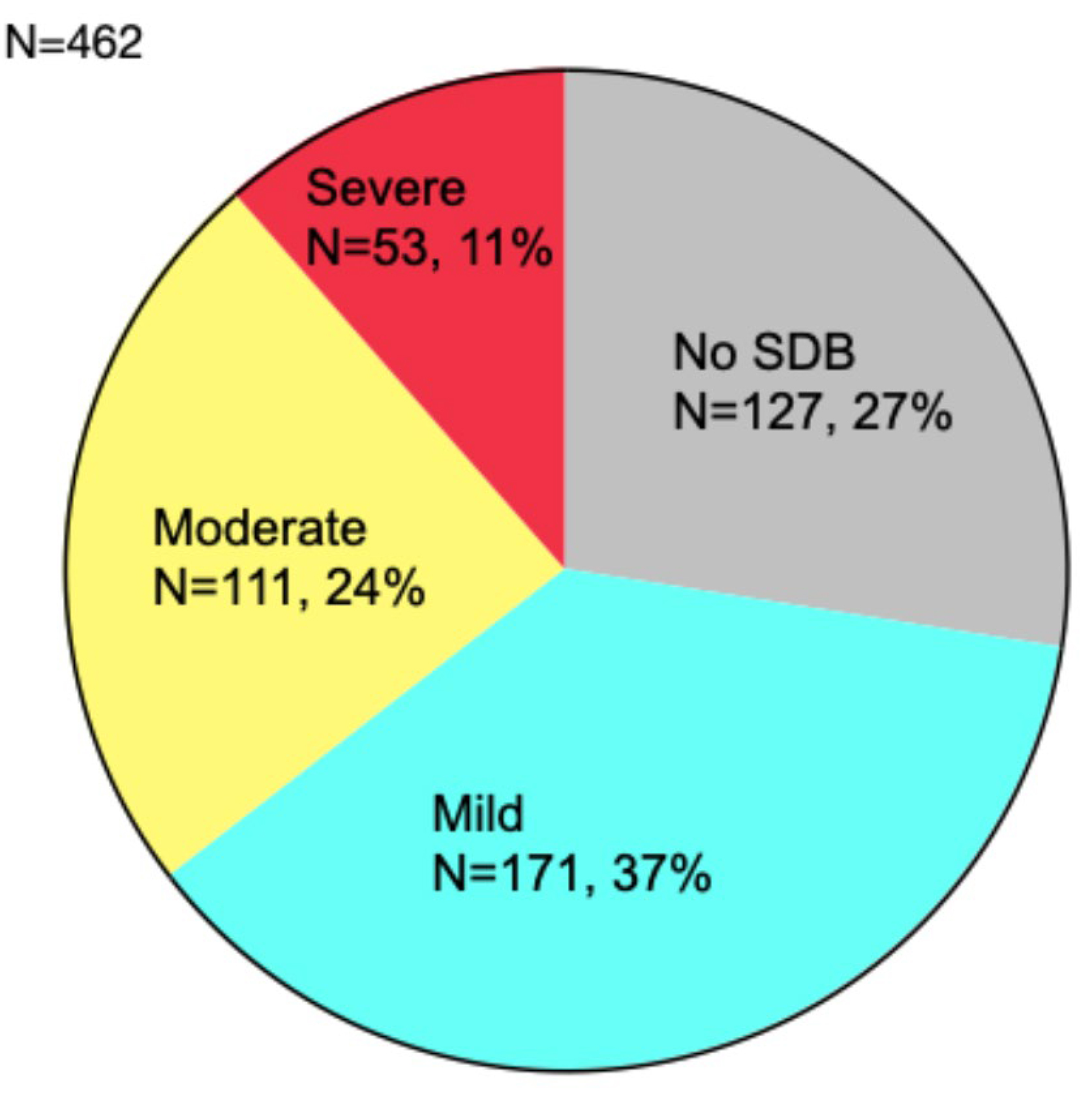 Fig. 1.
Fig. 1.
Severity and prevalence of SDB. SDB was observed in 335 patients (72.5%). SDB, sleep-disordered breathing.
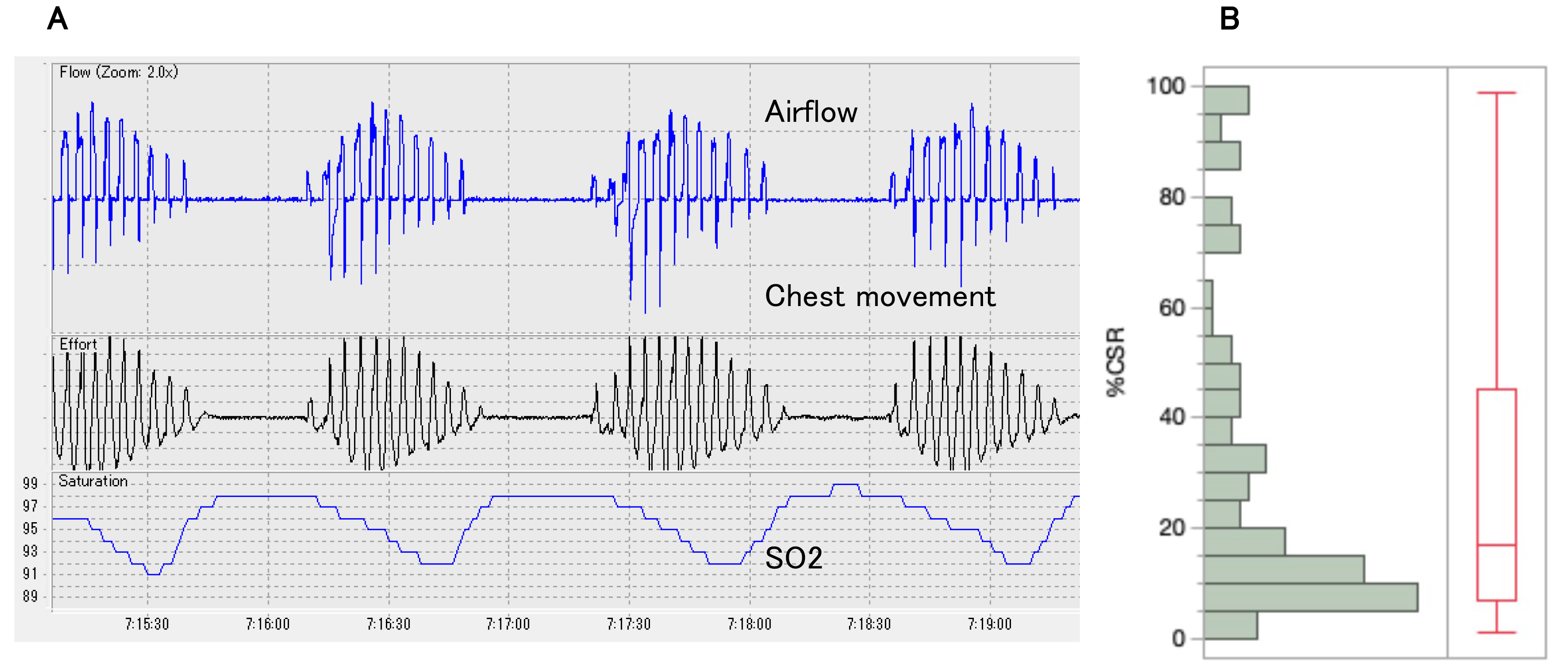 Fig. 2.
Fig. 2.
CSR observed in this study. (A) Typical CSR pattern: CSR is characterized by repetitive central apneas alternating with hyperventilation in a crescendo-decrescendo tidal volume pattern. (B) Distribution of %CSR: In patients with CSR (n = 107), the median %CSR was 17% (interquartile range, 7–45). CSR, Cheyne-Stokes respiration.
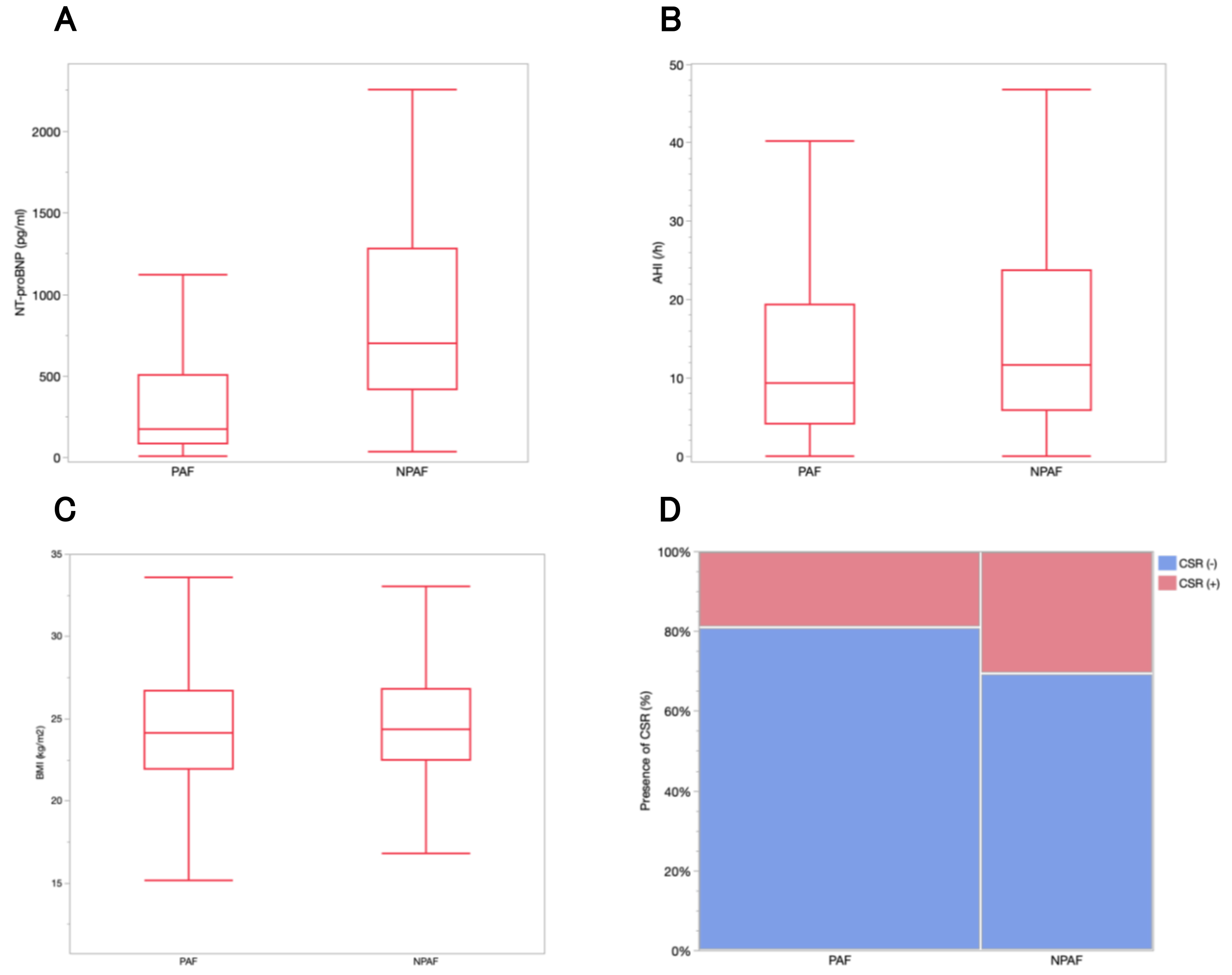 Fig. 3.
Fig. 3.
NT-proBNP levels, AHI, and the percentage of patients with CSR
according to AF type. (A) The median NT-proBNP level was 176 pg/mL
(interquartile range, 83.8–504.5) in patients with PAF and 700.5 pg/mL
(interquartile range, 414–1279.3) in those with NPAF (p
| Characteristic | All N = 462 | CSR (+) N = 107 | CSR (-) N = 355 | p-value |
| Age, years | 67.6 |
73.1 |
65.9 |
|
| Female sex, n (%) | 104 (23%) | 20 (19%) | 84 (24%) | 0.2747 |
| BMI, kg/m2 | 24.6 |
24.8 |
24.5 |
0.4968 |
| Non-paroxysmal AF, n (%) | 175 (38%) | 53 (50%) | 122 (34%) | 0.0049 |
| History of HF, n (%) | 111 (24%) | 40 (37%) | 71 (20%) | 0.0002 |
| History of stroke, n (%) | 39 (8%) | 9 (8%) | 30 (9%) | 0.9711 |
| Diabetes mellitus, n (%) | 96 (21%) | 35 (33%) | 61 (17%) | 0.0005 |
| Hypertension, n (%) | 252 (55%) | 66 (62%) | 186 (53%) | 0.0873 |
| Dyslipidemia, n (%) | 199 (43%) | 43 (41%) | 156 (44%) | 0.4945 |
| Hyperuricemia, n (%) | 117 (25%) | 30 (28%) | 87 (25%) | 0.4580 |
| Warfarin, n (%) | 43 (9%) | 14 (13%) | 29 (8%) | 0.1241 |
| DOAC, n (%) | 385 (84%) | 90 (85%) | 295 (84%) | 0.7864 |
| ACE/ARB/ARNI, n (%) | 189 (41%) | 56 (53%) | 133 (38%) | 0.0058 |
| Beta blocker, n (%) | 260 (57%) | 77 (73%) | 183 (52%) | 0.0002 |
| CCB, n (%) | 170 (37%) | 38 (35%) | 132 (38%) | 0.7577 |
| Diuretics, n (%) | 101 (22%) | 38 (36%) | 63 (18%) | |
| Statin, n (%) | 126 (27%) | 37 (35%) | 89 (25%) | 0.0518 |
| Antiarrhythmic drug, n (%) | 197 (43%) | 38 (36%) | 159 (45%) | 0.0890 |
| Hemoglobin, g/dL | 14.3 |
14.4 |
14.3 |
0.6326 |
| Creatinine, mg/dL | 0.9 |
1.0 |
0.9 |
0.0001 |
| eGFR, mL/min/1.73 cm2 | 66.4 |
59.7 |
68.3 |
|
| NT-proBNP, pg/mL | 342 (134.0–810.8) | 698 (284.5–1253.3) | 269 (116.5–677) |
Data are expressed as mean
AF, atrial fibrillation; ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; BMI, body mass index; CCB, calcium channel blocker; DOAC, direct oral anticoagulant; eGFR, estimated glomerular filtration rate; HF, heart failure; NT-proBNP, N-terminal prohormone of brain natriuretic peptide.
| Variable | N = 462 | CSR (+) N = 107 | CSR (-) N = 355 | p-value |
| LVDd, mm | 47.3 |
48.0 |
47.2 |
0.1778 |
| LVDs, mm | 31.1 |
32.4 |
30.8 |
0.0150 |
| IVSd, mm | 9.8 |
10.3 |
9.7 |
0.0002 |
| PWd, mm | 9.7 |
10.2 |
9.6 |
|
| LVEF, % | 62.9 |
60.6 |
63.6 |
0.0055 |
| LAVI, mL/m2 | 45.5 |
54.7 |
42.7 |
|
| DcT, ms | 186.4 |
187.6 |
186.3 |
0.8499 |
| E/e’ | 10.6 |
11.7 |
10.3 |
0.0162 |
| RVSP, mmHg | 25.7 |
27.0 |
25.2 |
0.0264 |
Data are expressed as mean
DcT, deceleration time; IVSd, interventricular septum (diastolic); LAVI, left atrial volume index; LVDd, left ventricular diameter (diastolic); LVDs, left ventricular diameter (systolic); PWd, posterior wall (diastolic); RVSP, right ventricular systolic pressure; LVEF, left ventricular ejection fraction.
| Variable | N = 462 |
| AHI (events per hour) | 10.3 (4.7–20.8) |
| 3% ODI (events per hour) | 13.5 (7.0–24.7) |
| Mean SpO2 (%) | 95.0 (94.0–96.0) |
| Lowest SpO2 (%) | 85.0 (80.0–89.0) |
| Coexisting CSR, n (%) | 107 (23.2%) |
Data are expressed as median (interquartile range) for continuous variables and numbers (%) for nominal variables.
AHI, apnea-hypopnea index; CSR, Cheyne-Stokes respiration; ODI, oxygen desaturation index; SpO2, oxyhemoglobin saturation.
Univariate analysis showed that age, sex, BMI, hypertension, left atrial volume index (LAVI), and non-paroxysmal AF were correlated with AHI. Multivariate analysis identified age, sex, BMI, and hypertension as independent correlates of AHI (Table 4).
| Univariable | Multivariable | |||
| Correlation coefficient | p | Partial correlation coefficient | p | |
| Age | 0.095 | 0.0406 | 0.122 | 0.0188 |
| Female sex | –0.174 | 0.0002 | –0.181 | 0.0002 |
| BMI | 0.250 | 0.230 | ||
| Hypertension | 0.198 | 0.099 | 0.0457 | |
| LAVI | 0.095 | 0.0479 | 0.055 | 0.2889 |
| non-paroxysmal AF | 0.098 | 0.0362 | –0.024 | 0.6296 |
BMI, body mass index; LAVI, left atrial volume index; AF, atrial fibrillation.
Univariate analysis showed that age, DM, angiotensin-converting enzyme (ACE)/angiotensin II receptor blocker (ARB)/angiotensin receptor neprilysin inhibitor (ARNI), beta-blockers, diuretics, eGFR, NT-proBNP, LVEF, and E/e’ were associated with %CSR. Multivariate analysis identified age, DM, and NT-proBNP level as independent correlates of %CSR (Table 5).
| Univariable | Multivariable | |||
| Correlation coefficient | p | Partial correlation coefficient | p | |
| Age | 0.284 | 0.246 | ||
| DM | 0.159 | 0.0006 | 0.138 | 0.0047 |
| ACE/ARB/ARNI | 0.153 | 0.0011 | 0.053 | 0.3263 |
| Beta-blocker | 0.179 | 0.0001 | 0.086 | 0.0995 |
| Diuretics | 0.211 | 0.058 | 0.3297 | |
| eGFR | 0.221 | 0.025 | 0.6830 | |
| NT-proBNP | 0.242 | 0.152 | 0.0095 | |
| LVEF | 0.150 | 0.0013 | 0.043 | 0.4475 |
| E/e’ | 0.121 | 0.0115 | 0.031 | 0.5892 |
DM, diabetes mellitus; ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; eGFR, estimated glomerular filtration rate; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; LVEF, left ventricular ejection fraction.
The findings of this study were as follows: (1) 72.5% of AF patients had SDB; (2) 23.1% of AF patients had CSR; (3) AHI was associated with age, sex, BMI, and hypertension; and (4) the presence of CSR was associated with older age, DM, and elevated NT-proBNP levels, suggesting that in patients with AF, SDB is highly prevalent, particularly among older, obese men with hypertension. CSR is also prevalent, particularly among older patients with diabetes and high LV filling pressure. Since specific data regarding CSR in AF patients without LV systolic dysfunction are lacking, the finding that 23% of these patients have CSR—along with the abovementioned correlates that are similar to those seen in other patient populations—represents a novel and significant contribution of the present study. The correlates of AHI and CSR identified in the present study are plausible. In the general population, factors such as age, male sex, BMI, and comorbid hypertension are associated with increased AHI. Additionally, in other patient populations, older patients with elevated LV filling pressure are more likely to exhibit CSR. Moreover, limited data suggest that patients with DM may experience CSR, even in the absence of AF [20, 21].
In terms of prevalence of SDB, Traaen et al. [22] found that 82.7% of
579 patients with paroxysmal AF had SDB (AHI
However, the relationship between AF and CSR may be bidirectional. On one hand,
CSR can contribute to the development of AF. Previous studies have reported that
central sleep apnea as suggestive of CSR is a predictor of incident AF, even in
cohort studies [28, 29]. For instance, Tung et al. [30] reported that
central sleep apnea as suggestives of CSR was associated with approximately a
twofold increase in the odds of developing AF. Similarly, May et al.
[31] reported that central sleep apnea as suggestives of CSR predicted a higher
risk of AF in older men, particularly those aged 76 years or older. In patients
with HF, one of the risk factors for CSR with central sleep apnea is AF,
alongside male sex, age
In the present study, older age was significantly associated with CSR. This aligns with previous reports indicating that central sleep apnea or CSR is more prevalent in older adults [12, 29, 30, 38], and this is true in AF patients without LV systolic dysfunction. Although the reasons for this age-related increase remain unclear, age-related diastolic dysfunction [39], which is associated with increased LV filling pressure, may partly explain this finding in our patient population. Our findings also suggest that DM is associated with an increased likelihood of CSR in patients with AF. This may be due to diabetes-associated autonomic dysfunction, which enhances chemoreceptor sensitivity [20]. Additionally, data from the Sleep Heart Health Study showed that periodic breathing patterns were more common in patients with DM [21]. Although the other CSR correlates found in the present study are similar to those in other patient populations, physicians should consider the relationship between CSR and DM when managing patients with AF.
A recent randomized controlled trial showed that short-term continuous positive airway pressure (CPAP) therapy did not significantly reduce AF burden in patients with paroxysmal AF and moderate-to-severe SDB, probably due to insufficient statistical power and a short follow-up period [40]. However, several observational studies have indicated that CPAP therapy for SDB may be beneficial for rhythm control in AF [41]. Thus, CPAP is generally considered a treatment option for managing SDB in patients with AF. Nevertheless, clinicians encounter patients with AF whose SDB cannot be sufficiently alleviated by CPAP. Considering the findings of the present study, the presence of CSR may explain why certain cases of SDB are resistant to CPAP in patients with AF. Furthermore, in patients with AF, SDB assessments are likely to be performed using polygraphy, which cannot detect CSR, contributing to this challenge. Although not specific to patients with AF, unsuppressed SDB by CPAP is associated with poor prognosis in patients with HF and systolic dysfunction [42, 43]. Thus, the therapeutic options for SDB in patients with AF require further discussion.
The main limitation of this study is its observational, single-academic center design, and the findings are based on a cross-sectional analysis. Therefore, the results do not support a cause-and-effect relationship between SDB or CSR and other factors. Furthermore, sleep studies were conducted using polygraphy, rather than polysomnography. Accurate diagnosis of CSR and differentiation of respiratory events ideally requires polysomnography with monitoring of the CO2 level or ventilation data. However, polysomnography is infeasible for all patients with AF because of the associated costs, limited access, and long waiting times [10]. Therefore, in the present study, we evaluated SDB and CSR using polygraphy, which is a simple, inexpensive, and feasible tool. Nevertheless, the absence of multi-night polygraphy recordings represents a limitation. Additionally, we did not perform systematic assessments of CSR before and after AF treatment, and electrocardiogram was not included in the polygraphy of this study. Therefore, the relationship between CSR and AF burden remains unclear. Finally, the specific effects of coexisting SDB or CSR on clinical outcomes, including incident HF or stroke, are still unknown in this patient population. Further studies are needed to investigate the effects of coexisting SDB and CSR on the clinical outcomes of patients with AF.
SDB is common in patients with AF, and its severity is associated with aging, male sex, increased BMI, and comorbid hypertension. CSR is also common and is linked to aging, DM, and increased NT-proBNP levels, suggesting the presence of pulmonary congestion.
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
HM and TKas designed this study. HM and AS performed the recruitment of participants. HM analyzed the data. HM and TKas drafted the manuscript. HM, TKas, AS, NS, SI, SY, JS, TKat, SS, RN, HH, TM and HD contributed to critical revision of the manuscript for important intellectual content and conception. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study protocol was approved by the Institutional Research Ethics Board (Hospital Ethics Committee Juntendo University Hospital; approval code: UMIN000029327), and the study complied with the ethical principles of the Declaration of Helsinki. All patients provided written informed consent for the use of their data, and all identifying information was removed.
Not applicable.
Funding support was provided by ResMed. In addition, this was partially supported by Grant to the Intractable Respiratory Diseases and Pulmonary Hypertension Research Group from the Ministry of Health, Labour and Welfare, Japan, Grant/Award Number: 23FC1031; JSPS KAKENHI, Grant/Award Number: 21K08116, 21K16034; research grant from the Japanese Center for Research on Women in Sport, Juntendo University.
Takatoshi Kasai and Nanako Shiroshita are affiliated with a department endowed with Philips, ResMed, Fukuda Denshi and the Paramount Bed. However, these companies had no role in the handling or conduct of the study. The authors had full access to all data in the study and take full responsibility for the integrity of the data and the accuracy of the data analysis. Takatoshi Kasai is serving as one of the Editorial Board members and Guest Editors of this journal. We declare that Takatoshi Kasai had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Karol E. Watson and Vladimir M. Pokrovskii.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.



